Photochemical reactions in cell-free environments.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photochemical Reactions Background and Objectives
Photochemical reactions, which involve the interaction of light with molecules to induce chemical transformations, have been studied extensively since the early 20th century. These reactions harness photon energy to overcome activation barriers, enabling chemical pathways that would be thermodynamically unfavorable or kinetically slow under conventional conditions. The field has evolved from basic photolysis studies to sophisticated applications in materials science, organic synthesis, and environmental remediation.
In cell-free environments, photochemical reactions offer unique advantages including precise spatial and temporal control, mild reaction conditions, and the ability to access high-energy intermediates without biological constraints. This controlled setting allows researchers to isolate and optimize specific photochemical processes without interference from cellular components, making it ideal for fundamental studies and industrial applications.
The evolution of photochemical technology has accelerated significantly in recent decades, driven by advances in light sources (from mercury lamps to LEDs and lasers), photocatalysts, and analytical techniques. Modern approaches incorporate principles from materials science, quantum chemistry, and engineering to develop more efficient and selective photochemical systems.
Current research objectives in cell-free photochemical reactions focus on several key areas. First, developing sustainable photocatalytic systems for chemical synthesis that operate under visible light, reducing energy requirements and environmental impact. Second, creating artificial photosynthetic systems that can efficiently convert solar energy into chemical energy, potentially addressing global energy challenges. Third, engineering photochemical processes for environmental applications such as water purification and CO2 reduction.
Additionally, researchers aim to understand fundamental photochemical mechanisms at unprecedented levels of detail using advanced spectroscopic techniques and computational modeling. This mechanistic understanding is crucial for rational design of more efficient photochemical systems and prediction of reaction outcomes.
The integration of photochemical reactions with flow chemistry, microfluidics, and automation represents another important objective, enabling precise control over reaction parameters and facilitating scale-up for industrial applications. These technological advances are expected to expand the practical utility of photochemical processes across multiple sectors.
Looking forward, the field seeks to bridge the gap between fundamental photochemical research and practical applications, developing standardized methodologies and robust systems that can be implemented in industrial settings. The ultimate goal is to establish photochemical reactions as a mainstream technology for sustainable chemical production, energy conversion, and environmental remediation in cell-free contexts.
In cell-free environments, photochemical reactions offer unique advantages including precise spatial and temporal control, mild reaction conditions, and the ability to access high-energy intermediates without biological constraints. This controlled setting allows researchers to isolate and optimize specific photochemical processes without interference from cellular components, making it ideal for fundamental studies and industrial applications.
The evolution of photochemical technology has accelerated significantly in recent decades, driven by advances in light sources (from mercury lamps to LEDs and lasers), photocatalysts, and analytical techniques. Modern approaches incorporate principles from materials science, quantum chemistry, and engineering to develop more efficient and selective photochemical systems.
Current research objectives in cell-free photochemical reactions focus on several key areas. First, developing sustainable photocatalytic systems for chemical synthesis that operate under visible light, reducing energy requirements and environmental impact. Second, creating artificial photosynthetic systems that can efficiently convert solar energy into chemical energy, potentially addressing global energy challenges. Third, engineering photochemical processes for environmental applications such as water purification and CO2 reduction.
Additionally, researchers aim to understand fundamental photochemical mechanisms at unprecedented levels of detail using advanced spectroscopic techniques and computational modeling. This mechanistic understanding is crucial for rational design of more efficient photochemical systems and prediction of reaction outcomes.
The integration of photochemical reactions with flow chemistry, microfluidics, and automation represents another important objective, enabling precise control over reaction parameters and facilitating scale-up for industrial applications. These technological advances are expected to expand the practical utility of photochemical processes across multiple sectors.
Looking forward, the field seeks to bridge the gap between fundamental photochemical research and practical applications, developing standardized methodologies and robust systems that can be implemented in industrial settings. The ultimate goal is to establish photochemical reactions as a mainstream technology for sustainable chemical production, energy conversion, and environmental remediation in cell-free contexts.
Market Applications of Cell-Free Photochemistry
Cell-free photochemistry applications are rapidly expanding across multiple market sectors, driven by their unique advantages in sustainability and efficiency. In pharmaceutical manufacturing, these systems enable the production of complex drug intermediates and active pharmaceutical ingredients through light-driven reactions that would be impossible or toxic within living cells. The market value for cell-free photocatalytic drug synthesis is growing as pharmaceutical companies seek greener chemistry approaches that reduce waste and energy consumption.
Biofuel production represents another significant market opportunity, where cell-free photochemical systems can directly convert CO2 and water into fuel precursors using solar energy. This approach circumvents the efficiency limitations of traditional biofuel production that relies on plant growth. Companies like Joule Unlimited and Algenol have demonstrated commercial interest in similar technologies, suggesting a receptive market for advanced cell-free photosynthetic systems.
The fine chemicals industry is increasingly adopting cell-free photochemistry for the synthesis of specialty chemicals, flavors, fragrances, and cosmetic ingredients. These applications benefit from the precise control over reaction conditions that cell-free environments provide, allowing for higher product purity and reduced downstream processing costs. Market analysis indicates growing demand for natural-equivalent compounds that can be produced through clean photochemical processes.
Environmental remediation represents an emerging application with significant market potential. Cell-free photochemical systems can degrade persistent organic pollutants in wastewater and contaminated soils through advanced oxidation processes. The global water treatment chemicals market, valued at over $30 billion, presents substantial opportunities for innovative photochemical solutions that offer cost advantages over traditional treatment methods.
Analytical and diagnostic applications constitute a high-value niche market. Cell-free photochemical reactions are being incorporated into biosensors and diagnostic platforms for detecting pathogens, toxins, and biomarkers. These systems offer advantages in stability, sensitivity, and shelf-life compared to conventional enzyme-based detection methods, addressing the growing market demand for point-of-care diagnostics.
Materials science applications are expanding rapidly, with cell-free photochemistry enabling the synthesis of advanced polymers, nanoparticles, and functional materials with precisely controlled properties. The specialty materials market is particularly receptive to these innovations, as they enable customization of material properties for specific industrial applications.
The agricultural sector presents opportunities for cell-free photochemical systems in fertilizer production, particularly nitrogen fixation processes that could reduce dependence on energy-intensive Haber-Bosch synthesis. With the global fertilizer market exceeding $150 billion, even incremental adoption of photochemical alternatives could represent significant market value.
Biofuel production represents another significant market opportunity, where cell-free photochemical systems can directly convert CO2 and water into fuel precursors using solar energy. This approach circumvents the efficiency limitations of traditional biofuel production that relies on plant growth. Companies like Joule Unlimited and Algenol have demonstrated commercial interest in similar technologies, suggesting a receptive market for advanced cell-free photosynthetic systems.
The fine chemicals industry is increasingly adopting cell-free photochemistry for the synthesis of specialty chemicals, flavors, fragrances, and cosmetic ingredients. These applications benefit from the precise control over reaction conditions that cell-free environments provide, allowing for higher product purity and reduced downstream processing costs. Market analysis indicates growing demand for natural-equivalent compounds that can be produced through clean photochemical processes.
Environmental remediation represents an emerging application with significant market potential. Cell-free photochemical systems can degrade persistent organic pollutants in wastewater and contaminated soils through advanced oxidation processes. The global water treatment chemicals market, valued at over $30 billion, presents substantial opportunities for innovative photochemical solutions that offer cost advantages over traditional treatment methods.
Analytical and diagnostic applications constitute a high-value niche market. Cell-free photochemical reactions are being incorporated into biosensors and diagnostic platforms for detecting pathogens, toxins, and biomarkers. These systems offer advantages in stability, sensitivity, and shelf-life compared to conventional enzyme-based detection methods, addressing the growing market demand for point-of-care diagnostics.
Materials science applications are expanding rapidly, with cell-free photochemistry enabling the synthesis of advanced polymers, nanoparticles, and functional materials with precisely controlled properties. The specialty materials market is particularly receptive to these innovations, as they enable customization of material properties for specific industrial applications.
The agricultural sector presents opportunities for cell-free photochemical systems in fertilizer production, particularly nitrogen fixation processes that could reduce dependence on energy-intensive Haber-Bosch synthesis. With the global fertilizer market exceeding $150 billion, even incremental adoption of photochemical alternatives could represent significant market value.
Current Challenges in Cell-Free Photochemical Systems
Despite significant advancements in cell-free photochemical systems, several critical challenges continue to impede their widespread application and efficiency. One fundamental obstacle remains the stability of photocatalysts in cell-free environments. Without the protective mechanisms of cellular structures, photocatalysts often experience rapid degradation under continuous light exposure, limiting reaction duration and yield. This photodegradation is particularly problematic for organic photocatalysts and certain metal complexes that form reactive oxygen species, which subsequently attack the catalyst structure.
Scaling presents another significant hurdle. While laboratory-scale reactions demonstrate promising results, translating these systems to industrial scales introduces complications in light penetration and distribution. As reaction volumes increase, light attenuation becomes more pronounced, creating heterogeneous reaction environments where only the outer layers receive sufficient photonic energy. This limitation necessitates specialized reactor designs that many current manufacturing facilities are not equipped to implement.
Energy efficiency remains suboptimal in most cell-free photochemical systems. Many current setups utilize broad-spectrum light sources that waste significant energy on wavelengths not absorbed by the photocatalyst. Additionally, the quantum yield—the ratio of product molecules formed to photons absorbed—rarely approaches theoretical maximums due to competing non-productive pathways and energy dissipation mechanisms.
The selectivity of photochemical reactions in cell-free environments poses another substantial challenge. Without the precise spatial organization and enzyme specificity found in biological systems, side reactions frequently occur, reducing yield and complicating downstream purification processes. This issue becomes particularly pronounced when working with complex substrates containing multiple reactive sites.
Oxygen sensitivity represents a persistent problem for many photochemical systems. Molecular oxygen readily quenches excited states of photocatalysts and can initiate radical chain reactions that interfere with desired transformation pathways. Creating and maintaining anaerobic conditions at scale introduces additional complexity and cost to system design.
Water compatibility remains problematic for numerous photochemical reactions that require organic solvents due to substrate solubility constraints. This limitation contradicts green chemistry principles and creates environmental concerns for large-scale applications. Developing aqueous-compatible photocatalytic systems that maintain efficiency while accommodating hydrophobic substrates continues to challenge researchers.
Finally, the analytical complexity of monitoring photochemical reactions in real-time presents significant obstacles to process optimization. Current techniques often provide limited information about reaction intermediates and kinetics, hampering systematic improvement efforts and mechanistic understanding necessary for rational design of next-generation photochemical systems.
Scaling presents another significant hurdle. While laboratory-scale reactions demonstrate promising results, translating these systems to industrial scales introduces complications in light penetration and distribution. As reaction volumes increase, light attenuation becomes more pronounced, creating heterogeneous reaction environments where only the outer layers receive sufficient photonic energy. This limitation necessitates specialized reactor designs that many current manufacturing facilities are not equipped to implement.
Energy efficiency remains suboptimal in most cell-free photochemical systems. Many current setups utilize broad-spectrum light sources that waste significant energy on wavelengths not absorbed by the photocatalyst. Additionally, the quantum yield—the ratio of product molecules formed to photons absorbed—rarely approaches theoretical maximums due to competing non-productive pathways and energy dissipation mechanisms.
The selectivity of photochemical reactions in cell-free environments poses another substantial challenge. Without the precise spatial organization and enzyme specificity found in biological systems, side reactions frequently occur, reducing yield and complicating downstream purification processes. This issue becomes particularly pronounced when working with complex substrates containing multiple reactive sites.
Oxygen sensitivity represents a persistent problem for many photochemical systems. Molecular oxygen readily quenches excited states of photocatalysts and can initiate radical chain reactions that interfere with desired transformation pathways. Creating and maintaining anaerobic conditions at scale introduces additional complexity and cost to system design.
Water compatibility remains problematic for numerous photochemical reactions that require organic solvents due to substrate solubility constraints. This limitation contradicts green chemistry principles and creates environmental concerns for large-scale applications. Developing aqueous-compatible photocatalytic systems that maintain efficiency while accommodating hydrophobic substrates continues to challenge researchers.
Finally, the analytical complexity of monitoring photochemical reactions in real-time presents significant obstacles to process optimization. Current techniques often provide limited information about reaction intermediates and kinetics, hampering systematic improvement efforts and mechanistic understanding necessary for rational design of next-generation photochemical systems.
Current Methodologies for Cell-Free Photochemical Reactions
01 Photochemical reaction systems and apparatus
Various systems and apparatus have been developed for conducting photochemical reactions efficiently. These include specialized reactors, light sources, and control mechanisms designed to optimize the interaction between light and reactants. Such systems often incorporate features for temperature control, mixing, and precise light delivery to ensure consistent and efficient photochemical transformations.- Photochemical reaction systems and apparatus: Various systems and apparatus have been developed for conducting photochemical reactions. These include specialized reactors, chambers, and equipment designed to facilitate controlled exposure to light sources for initiating and maintaining photochemical processes. The designs focus on optimizing light distribution, reaction efficiency, and process control for various industrial and research applications.
- Photocatalytic processes and materials: Photocatalytic materials and processes utilize light energy to accelerate chemical reactions without being consumed in the process. These technologies employ semiconductors or other photosensitive materials that, when exposed to appropriate wavelengths of light, generate reactive species capable of driving chemical transformations. Applications include environmental remediation, water treatment, and sustainable chemical synthesis.
- Photoelectrochemical systems: Photoelectrochemical systems combine photochemical and electrochemical processes to convert light energy into chemical or electrical energy. These systems typically involve semiconductors, electrodes, and electrolytes arranged to facilitate light-induced charge separation and subsequent chemical reactions. Applications include solar fuel production, water splitting for hydrogen generation, and advanced oxidation processes.
- Light-sensitive materials and photopolymerization: Light-sensitive materials undergo chemical changes when exposed to specific wavelengths of light. Photopolymerization involves the use of light to initiate polymerization reactions, converting monomers into polymers. These processes are fundamental to applications such as photolithography, 3D printing, dental materials, coatings, and adhesives where spatial control of polymerization is desired.
- Advanced photochemical applications and innovations: Recent innovations in photochemistry have led to advanced applications across multiple fields. These include novel photochemical synthesis methods, light-controlled drug delivery systems, photodynamic therapy for medical treatments, and photochemical approaches to renewable energy. These innovations leverage specific wavelengths, pulsed light sources, and specialized photosensitizers to achieve precise control over chemical transformations.
02 Photocatalytic processes and materials
Photocatalytic materials and processes utilize light energy to accelerate chemical reactions. These typically involve semiconductors or other materials that can absorb photons and generate reactive species. Applications include water treatment, air purification, and organic synthesis, where photocatalysts enable reactions to proceed under milder conditions than conventional methods, often with improved selectivity and reduced waste.Expand Specific Solutions03 Solar-driven photochemical reactions
Solar energy can be harnessed to drive photochemical reactions, offering sustainable alternatives to conventional chemical processes. These reactions utilize natural sunlight as the energy source for chemical transformations, including water splitting, carbon dioxide reduction, and organic synthesis. Solar photochemical systems often incorporate concentrators, filters, or tracking mechanisms to maximize light capture and conversion efficiency.Expand Specific Solutions04 Photochemical reaction monitoring and control methods
Advanced methods for monitoring and controlling photochemical reactions ensure optimal performance and product quality. These include real-time spectroscopic techniques, automated feedback systems, and computational models that predict reaction outcomes. Such methods allow for precise adjustment of reaction parameters like light intensity, wavelength, exposure time, and reagent concentrations to achieve desired conversion rates and selectivity.Expand Specific Solutions05 Novel applications of photochemical reactions
Emerging applications of photochemical reactions span diverse fields including pharmaceuticals, materials science, and environmental remediation. These include light-activated drug delivery systems, photopatterning of surfaces, photodynamic therapy for medical treatments, and photochemical approaches to waste degradation. Such applications leverage the spatial and temporal control offered by light-initiated processes to achieve outcomes difficult to obtain through conventional chemistry.Expand Specific Solutions
Leading Research Groups and Companies in Photochemistry
Photochemical reactions in cell-free environments are advancing through a competitive landscape characterized by early commercial development and growing research interest. The market is expanding as companies like Debut Biotechnology, Synvitrobio (Tierra Biosciences), and GreenLight Biosciences leverage cell-free systems for sustainable biomanufacturing applications. Technical maturity varies significantly across players, with pharmaceutical companies (Novartis, Millennium Pharmaceuticals) exploring drug discovery applications, while specialized biotechnology firms (Spiber, Snap Bio) focus on novel biomaterials and therapeutics. Research institutions like RIKEN, AIST, and universities (Northwestern, Soochow) are driving fundamental innovations, suggesting this field is transitioning from academic research to industrial application with significant growth potential.
Debut Biotechnology, Inc.
Technical Solution: Debut Biotechnology has pioneered a continuous-flow biomanufacturing platform that enables photochemical reactions in cell-free environments. Their technology utilizes immobilized enzymes on solid supports within flow reactors that incorporate light-driven catalysis. This approach allows precise control of reaction parameters including light intensity, wavelength, and exposure time. The platform integrates photosensitizers with enzymatic catalysts to perform complex transformations that would be challenging or impossible in traditional cellular systems. By removing cellular constraints, Debut's system can operate under conditions optimized specifically for photochemical reactions, including non-physiological pH ranges and solvent systems that would be toxic to living cells[1]. Their technology enables the synthesis of high-value compounds through multi-step enzymatic cascades coupled with photochemical transformations, significantly expanding the chemical space accessible through biomanufacturing approaches.
Strengths: Enables reactions that would be toxic or impossible in living cells; provides precise control over reaction parameters; allows continuous production rather than batch processing; reduces waste and improves yield through optimized reaction conditions. Weaknesses: Requires specialized equipment and expertise; may face challenges in scaling up complex photochemical processes; potentially higher initial capital investment compared to traditional biomanufacturing approaches.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free RNA production platform that incorporates photochemical reaction control for enhanced precision and yield. Their system utilizes light-activated transcription regulators and photocaged nucleotides that can be selectively activated using specific wavelengths of light. This approach enables spatial and temporal control over RNA synthesis reactions in their cell-free environment. The platform employs a proprietary mix of enzymes, energy systems, and building blocks optimized for high-yield RNA production without cellular constraints. By incorporating photochemical triggers, GreenLight can precisely initiate and terminate reactions, control reaction rates, and even perform sequential reactions in the same reaction vessel[2]. Their technology has been particularly valuable for producing mRNA therapeutics and agricultural RNA products that require high purity and specific modifications. The cell-free nature of their system eliminates contamination risks from host cell proteins and nucleic acids while allowing incorporation of non-natural nucleotides that would be rejected by cellular machinery.
Strengths: Achieves high purity RNA production without cellular contaminants; enables precise spatial and temporal control of reactions; allows incorporation of modified nucleotides; scales more predictably than cell-based systems. Weaknesses: Energy-intensive process requiring continuous ATP regeneration; potentially higher production costs compared to some cell-based systems; limited to RNA-based products rather than full protein synthesis.
Key Photocatalysts and Reaction Mechanisms Analysis
Cell-free protein synthesis method using photothermal effect
PatentWO2025105584A1
Innovation
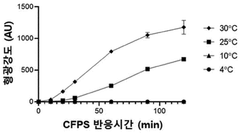
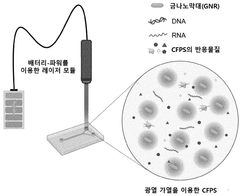
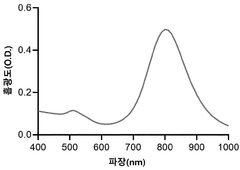
- A cell-free protein synthesis method utilizing the photothermal effect, where a PEG-GNRs (polyethylene glycol-conjugated gold nanorods) complex is used to generate heat upon laser irradiation, effectively increasing the reaction solution temperature to 30-37°C, facilitating faster protein synthesis.
Photocatalytic thermal barrier coating
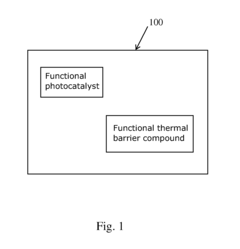
PatentInactiveUS20160346775A1
Innovation
- A thermally insulated photocatalytic coating is developed by combining a photocatalyst material with a thermal barrier compound, such as indium tin oxide or ceramic particles, to reflect infrared radiation and reduce surface temperature, enhancing the production of hydroxyl radicals and the overall efficiency of the photocatalytic process.
Sustainability Impact of Cell-Free Photochemical Processes
Cell-free photochemical processes represent a significant advancement in sustainable chemical manufacturing, offering numerous environmental benefits compared to traditional methods. These systems harness light energy to drive chemical reactions without requiring living cells, thereby eliminating the resource-intensive maintenance of cellular machinery and reducing associated waste streams.
The sustainability impact of these processes is particularly evident in their reduced carbon footprint. By operating at ambient temperatures and pressures, cell-free photochemical reactions consume substantially less energy than conventional chemical synthesis methods that often require high temperatures and pressures. Studies indicate energy savings of up to 60-70% in certain reaction pathways, directly translating to reduced greenhouse gas emissions.
Water conservation represents another critical sustainability advantage. Cell-free systems typically require significantly less water than cell-based bioproduction platforms, which need substantial volumes for cell maintenance and product separation. This reduction in water usage becomes increasingly important as water scarcity affects more regions globally.
The elimination of toxic catalysts and solvents further enhances the environmental profile of these processes. Many cell-free photochemical reactions can be conducted in water or benign solvents, replacing hazardous organic solvents commonly used in traditional chemical synthesis. This shift reduces both environmental contamination risks and workplace hazards.
Waste reduction constitutes a fundamental sustainability benefit of cell-free photochemical processes. These systems generate fewer byproducts and contaminants compared to both traditional chemical synthesis and cell-based production methods. The high selectivity of many photochemical reactions minimizes side reactions, thereby increasing atom economy and reducing purification requirements.
From a life cycle perspective, cell-free photochemical processes demonstrate favorable sustainability metrics. Recent life cycle assessments reveal that products manufactured via these methods can achieve 30-50% reductions in environmental impact categories including ecotoxicity, eutrophication potential, and resource depletion compared to conventional manufacturing routes.
The scalability of these processes further amplifies their sustainability impact. Unlike many green chemistry approaches that work well in laboratories but fail at industrial scales, cell-free photochemical reactions have demonstrated successful scaling in several commercial applications, particularly in pharmaceutical and fine chemical production sectors.
The sustainability impact of these processes is particularly evident in their reduced carbon footprint. By operating at ambient temperatures and pressures, cell-free photochemical reactions consume substantially less energy than conventional chemical synthesis methods that often require high temperatures and pressures. Studies indicate energy savings of up to 60-70% in certain reaction pathways, directly translating to reduced greenhouse gas emissions.
Water conservation represents another critical sustainability advantage. Cell-free systems typically require significantly less water than cell-based bioproduction platforms, which need substantial volumes for cell maintenance and product separation. This reduction in water usage becomes increasingly important as water scarcity affects more regions globally.
The elimination of toxic catalysts and solvents further enhances the environmental profile of these processes. Many cell-free photochemical reactions can be conducted in water or benign solvents, replacing hazardous organic solvents commonly used in traditional chemical synthesis. This shift reduces both environmental contamination risks and workplace hazards.
Waste reduction constitutes a fundamental sustainability benefit of cell-free photochemical processes. These systems generate fewer byproducts and contaminants compared to both traditional chemical synthesis and cell-based production methods. The high selectivity of many photochemical reactions minimizes side reactions, thereby increasing atom economy and reducing purification requirements.
From a life cycle perspective, cell-free photochemical processes demonstrate favorable sustainability metrics. Recent life cycle assessments reveal that products manufactured via these methods can achieve 30-50% reductions in environmental impact categories including ecotoxicity, eutrophication potential, and resource depletion compared to conventional manufacturing routes.
The scalability of these processes further amplifies their sustainability impact. Unlike many green chemistry approaches that work well in laboratories but fail at industrial scales, cell-free photochemical reactions have demonstrated successful scaling in several commercial applications, particularly in pharmaceutical and fine chemical production sectors.
Scale-up Considerations for Industrial Applications
Scaling up photochemical reactions from laboratory to industrial scale presents unique challenges that require careful engineering considerations. The transition from small-scale batch processes to continuous flow systems demands significant modifications in reactor design, light source configuration, and process control mechanisms. Industrial implementation necessitates addressing issues related to light penetration depth, which becomes increasingly problematic as reactor dimensions expand due to the Beer-Lambert law limitations.
Material selection for large-scale photoreactors requires balancing optical transparency, chemical resistance, and mechanical durability. Quartz and specialized borosilicate glass remain preferred for UV applications, while more economical polymeric materials may suffice for visible light reactions. The cost implications of these materials become significant at industrial scales, necessitating optimization between performance and economic feasibility.
Energy efficiency represents a critical factor in industrial photochemical processes. Conventional mercury lamps, while powerful, consume substantial energy and generate excessive heat requiring sophisticated cooling systems. LED technology offers promising alternatives with lower energy consumption, longer operational lifetimes, and reduced thermal management requirements. Recent developments in high-power LED arrays specifically designed for photochemical applications have significantly improved the economic viability of large-scale operations.
Process control and monitoring systems require substantial adaptation when scaling up photochemical reactions. Real-time spectroscopic analysis, automated feedback mechanisms, and precise control of reaction parameters become essential for maintaining consistent product quality. The integration of advanced process analytical technology (PAT) enables continuous monitoring of reaction progress and immediate adjustment of operational parameters.
Safety considerations escalate dramatically at industrial scale, particularly regarding photosensitizers and reactive intermediates. Containment systems, emergency shutdown protocols, and specialized handling procedures must be implemented to mitigate risks associated with large volumes of photochemically active materials. Regulatory compliance frameworks vary significantly across regions, adding complexity to global implementation strategies.
Economic viability ultimately determines industrial adoption of cell-free photochemical processes. Capital expenditure for specialized equipment must be balanced against operational benefits including reduced catalyst costs, simplified downstream processing, and potential access to novel high-value products. Recent techno-economic analyses suggest that certain photochemical pathways become economically competitive at scales exceeding 100 tons annually, particularly for pharmaceutical intermediates and specialty chemicals.
Material selection for large-scale photoreactors requires balancing optical transparency, chemical resistance, and mechanical durability. Quartz and specialized borosilicate glass remain preferred for UV applications, while more economical polymeric materials may suffice for visible light reactions. The cost implications of these materials become significant at industrial scales, necessitating optimization between performance and economic feasibility.
Energy efficiency represents a critical factor in industrial photochemical processes. Conventional mercury lamps, while powerful, consume substantial energy and generate excessive heat requiring sophisticated cooling systems. LED technology offers promising alternatives with lower energy consumption, longer operational lifetimes, and reduced thermal management requirements. Recent developments in high-power LED arrays specifically designed for photochemical applications have significantly improved the economic viability of large-scale operations.
Process control and monitoring systems require substantial adaptation when scaling up photochemical reactions. Real-time spectroscopic analysis, automated feedback mechanisms, and precise control of reaction parameters become essential for maintaining consistent product quality. The integration of advanced process analytical technology (PAT) enables continuous monitoring of reaction progress and immediate adjustment of operational parameters.
Safety considerations escalate dramatically at industrial scale, particularly regarding photosensitizers and reactive intermediates. Containment systems, emergency shutdown protocols, and specialized handling procedures must be implemented to mitigate risks associated with large volumes of photochemically active materials. Regulatory compliance frameworks vary significantly across regions, adding complexity to global implementation strategies.
Economic viability ultimately determines industrial adoption of cell-free photochemical processes. Capital expenditure for specialized equipment must be balanced against operational benefits including reduced catalyst costs, simplified downstream processing, and potential access to novel high-value products. Recent techno-economic analyses suggest that certain photochemical pathways become economically competitive at scales exceeding 100 tons annually, particularly for pharmaceutical intermediates and specialty chemicals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!