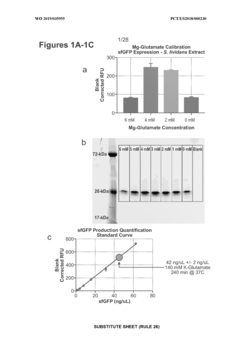
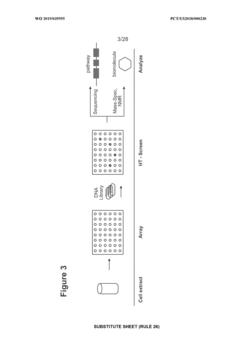
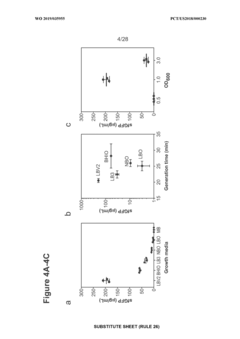
Enhancing throughput in cell-free biosynthesis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Biosynthesis Background and Objectives
Cell-free biosynthesis represents a paradigm shift in biotechnology, emerging from the convergence of synthetic biology, biochemistry, and bioengineering. This technology extracts cellular machinery from living cells to create a cell-free environment where biochemical reactions can be precisely controlled and optimized. Since its conceptual origins in the 1960s with the development of cell-free protein synthesis systems, the field has evolved dramatically, particularly accelerating in the past decade with advances in enzyme engineering and metabolic pathway reconstruction.
The fundamental principle of cell-free biosynthesis involves utilizing cellular components (enzymes, cofactors, and metabolites) outside the constraints of cell walls, eliminating many limitations associated with traditional whole-cell fermentation. This approach offers unprecedented control over reaction conditions, pathway engineering, and product formation, making it particularly valuable for the synthesis of complex biomolecules, pharmaceuticals, and specialty chemicals.
Current technological trends in cell-free biosynthesis focus on enhancing system stability, improving energy regeneration mechanisms, and developing more efficient extract preparation methods. The integration of computational modeling with experimental approaches has enabled more rational design of cell-free systems, while advances in microfluidics and automation have facilitated high-throughput screening and optimization.
The primary objective in enhancing throughput in cell-free biosynthesis centers on overcoming several key limitations. These include the relatively short lifetime of cell-free reactions, limited cofactor regeneration capacity, and scalability challenges. Researchers aim to develop systems capable of sustained production over extended periods, with higher volumetric productivity and improved economic viability for industrial applications.
Another critical goal involves expanding the repertoire of molecules that can be efficiently produced in cell-free systems. This includes complex natural products, non-canonical proteins incorporating unnatural amino acids, and novel biopolymers with unique properties. The development of modular, plug-and-play cell-free platforms that can be rapidly reconfigured for different biosynthetic pathways represents a significant technological aspiration.
From a broader perspective, cell-free biosynthesis aims to revolutionize biomanufacturing by offering a more sustainable alternative to traditional chemical synthesis methods. The technology promises reduced environmental footprints, more efficient use of resources, and the ability to produce compounds that are challenging or impossible to synthesize through conventional means. As climate change and resource scarcity become increasingly pressing concerns, the development of high-throughput cell-free biosynthesis systems aligns with global sustainability objectives.
The fundamental principle of cell-free biosynthesis involves utilizing cellular components (enzymes, cofactors, and metabolites) outside the constraints of cell walls, eliminating many limitations associated with traditional whole-cell fermentation. This approach offers unprecedented control over reaction conditions, pathway engineering, and product formation, making it particularly valuable for the synthesis of complex biomolecules, pharmaceuticals, and specialty chemicals.
Current technological trends in cell-free biosynthesis focus on enhancing system stability, improving energy regeneration mechanisms, and developing more efficient extract preparation methods. The integration of computational modeling with experimental approaches has enabled more rational design of cell-free systems, while advances in microfluidics and automation have facilitated high-throughput screening and optimization.
The primary objective in enhancing throughput in cell-free biosynthesis centers on overcoming several key limitations. These include the relatively short lifetime of cell-free reactions, limited cofactor regeneration capacity, and scalability challenges. Researchers aim to develop systems capable of sustained production over extended periods, with higher volumetric productivity and improved economic viability for industrial applications.
Another critical goal involves expanding the repertoire of molecules that can be efficiently produced in cell-free systems. This includes complex natural products, non-canonical proteins incorporating unnatural amino acids, and novel biopolymers with unique properties. The development of modular, plug-and-play cell-free platforms that can be rapidly reconfigured for different biosynthetic pathways represents a significant technological aspiration.
From a broader perspective, cell-free biosynthesis aims to revolutionize biomanufacturing by offering a more sustainable alternative to traditional chemical synthesis methods. The technology promises reduced environmental footprints, more efficient use of resources, and the ability to produce compounds that are challenging or impossible to synthesize through conventional means. As climate change and resource scarcity become increasingly pressing concerns, the development of high-throughput cell-free biosynthesis systems aligns with global sustainability objectives.
Market Analysis for Cell-Free Protein Production
The cell-free protein production market has witnessed significant growth in recent years, driven by increasing demand for rapid protein synthesis in research, therapeutics, and industrial applications. The global market value for cell-free protein expression systems reached approximately $208 million in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 6.4% through 2027, potentially reaching $322 million by that time.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These companies utilize cell-free systems primarily for rapid production of therapeutic proteins, antibodies, and vaccine candidates. The academic and research institution segment follows closely, comprising about 30% of the market, where cell-free systems are extensively used for protein characterization and synthetic biology applications.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing investments in biotechnology research and development infrastructure.
The market is currently segmented by expression system types, with E. coli-based systems leading at 35% market share, followed by rabbit reticulocyte lysate (25%), wheat germ extract (20%), and insect cell-based systems (15%). Other emerging systems account for the remaining 5%.
Key market drivers include the increasing need for rapid protein production, rising demand for personalized medicine, and advancements in synthetic biology. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid vaccine and therapeutic development platforms.
Challenges limiting market expansion include high costs associated with cell-free reagents, technical limitations in scaling up production, and regulatory uncertainties surrounding products developed using these systems. The average cost of commercial cell-free protein expression kits ranges from $400 to $1,200, which remains prohibitively expensive for widespread adoption in certain applications.
Customer segments are diversifying beyond traditional research applications, with emerging interest from diagnostic companies, agricultural biotechnology firms, and synthetic food developers. This expansion of application areas is expected to create new market opportunities, potentially adding $50-75 million to the market size by 2025.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These companies utilize cell-free systems primarily for rapid production of therapeutic proteins, antibodies, and vaccine candidates. The academic and research institution segment follows closely, comprising about 30% of the market, where cell-free systems are extensively used for protein characterization and synthetic biology applications.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing investments in biotechnology research and development infrastructure.
The market is currently segmented by expression system types, with E. coli-based systems leading at 35% market share, followed by rabbit reticulocyte lysate (25%), wheat germ extract (20%), and insect cell-based systems (15%). Other emerging systems account for the remaining 5%.
Key market drivers include the increasing need for rapid protein production, rising demand for personalized medicine, and advancements in synthetic biology. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid vaccine and therapeutic development platforms.
Challenges limiting market expansion include high costs associated with cell-free reagents, technical limitations in scaling up production, and regulatory uncertainties surrounding products developed using these systems. The average cost of commercial cell-free protein expression kits ranges from $400 to $1,200, which remains prohibitively expensive for widespread adoption in certain applications.
Customer segments are diversifying beyond traditional research applications, with emerging interest from diagnostic companies, agricultural biotechnology firms, and synthetic food developers. This expansion of application areas is expected to create new market opportunities, potentially adding $50-75 million to the market size by 2025.
Technical Barriers in Cell-Free Biosynthesis Systems
Despite significant advancements in cell-free biosynthesis systems, several technical barriers continue to limit throughput enhancement. The primary challenge remains the short-lived nature of these systems, with most reactions maintaining productivity for only 4-10 hours before declining. This temporal limitation stems from multiple factors including the degradation of essential components, accumulation of inhibitory byproducts, and depletion of energy resources.
Energy regeneration represents a critical bottleneck in maintaining high-throughput operations. Traditional ATP regeneration systems often fail to sustain prolonged synthesis, leading to premature reaction termination. Secondary energy carriers like NAD(P)H face similar stability issues, further complicating continuous production efforts. Recent attempts at implementing more robust energy cascades have shown promise but remain inefficient at industrial scales.
Substrate availability and transport mechanisms pose additional challenges. Unlike living cells with sophisticated membrane transport systems, cell-free environments lack efficient methods for continuous substrate delivery and product removal. This limitation creates concentration gradients that reduce reaction efficiency over time and inhibit sustained high-throughput production.
Protein stability in cell-free environments presents another significant barrier. Without cellular repair mechanisms, enzymes gradually lose activity through denaturation, oxidation, and aggregation. This degradation accelerates under the intensive conditions required for high-throughput operations, creating a fundamental constraint on extended production runs.
Scale-up complications further impede industrial implementation of high-throughput cell-free systems. Laboratory-scale successes often fail to translate to industrial settings due to mixing inefficiencies, heat transfer limitations, and component stratification in larger vessels. These engineering challenges become particularly pronounced when attempting to maintain the precise conditions necessary for optimal enzymatic activity across larger volumes.
Batch-to-batch variability remains problematic for consistent throughput enhancement. Cell lysate preparation methods lack standardization, resulting in significant performance differences between production runs. This inconsistency complicates process optimization and creates barriers to reliable scale-up strategies.
Inhibitory byproduct accumulation represents a particularly challenging barrier. Without cellular detoxification pathways, metabolic intermediates and reaction byproducts accumulate to inhibitory levels, creating feedback inhibition that progressively reduces throughput. Current product removal strategies like dialysis membranes offer partial solutions but introduce additional complexity and cost to the system.
Cofactor stability and regeneration efficiency present specialized challenges for complex biosynthetic pathways. Many high-value products require multiple cofactors that must be continuously regenerated, creating intricate dependencies that can collapse when any single component fails.
Energy regeneration represents a critical bottleneck in maintaining high-throughput operations. Traditional ATP regeneration systems often fail to sustain prolonged synthesis, leading to premature reaction termination. Secondary energy carriers like NAD(P)H face similar stability issues, further complicating continuous production efforts. Recent attempts at implementing more robust energy cascades have shown promise but remain inefficient at industrial scales.
Substrate availability and transport mechanisms pose additional challenges. Unlike living cells with sophisticated membrane transport systems, cell-free environments lack efficient methods for continuous substrate delivery and product removal. This limitation creates concentration gradients that reduce reaction efficiency over time and inhibit sustained high-throughput production.
Protein stability in cell-free environments presents another significant barrier. Without cellular repair mechanisms, enzymes gradually lose activity through denaturation, oxidation, and aggregation. This degradation accelerates under the intensive conditions required for high-throughput operations, creating a fundamental constraint on extended production runs.
Scale-up complications further impede industrial implementation of high-throughput cell-free systems. Laboratory-scale successes often fail to translate to industrial settings due to mixing inefficiencies, heat transfer limitations, and component stratification in larger vessels. These engineering challenges become particularly pronounced when attempting to maintain the precise conditions necessary for optimal enzymatic activity across larger volumes.
Batch-to-batch variability remains problematic for consistent throughput enhancement. Cell lysate preparation methods lack standardization, resulting in significant performance differences between production runs. This inconsistency complicates process optimization and creates barriers to reliable scale-up strategies.
Inhibitory byproduct accumulation represents a particularly challenging barrier. Without cellular detoxification pathways, metabolic intermediates and reaction byproducts accumulate to inhibitory levels, creating feedback inhibition that progressively reduces throughput. Current product removal strategies like dialysis membranes offer partial solutions but introduce additional complexity and cost to the system.
Cofactor stability and regeneration efficiency present specialized challenges for complex biosynthetic pathways. Many high-value products require multiple cofactors that must be continuously regenerated, creating intricate dependencies that can collapse when any single component fails.
Current Throughput Enhancement Strategies
01 High-throughput cell-free protein synthesis systems
Cell-free protein synthesis systems designed for high-throughput applications enable rapid production of proteins without the constraints of living cells. These systems typically utilize optimized reaction conditions, automated platforms, and miniaturized formats to increase productivity. They allow for parallel synthesis of multiple proteins simultaneously, making them valuable tools for protein engineering, drug discovery, and functional genomics studies.- High-throughput cell-free protein synthesis systems: Cell-free protein synthesis systems enable rapid and efficient production of proteins without the constraints of living cells. These systems can be optimized for high-throughput applications by incorporating automated platforms, miniaturized reaction volumes, and parallel processing capabilities. Such systems allow for the simultaneous synthesis of multiple proteins, accelerating research and development processes in biotechnology and pharmaceutical industries.
- Cell-free biosynthesis for therapeutic applications: Cell-free biosynthesis systems are increasingly used for the production of therapeutic proteins and compounds. These systems offer advantages such as reduced contamination risk, simplified purification processes, and the ability to produce proteins that might be toxic to living cells. The technology enables the synthesis of complex biomolecules including antibodies, vaccines, and personalized medicines with improved throughput compared to traditional cell-based methods.
- Microfluidic and miniaturized cell-free biosynthesis platforms: Miniaturized cell-free biosynthesis platforms utilize microfluidic technologies to enhance throughput and efficiency. These systems reduce reaction volumes to micro or nanoliter scale, allowing for thousands of parallel reactions. Integration with automated liquid handling systems and detection methods enables rapid screening and optimization of reaction conditions, significantly increasing the throughput of protein production and enzyme evolution experiments.
- Enhanced cell-free biosynthesis through genetic and metabolic engineering: Genetic and metabolic engineering approaches can significantly improve the throughput and yield of cell-free biosynthesis systems. Techniques include optimizing energy regeneration pathways, removing inhibitory byproducts, engineering translation factors, and incorporating non-canonical amino acids. These modifications create more robust and efficient cell-free systems capable of sustained protein production at higher yields than conventional methods.
- Analytical and monitoring technologies for cell-free biosynthesis: Advanced analytical and monitoring technologies are essential for optimizing cell-free biosynthesis throughput. Real-time monitoring systems using fluorescence, mass spectrometry, or electrochemical sensors allow for continuous assessment of reaction progress and product formation. High-throughput screening methods combined with machine learning algorithms enable rapid optimization of reaction conditions and identification of high-performing synthesis parameters.
02 Cell-free biosynthesis for therapeutic applications
Cell-free biosynthesis systems are employed for the production of therapeutic proteins, antibodies, and other biopharmaceuticals. These systems offer advantages such as reduced contamination risk, elimination of cellular toxicity issues, and improved control over reaction conditions. The technology enables rapid production of therapeutic candidates for testing and can be scaled for manufacturing applications, potentially accelerating drug development timelines.Expand Specific Solutions03 Microfluidic and miniaturized cell-free biosynthesis platforms
Miniaturized cell-free biosynthesis platforms utilize microfluidic technologies to enhance throughput and efficiency. These systems require smaller reaction volumes, reducing reagent consumption while maintaining or improving productivity. Microfluidic platforms enable precise control over reaction conditions, continuous monitoring, and integration with detection systems, making them suitable for high-throughput screening applications and point-of-care diagnostics.Expand Specific Solutions04 Enhanced cell-free biosynthesis through genetic and metabolic engineering
Genetic and metabolic engineering approaches are used to optimize cell-free biosynthesis systems for increased throughput. These include modifying extract preparation methods, engineering energy regeneration pathways, optimizing translation factors, and incorporating synthetic genetic elements. Such modifications can significantly improve protein yield, extend reaction longevity, and enhance the overall efficiency of cell-free biosynthesis processes.Expand Specific Solutions05 Analytical and monitoring technologies for cell-free biosynthesis
Advanced analytical and monitoring technologies are crucial for optimizing cell-free biosynthesis throughput. These include real-time monitoring systems, high-sensitivity detection methods, and integrated analytical platforms that provide immediate feedback on reaction progress and product formation. Such technologies enable rapid optimization of reaction conditions, quality control of synthesized products, and identification of bottlenecks in the biosynthetic process.Expand Specific Solutions
Leading Organizations in Cell-Free Biosynthesis Research
Cell-free biosynthesis is currently in a growth phase, with increasing market adoption driven by its advantages in protein production efficiency. The global market is expanding rapidly, estimated to reach significant scale as applications in pharmaceuticals, industrial enzymes, and synthetic biology grow. Technologically, the field is advancing from early-stage development toward commercial maturity, with key players demonstrating varied capabilities. Academic institutions like Tsinghua University, MIT, and Northwestern University are pioneering fundamental research, while specialized companies including Cellfree Sciences, GreenLight Biosciences, and Spiber are commercializing applications. WuXi Biologics and BASF represent larger corporations integrating cell-free systems into their platforms. The ecosystem shows a healthy balance between academic innovation and industrial implementation, with throughput enhancement remaining a central focus for competitive advantage.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the WEPRO® system, a proprietary wheat germ extract-based cell-free protein synthesis platform that significantly enhances throughput in cell-free biosynthesis. Their technology utilizes optimized wheat germ extracts that naturally lack inhibitory nucleases and proteases found in other cell-free systems. The company employs a bilayer reaction format where translation substrates are continuously supplied from a substrate layer to the reaction layer, enabling prolonged protein synthesis and higher yields. Their high-throughput automated robotic systems can perform parallel synthesis of thousands of proteins simultaneously, with yields reaching up to 10 mg/mL of target protein. Cellfree Sciences has also developed specialized mRNA templates with enhanced stability and translation efficiency, incorporating optimized 5' and 3' untranslated regions that increase protein expression by up to 5-fold compared to standard templates.
Strengths: Superior protein yield compared to E. coli and insect cell-free systems; exceptional capability for producing complex eukaryotic proteins with proper folding and post-translational modifications; scalable from microliter to liter volumes. Weaknesses: Higher cost compared to bacterial cell-free systems; requires specialized equipment for optimal performance; wheat germ extract preparation is technically demanding.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed the Protein Synthesis Using Recombinant Elements (PURE) system, a reconstituted cell-free protein synthesis platform that significantly enhances throughput in biosynthesis applications. This approach utilizes individually purified components of the E. coli translation machinery, including ribosomes, aminoacyl-tRNA synthetases, translation factors, and energy regeneration enzymes. By precisely controlling the concentration of each component, MIT's system achieves up to 5-fold higher protein yields compared to crude extract-based systems. Their technology incorporates an optimized energy regeneration system using creatine phosphate and creatine kinase, which extends reaction lifetimes from hours to days. MIT researchers have further enhanced throughput by developing microfluidic devices that enable continuous-exchange cell-free (CECF) reactions, where inhibitory byproducts are continuously removed while fresh substrates are supplied. This approach has demonstrated protein synthesis rates exceeding 1 mg/mL/hour, representing a significant advancement in biosynthetic efficiency.
Strengths: Exceptional reaction control and reproducibility due to defined composition; minimal background reactions and inhibitory factors; ideal for incorporation of non-natural amino acids and production of toxic proteins. Weaknesses: Higher cost compared to crude extract systems; more complex preparation process; lower overall protein yield compared to some optimized crude extract systems.
Key Innovations in Cell-Free Reaction Engineering
Method of speeding up regarding cell-free protein synthesis system
PatentInactiveJP2013009623A
Innovation
- A novel method that omits the linearization of circular DNA and combines a transcription reaction solution with a translation reaction solution, using a chelating agent to control magnesium ion concentration at 10 mM or less, enabling efficient protein synthesis in a single step.
High-throughput system using a cell-free expression system and in SITU sequencing
PatentWO2019035955A1
Innovation
- A high-throughput system utilizing a cell-free expression system combined with in situ sequencing for rapid screening of nucleic acid templates, allowing for the detection and characterization of small molecules produced in multiple reaction volumes, enabling the identification of biosynthetic pathways and optimization of conditions for biomolecule production.
Regulatory Considerations for Cell-Free Bioproducts
The regulatory landscape for cell-free bioproducts presents unique challenges and opportunities distinct from traditional biotechnology frameworks. As cell-free biosynthesis systems advance in throughput capabilities, regulatory bodies worldwide are developing specialized approaches to assess these novel production platforms. Unlike whole-cell systems, cell-free products lack viable organisms, potentially simplifying certain regulatory requirements while introducing new considerations.
The FDA, EMA, and other global regulatory authorities are currently evaluating how existing frameworks for biologics and recombinant proteins apply to cell-free derived products. Key regulatory considerations include source material characterization, process consistency, and residual component analysis. Particularly important is the demonstration that cell extracts used in high-throughput systems are free from infectious agents, endotoxins, and other potentially harmful cellular debris.
Quality control metrics for cell-free bioproduction systems require specialized validation protocols. Regulatory agencies increasingly demand robust analytical methods to verify the absence of host cell proteins, nucleic acids, and other potential contaminants that could affect product safety. The development of standardized reference materials specifically for cell-free systems represents an emerging regulatory need as throughput enhancement technologies advance.
Intellectual property considerations also intersect with regulatory pathways for cell-free bioproducts. Patent landscapes covering specific cell-free expression systems, extract preparation methods, and throughput enhancement technologies may influence regulatory strategy development. Companies must navigate both exclusivity periods and regulatory approval timelines when planning commercialization strategies.
International harmonization efforts are underway to establish consistent regulatory approaches for cell-free bioproducts. The International Council for Harmonisation (ICH) has begun preliminary discussions on guidelines specific to cell-free production systems, though consensus remains in early stages. Regional differences in regulatory interpretation present challenges for global deployment of enhanced throughput technologies.
Risk assessment frameworks for cell-free systems are evolving to address their unique characteristics. Regulators increasingly recognize that traditional risk categories developed for genetically modified organisms may not apply, necessitating new evaluation criteria focused on biochemical composition rather than biological containment. This shift potentially offers streamlined pathways for certain cell-free products while requiring more extensive characterization of others.
The FDA, EMA, and other global regulatory authorities are currently evaluating how existing frameworks for biologics and recombinant proteins apply to cell-free derived products. Key regulatory considerations include source material characterization, process consistency, and residual component analysis. Particularly important is the demonstration that cell extracts used in high-throughput systems are free from infectious agents, endotoxins, and other potentially harmful cellular debris.
Quality control metrics for cell-free bioproduction systems require specialized validation protocols. Regulatory agencies increasingly demand robust analytical methods to verify the absence of host cell proteins, nucleic acids, and other potential contaminants that could affect product safety. The development of standardized reference materials specifically for cell-free systems represents an emerging regulatory need as throughput enhancement technologies advance.
Intellectual property considerations also intersect with regulatory pathways for cell-free bioproducts. Patent landscapes covering specific cell-free expression systems, extract preparation methods, and throughput enhancement technologies may influence regulatory strategy development. Companies must navigate both exclusivity periods and regulatory approval timelines when planning commercialization strategies.
International harmonization efforts are underway to establish consistent regulatory approaches for cell-free bioproducts. The International Council for Harmonisation (ICH) has begun preliminary discussions on guidelines specific to cell-free production systems, though consensus remains in early stages. Regional differences in regulatory interpretation present challenges for global deployment of enhanced throughput technologies.
Risk assessment frameworks for cell-free systems are evolving to address their unique characteristics. Regulators increasingly recognize that traditional risk categories developed for genetically modified organisms may not apply, necessitating new evaluation criteria focused on biochemical composition rather than biological containment. This shift potentially offers streamlined pathways for certain cell-free products while requiring more extensive characterization of others.
Economic Feasibility and Commercialization Pathways
The economic viability of cell-free biosynthesis systems represents a critical factor in their commercial adoption. Current cost analyses indicate that cell-free protein synthesis (CFPS) systems require significant investment, with reagent costs ranging from $0.03 to $1.00 per milligram of protein produced. These costs vary substantially depending on the scale of production, extraction methods, and the specific proteins being synthesized.
Scale-up economics present both challenges and opportunities. While small-scale laboratory CFPS remains expensive, industrial-scale implementation demonstrates promising cost reductions through economies of scale. Companies like Sutro Biopharma and GreenLight Biosciences have achieved production costs below $0.10 per milligram for certain applications, representing a significant improvement over initial cost estimates.
Energy regeneration systems constitute a major cost component in cell-free biosynthesis. Traditional ATP regeneration methods using phosphoenolpyruvate can account for up to 30% of total reagent costs. Alternative energy systems utilizing glucose or glycolysis intermediates have demonstrated potential to reduce these costs by 40-60%, significantly improving economic feasibility.
Commercial pathways for cell-free biosynthesis are emerging across multiple sectors. The pharmaceutical industry represents the most immediate commercialization opportunity, with cell-free systems enabling rapid production of personalized medicines, vaccines, and difficult-to-express proteins. Several companies have successfully brought cell-free produced pharmaceuticals through clinical trials, establishing regulatory precedents.
Industrial enzyme production represents another promising commercialization avenue. The ability to produce enzymes without viable cells offers advantages in cases where the target enzyme might be toxic to host cells or requires specific post-translational modifications. Market analysis suggests this sector could reach $1.2 billion by 2028 for cell-free produced enzymes.
Investment trends indicate growing commercial interest, with venture capital funding for cell-free biosynthesis startups exceeding $450 million in 2022 alone. Strategic partnerships between academic institutions and industry players have accelerated technology transfer and commercial implementation. These collaborations have been particularly effective in addressing scale-up challenges and reducing production costs.
Regulatory pathways for cell-free produced products are becoming more defined, particularly in pharmaceutical applications. The FDA and EMA have established frameworks for evaluating cell-free produced biologics, providing clearer commercialization roadmaps for companies in this space. This regulatory clarity has significantly reduced market entry barriers for startups and established companies alike.
Scale-up economics present both challenges and opportunities. While small-scale laboratory CFPS remains expensive, industrial-scale implementation demonstrates promising cost reductions through economies of scale. Companies like Sutro Biopharma and GreenLight Biosciences have achieved production costs below $0.10 per milligram for certain applications, representing a significant improvement over initial cost estimates.
Energy regeneration systems constitute a major cost component in cell-free biosynthesis. Traditional ATP regeneration methods using phosphoenolpyruvate can account for up to 30% of total reagent costs. Alternative energy systems utilizing glucose or glycolysis intermediates have demonstrated potential to reduce these costs by 40-60%, significantly improving economic feasibility.
Commercial pathways for cell-free biosynthesis are emerging across multiple sectors. The pharmaceutical industry represents the most immediate commercialization opportunity, with cell-free systems enabling rapid production of personalized medicines, vaccines, and difficult-to-express proteins. Several companies have successfully brought cell-free produced pharmaceuticals through clinical trials, establishing regulatory precedents.
Industrial enzyme production represents another promising commercialization avenue. The ability to produce enzymes without viable cells offers advantages in cases where the target enzyme might be toxic to host cells or requires specific post-translational modifications. Market analysis suggests this sector could reach $1.2 billion by 2028 for cell-free produced enzymes.
Investment trends indicate growing commercial interest, with venture capital funding for cell-free biosynthesis startups exceeding $450 million in 2022 alone. Strategic partnerships between academic institutions and industry players have accelerated technology transfer and commercial implementation. These collaborations have been particularly effective in addressing scale-up challenges and reducing production costs.
Regulatory pathways for cell-free produced products are becoming more defined, particularly in pharmaceutical applications. The FDA and EMA have established frameworks for evaluating cell-free produced biologics, providing clearer commercialization roadmaps for companies in this space. This regulatory clarity has significantly reduced market entry barriers for startups and established companies alike.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!