Cell-free systems for rare enzyme production.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Enzyme Production Background and Objectives
Cell-free systems represent a revolutionary approach in biotechnology that has evolved significantly over the past decades. Initially developed as a research tool for understanding fundamental biological processes, these systems have now emerged as powerful platforms for protein synthesis outside living cells. The evolution of cell-free technology began with crude extracts and has progressed to highly engineered systems capable of producing complex proteins with remarkable efficiency.
The field has witnessed several transformative milestones, including the development of the PURE (Protein synthesis Using Recombinant Elements) system, which utilizes purified components rather than crude extracts, offering unprecedented control over reaction conditions. Recent advances in synthetic biology and metabolic engineering have further enhanced the capabilities of cell-free systems, enabling the production of proteins that are difficult to express in conventional cellular systems.
Rare enzymes present particular challenges for traditional production methods. These enzymes often exhibit toxicity to host cells, require specific post-translational modifications, or yield poor expression levels in cellular systems. Cell-free production offers a promising alternative by circumventing cellular viability constraints and allowing direct manipulation of the biochemical environment to optimize enzyme production.
The primary objectives of cell-free enzyme production technology are multifaceted. First, to develop robust and scalable platforms capable of producing rare enzymes with high yield and activity. Second, to enhance the economic viability of the process by reducing costs associated with reagents and improving reaction longevity. Third, to expand the repertoire of enzymes that can be efficiently produced, particularly those with industrial or pharmaceutical significance.
Technical goals include optimizing energy regeneration systems to sustain longer reaction times, developing methods for efficient post-translational modifications, and creating standardized protocols that ensure reproducibility across different laboratory settings. Additionally, there is a growing focus on miniaturization and automation to enable high-throughput screening of enzyme variants.
The trajectory of cell-free technology suggests a convergence with other cutting-edge fields such as microfluidics, artificial intelligence for reaction optimization, and novel biomaterials for reaction compartmentalization. These integrations promise to further expand the capabilities and applications of cell-free systems for rare enzyme production.
As industrial and pharmaceutical demands for specialized enzymes continue to grow, cell-free systems are positioned to become an essential technology in the biomanufacturing toolkit, offering solutions to production challenges that have long constrained the availability and application of rare enzymes.
The field has witnessed several transformative milestones, including the development of the PURE (Protein synthesis Using Recombinant Elements) system, which utilizes purified components rather than crude extracts, offering unprecedented control over reaction conditions. Recent advances in synthetic biology and metabolic engineering have further enhanced the capabilities of cell-free systems, enabling the production of proteins that are difficult to express in conventional cellular systems.
Rare enzymes present particular challenges for traditional production methods. These enzymes often exhibit toxicity to host cells, require specific post-translational modifications, or yield poor expression levels in cellular systems. Cell-free production offers a promising alternative by circumventing cellular viability constraints and allowing direct manipulation of the biochemical environment to optimize enzyme production.
The primary objectives of cell-free enzyme production technology are multifaceted. First, to develop robust and scalable platforms capable of producing rare enzymes with high yield and activity. Second, to enhance the economic viability of the process by reducing costs associated with reagents and improving reaction longevity. Third, to expand the repertoire of enzymes that can be efficiently produced, particularly those with industrial or pharmaceutical significance.
Technical goals include optimizing energy regeneration systems to sustain longer reaction times, developing methods for efficient post-translational modifications, and creating standardized protocols that ensure reproducibility across different laboratory settings. Additionally, there is a growing focus on miniaturization and automation to enable high-throughput screening of enzyme variants.
The trajectory of cell-free technology suggests a convergence with other cutting-edge fields such as microfluidics, artificial intelligence for reaction optimization, and novel biomaterials for reaction compartmentalization. These integrations promise to further expand the capabilities and applications of cell-free systems for rare enzyme production.
As industrial and pharmaceutical demands for specialized enzymes continue to grow, cell-free systems are positioned to become an essential technology in the biomanufacturing toolkit, offering solutions to production challenges that have long constrained the availability and application of rare enzymes.
Market Analysis for Rare Enzyme Applications
The global market for rare enzymes has been experiencing significant growth, driven by advancements in biotechnology and increasing applications across multiple industries. The current market size for industrial enzymes is estimated at $7 billion, with rare and specialized enzymes comprising approximately 15% of this market. This segment is projected to grow at a compound annual growth rate (CAGR) of 9.5% through 2028, outpacing the broader enzyme market's growth rate of 6.7%.
Healthcare and pharmaceutical sectors represent the largest application areas for rare enzymes, accounting for 42% of the market share. These enzymes are critical components in diagnostic kits, therapeutic treatments for rare diseases, and as catalysts in pharmaceutical manufacturing processes. The demand for enzyme replacement therapies alone has created a $2.5 billion market subset with double-digit growth projections.
Food and beverage industries constitute the second-largest application segment at 28% market share. Here, rare enzymes are increasingly utilized for specialized food processing, flavor enhancement, and the development of functional foods. The clean-label trend and consumer demand for natural ingredients have accelerated adoption in this sector, with particular growth in plant-based protein processing applications.
Research and development applications represent a smaller but rapidly growing segment at 17% market share. Academic institutions and biotechnology companies are driving demand for novel enzymes to enable breakthrough research in synthetic biology, metabolic engineering, and biocatalysis. The emergence of cell-free systems has particularly stimulated this segment, as these platforms require diverse enzymatic components.
Geographically, North America leads the market with 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 11.3% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea.
A significant market constraint remains the high production costs associated with rare enzymes, with prices ranging from $500 to $10,000 per gram depending on purity and specificity requirements. This cost barrier has limited broader adoption in price-sensitive applications and emerging markets. Cell-free production systems represent a promising solution to this constraint, potentially reducing production costs by 30-60% while increasing accessibility.
Customer segments are increasingly diversifying, with small and medium biotechnology enterprises now accounting for 35% of market demand, up from 22% five years ago. This democratization of access indicates a maturing market with expanding applications beyond traditional large pharmaceutical and industrial users.
Healthcare and pharmaceutical sectors represent the largest application areas for rare enzymes, accounting for 42% of the market share. These enzymes are critical components in diagnostic kits, therapeutic treatments for rare diseases, and as catalysts in pharmaceutical manufacturing processes. The demand for enzyme replacement therapies alone has created a $2.5 billion market subset with double-digit growth projections.
Food and beverage industries constitute the second-largest application segment at 28% market share. Here, rare enzymes are increasingly utilized for specialized food processing, flavor enhancement, and the development of functional foods. The clean-label trend and consumer demand for natural ingredients have accelerated adoption in this sector, with particular growth in plant-based protein processing applications.
Research and development applications represent a smaller but rapidly growing segment at 17% market share. Academic institutions and biotechnology companies are driving demand for novel enzymes to enable breakthrough research in synthetic biology, metabolic engineering, and biocatalysis. The emergence of cell-free systems has particularly stimulated this segment, as these platforms require diverse enzymatic components.
Geographically, North America leads the market with 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 11.3% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea.
A significant market constraint remains the high production costs associated with rare enzymes, with prices ranging from $500 to $10,000 per gram depending on purity and specificity requirements. This cost barrier has limited broader adoption in price-sensitive applications and emerging markets. Cell-free production systems represent a promising solution to this constraint, potentially reducing production costs by 30-60% while increasing accessibility.
Customer segments are increasingly diversifying, with small and medium biotechnology enterprises now accounting for 35% of market demand, up from 22% five years ago. This democratization of access indicates a maturing market with expanding applications beyond traditional large pharmaceutical and industrial users.
Technical Barriers in Cell-free Enzyme Production
Despite significant advancements in cell-free systems for enzyme production, several technical barriers continue to impede the widespread adoption and commercialization of this technology, particularly for rare enzymes. The primary challenge remains the limited stability of cell-free reaction components, with many systems exhibiting activity decline within hours. This instability stems from factors including nuclease degradation of DNA templates, proteolytic enzyme degradation, and metabolite depletion, necessitating either continuous supplementation or development of stabilization strategies.
Energy regeneration presents another significant hurdle, as cell-free systems lack the sophisticated metabolic networks found in living cells. Current ATP regeneration systems often rely on expensive phosphate donors like phosphoenolpyruvate or creatine phosphate, making large-scale production economically unfeasible. For rare enzymes requiring extended expression periods, this energy limitation becomes particularly problematic.
Scalability challenges persist across the field, with most successful cell-free systems operating at laboratory scales (milliliters to liters). Translation to industrial volumes introduces complications in mixing, temperature control, and oxygen transfer that can dramatically reduce productivity. These engineering challenges are compounded for rare enzymes that may require specialized reaction conditions or extended production times.
Extract preparation consistency represents a critical barrier, as variations between batches can significantly impact enzyme yield and activity. Current protocols often produce extracts with varying levels of endogenous nucleases, proteases, and metabolic enzymes, creating reproducibility issues that are particularly problematic for rare enzyme production where optimization parameters may be less established.
Post-translational modifications (PTMs) present unique challenges for complex enzymes requiring specific folding assistance or modifications. While some cell-free systems incorporate chaperones and modification enzymes, many rare enzymes with complex structural requirements remain difficult to produce in active form. This limitation is especially relevant for eukaryotic enzymes that require glycosylation or other sophisticated PTMs.
Regulatory and intellectual property barriers further complicate commercialization efforts. The emerging nature of cell-free technology has created a complex patent landscape, with overlapping claims on extract preparation methods, energy regeneration systems, and specific applications. For rare enzyme production, navigating these legal constraints while developing economically viable processes presents additional challenges.
Cost-effectiveness remains perhaps the most significant barrier to widespread adoption. Current cell-free systems require expensive components including nucleotides, amino acids, and energy sources. For rare enzymes with limited market demand, these high production costs often cannot be justified compared to traditional fermentation approaches, despite potential advantages in speed and specificity.
Energy regeneration presents another significant hurdle, as cell-free systems lack the sophisticated metabolic networks found in living cells. Current ATP regeneration systems often rely on expensive phosphate donors like phosphoenolpyruvate or creatine phosphate, making large-scale production economically unfeasible. For rare enzymes requiring extended expression periods, this energy limitation becomes particularly problematic.
Scalability challenges persist across the field, with most successful cell-free systems operating at laboratory scales (milliliters to liters). Translation to industrial volumes introduces complications in mixing, temperature control, and oxygen transfer that can dramatically reduce productivity. These engineering challenges are compounded for rare enzymes that may require specialized reaction conditions or extended production times.
Extract preparation consistency represents a critical barrier, as variations between batches can significantly impact enzyme yield and activity. Current protocols often produce extracts with varying levels of endogenous nucleases, proteases, and metabolic enzymes, creating reproducibility issues that are particularly problematic for rare enzyme production where optimization parameters may be less established.
Post-translational modifications (PTMs) present unique challenges for complex enzymes requiring specific folding assistance or modifications. While some cell-free systems incorporate chaperones and modification enzymes, many rare enzymes with complex structural requirements remain difficult to produce in active form. This limitation is especially relevant for eukaryotic enzymes that require glycosylation or other sophisticated PTMs.
Regulatory and intellectual property barriers further complicate commercialization efforts. The emerging nature of cell-free technology has created a complex patent landscape, with overlapping claims on extract preparation methods, energy regeneration systems, and specific applications. For rare enzyme production, navigating these legal constraints while developing economically viable processes presents additional challenges.
Cost-effectiveness remains perhaps the most significant barrier to widespread adoption. Current cell-free systems require expensive components including nucleotides, amino acids, and energy sources. For rare enzymes with limited market demand, these high production costs often cannot be justified compared to traditional fermentation approaches, despite potential advantages in speed and specificity.
Current Cell-free Expression Platforms
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems allow for the production of enzymes without the need for living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, tRNAs, amino acids, and energy sources. By eliminating the constraints of cell viability, these systems can produce enzymes that might be toxic to living cells and allow for rapid protein production with high yields.- Cell-free protein synthesis systems: Cell-free protein synthesis systems enable the production of enzymes without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, tRNAs, amino acids, and energy sources. By eliminating the constraints of cell viability, these systems allow for the production of proteins that might be toxic to living cells and enable rapid protein synthesis with high yields.
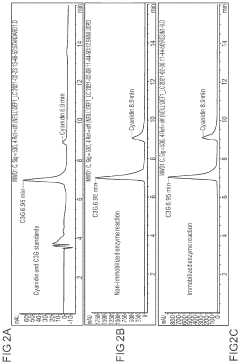
- Enzyme immobilization techniques: Immobilization of enzymes in cell-free systems enhances stability and allows for reuse of the biocatalysts. Various immobilization techniques include adsorption onto solid supports, covalent binding to carriers, entrapment in polymeric matrices, and encapsulation in membranes. These methods can improve enzyme performance by protecting them from harsh conditions and enabling continuous production processes with extended operational lifetimes.
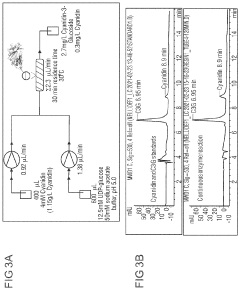
- Bioreactor design for cell-free enzyme production: Specialized bioreactor designs optimize cell-free enzyme production by controlling parameters such as temperature, pH, and substrate concentration. Continuous-flow systems allow for sustained production while membrane reactors enable separation of enzymes from products. Advanced bioreactors may incorporate monitoring systems for real-time process control and optimization, resulting in higher yields and improved enzyme quality.
- Energy regeneration systems: Energy regeneration systems are crucial for sustained enzyme production in cell-free environments. These systems replenish ATP and other energy molecules required for protein synthesis and enzymatic reactions. Various approaches include substrate-level phosphorylation, light-driven systems, and enzymatic cascades that recycle spent energy carriers. Efficient energy regeneration significantly extends the productive lifetime of cell-free systems and improves overall yield.
- Genetic optimization for cell-free expression: Genetic optimization techniques enhance enzyme production in cell-free systems through modification of expression templates. These include codon optimization, incorporation of strong promoters, engineering of untranslated regions, and removal of regulatory elements that might inhibit expression. Advanced genetic designs may include synthetic operons that coordinate the expression of multiple enzymes or metabolic pathways in cell-free environments.
02 Enzyme immobilization techniques
Immobilization of enzymes in cell-free systems enhances stability and allows for reuse of the biocatalysts. Various techniques include physical adsorption, covalent binding, entrapment, and encapsulation. Immobilized enzymes often show improved thermal stability, pH tolerance, and operational stability compared to their free counterparts, making them valuable for industrial applications where repeated use and extended shelf life are important.Expand Specific Solutions03 Continuous flow cell-free systems
Continuous flow cell-free systems allow for sustained enzyme production by continuously supplying substrates and removing inhibitory byproducts. These systems overcome limitations of batch processes by maintaining optimal reaction conditions over extended periods. They can be designed with various compartments or membrane-based separations to enhance productivity and enable the production of complex enzymes requiring multiple processing steps.Expand Specific Solutions04 Genetic optimization for cell-free expression
Genetic optimization techniques enhance enzyme production in cell-free systems by modifying gene sequences for optimal expression. This includes codon optimization, removal of regulatory elements that might inhibit expression, and addition of specific tags or signal sequences. These modifications can significantly increase protein yield and activity in cell-free environments, allowing for more efficient production of industrial enzymes.Expand Specific Solutions05 Energy regeneration systems
Energy regeneration systems are crucial for sustained enzyme production in cell-free environments. These systems replenish ATP and other energy molecules that drive protein synthesis and enzyme activity. Various approaches include enzymatic cascades that recycle spent energy carriers, supplementation with energy-rich compounds, and coupling with secondary reactions that generate energy. Effective energy regeneration significantly extends the productive lifetime of cell-free systems.Expand Specific Solutions
Industry Leaders in Cell-free Biotechnology
Cell-free systems for rare enzyme production are emerging as a disruptive technology in the early growth phase of market development. The global market is expanding rapidly, driven by applications in pharmaceuticals, diagnostics, and industrial biotechnology. Key players demonstrate varying levels of technical maturity: established companies like GreenLight Biosciences and Debut Biotechnology have developed commercial platforms, while Nuprotein and Nature's Toolbox are advancing novel cell-free synthesis approaches. Academic institutions including Tsinghua University, Northwestern University, and Cornell University provide fundamental research support. The competitive landscape is diversifying with specialized entrants like Kangma Biological Technology focusing on diagnostic applications and Tierra Biosciences (formerly Synvitrobio) leveraging AI with cell-free systems for high-throughput protein manufacturing.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free protein synthesis platform specifically optimized for rare enzyme production. Their technology utilizes a highly engineered cell lysate system with enhanced transcription-translation capabilities that can produce complex enzymes at commercially viable scales. The platform incorporates proprietary RNA technology that enables precise control over protein expression rates and folding dynamics, critical for rare enzymes with complex structures[1]. Their system employs continuous-exchange cell-free (CECF) methodology that allows for extended reaction times (up to 24 hours) and higher protein yields compared to batch processes[2]. GreenLight has also developed specialized microfluidic bioreactors that optimize reagent exchange and energy regeneration systems, addressing key limitations in traditional cell-free systems for industrial-scale enzyme production[3].
Strengths: Scalable production system capable of commercial volumes; proprietary RNA technology enabling precise expression control; extended reaction durations through CECF methodology. Weaknesses: Higher production costs compared to cell-based systems; potential challenges with post-translational modifications required for some enzyme classes; dependency on continuous supply of expensive energy substrates.
Debut Biotechnology, Inc.
Technical Solution: Debut Biotechnology has pioneered a continuous-flow cell-free system specifically designed for rare enzyme production. Their proprietary platform utilizes immobilized enzyme cascades in microfluidic channels that enable the synthesis of complex enzymes without cellular constraints. The technology incorporates specialized synthetic cofactor regeneration systems that maintain optimal reaction conditions for extended periods, significantly increasing yields of difficult-to-express enzymes[1]. Debut's approach features modular reaction chambers with precise temperature and pH control, allowing for the optimization of conditions for each enzymatic step in multi-enzyme synthesis pathways[2]. Their system also employs advanced stabilization techniques including engineered chaperone proteins and customized reaction environments that preserve enzyme activity during production. The platform can be rapidly reconfigured for different enzyme targets without the extensive development time required for cell-based expression systems[3].
Strengths: Continuous-flow system enables higher productivity than batch processes; modular design allows rapid adaptation to different enzyme targets; elimination of cellular toxicity constraints. Weaknesses: Higher initial capital investment requirements; potential challenges with complex post-translational modifications; more complex operational requirements compared to traditional batch systems.
Key Patents in Rare Enzyme Synthesis
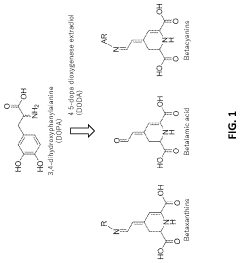
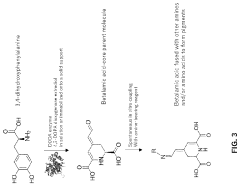
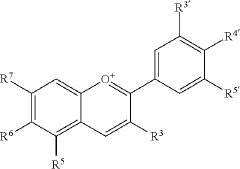
Cell-free methods of producing pigments
PatentPendingUS20230407353A1
Innovation
- A cell-free method using enzymes and cofactors in a bioreactor system to produce betalains, allowing for higher reaction concentrations and eliminating barriers like cell walls and byproduct formation, with the option for immobilized enzymes for simplified purification and recycling.
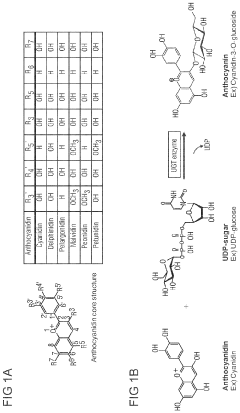
Anthocyanin bioproduction in a cell-free manufacturing system
PatentPendingUS20240191273A1
Innovation
- A cell-free biosynthesis platform using glycotransferase enzymes to convert anthocyanidins into anthocyanidin glycosides, allowing for higher reaction concentrations and flexibility in producing various anthocyanins by adjusting enzymes and UDP-sugars, with enzyme immobilization enhancing stability and efficiency.
Scalability Challenges and Solutions
Scaling up cell-free systems for rare enzyme production represents one of the most significant challenges in transitioning from laboratory-scale demonstrations to industrial applications. Current cell-free protein synthesis (CFPS) platforms typically operate efficiently at microliter to milliliter scales, but encounter substantial hurdles when expanded to industrial volumes necessary for commercial viability.
The primary scalability challenge stems from the inherent batch-to-batch variability in extract quality and activity. As production volumes increase, maintaining consistent extract performance becomes increasingly difficult, resulting in unpredictable yields and product quality. This variability is particularly problematic for rare enzymes that require precise post-translational modifications or specific folding environments to maintain functionality.
Economic considerations also present significant barriers to scale-up. The high cost of energy-rich compounds like ATP, GTP, and other nucleoside triphosphates becomes prohibitive at larger scales. Additionally, the expense of specialized components such as tRNA, amino acids, and cofactors necessary for rare enzyme production contributes substantially to the overall production costs, making industrial-scale operations economically challenging.
Several promising solutions have emerged to address these scalability issues. Continuous-exchange cell-free (CECF) systems represent a significant advancement, allowing for the replenishment of energy sources and removal of inhibitory byproducts during the production process. This approach has demonstrated up to 10-fold improvements in protein yields compared to batch reactions while maintaining consistent quality across larger volumes.
Microfluidic technologies offer another avenue for scaling cell-free production through parallelization rather than volume increase. By operating thousands of microscale reactions simultaneously, these systems maintain the benefits of small-scale precision while achieving industrially relevant total outputs. Companies like Sutro Biopharma have successfully implemented such approaches for therapeutic protein production.
Recent developments in lyophilized cell-free systems present perhaps the most promising solution for industrial scalability. These freeze-dried preparations maintain activity for months at ambient temperatures, dramatically reducing cold-chain requirements and allowing for standardized, modular production units that can be deployed as needed. This approach effectively decouples extract preparation from protein production, enabling centralized quality control while permitting distributed manufacturing.
Computational modeling and machine learning algorithms are increasingly being employed to optimize reaction conditions and predict scalability challenges before physical implementation. These in silico approaches significantly reduce development time and resources by identifying potential bottlenecks and suggesting mitigation strategies prior to large-scale production attempts.
The primary scalability challenge stems from the inherent batch-to-batch variability in extract quality and activity. As production volumes increase, maintaining consistent extract performance becomes increasingly difficult, resulting in unpredictable yields and product quality. This variability is particularly problematic for rare enzymes that require precise post-translational modifications or specific folding environments to maintain functionality.
Economic considerations also present significant barriers to scale-up. The high cost of energy-rich compounds like ATP, GTP, and other nucleoside triphosphates becomes prohibitive at larger scales. Additionally, the expense of specialized components such as tRNA, amino acids, and cofactors necessary for rare enzyme production contributes substantially to the overall production costs, making industrial-scale operations economically challenging.
Several promising solutions have emerged to address these scalability issues. Continuous-exchange cell-free (CECF) systems represent a significant advancement, allowing for the replenishment of energy sources and removal of inhibitory byproducts during the production process. This approach has demonstrated up to 10-fold improvements in protein yields compared to batch reactions while maintaining consistent quality across larger volumes.
Microfluidic technologies offer another avenue for scaling cell-free production through parallelization rather than volume increase. By operating thousands of microscale reactions simultaneously, these systems maintain the benefits of small-scale precision while achieving industrially relevant total outputs. Companies like Sutro Biopharma have successfully implemented such approaches for therapeutic protein production.
Recent developments in lyophilized cell-free systems present perhaps the most promising solution for industrial scalability. These freeze-dried preparations maintain activity for months at ambient temperatures, dramatically reducing cold-chain requirements and allowing for standardized, modular production units that can be deployed as needed. This approach effectively decouples extract preparation from protein production, enabling centralized quality control while permitting distributed manufacturing.
Computational modeling and machine learning algorithms are increasingly being employed to optimize reaction conditions and predict scalability challenges before physical implementation. These in silico approaches significantly reduce development time and resources by identifying potential bottlenecks and suggesting mitigation strategies prior to large-scale production attempts.
Regulatory Framework for Biocatalyst Production
The regulatory landscape governing biocatalyst production, particularly for cell-free systems producing rare enzymes, presents a complex framework that manufacturers must navigate. Regulations vary significantly across different regions, with the United States FDA, European Medicines Agency (EMA), and similar bodies in Asia establishing distinct requirements for biocatalyst development, production, and commercialization. These frameworks typically address safety assessments, quality control measures, and environmental impact considerations.
For cell-free enzyme production systems, regulatory considerations focus primarily on the source materials, production processes, and final product characterization. The absence of whole cells in the final product may simplify certain regulatory aspects compared to traditional microbial fermentation approaches, particularly regarding containment and biosafety levels. However, this advantage is counterbalanced by increased scrutiny on process validation and product consistency.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance for biocatalyst production. Manufacturers must demonstrate thorough understanding of critical quality attributes and process parameters that influence enzyme functionality, stability, and purity. For rare enzymes produced in cell-free systems, establishing appropriate reference standards presents a particular challenge due to limited availability of comparator materials.
Intellectual property considerations intersect with regulatory frameworks, especially for novel cell-free expression systems. Patent protection strategies must account for regulatory timelines, as extended approval processes may significantly reduce effective market exclusivity periods. Cross-licensing agreements between technology platform providers and enzyme developers have emerged as common strategies to navigate this complex landscape.
Environmental regulations also impact cell-free enzyme production, with increasing focus on sustainable manufacturing practices. While cell-free systems generally have reduced environmental footprints compared to whole-cell fermentation, waste management protocols for reaction components and byproducts remain subject to strict oversight. Recent regulatory trends indicate movement toward lifecycle assessment approaches that consider environmental impacts from raw material sourcing through product disposal.
International harmonization efforts, such as those through the International Council for Harmonisation (ICH), are gradually addressing regulatory divergences across markets. However, significant regional differences persist, necessitating tailored regulatory strategies for global commercialization of rare enzymes produced via cell-free systems. Regulatory intelligence and early engagement with authorities have become essential components of successful biocatalyst development programs.
For cell-free enzyme production systems, regulatory considerations focus primarily on the source materials, production processes, and final product characterization. The absence of whole cells in the final product may simplify certain regulatory aspects compared to traditional microbial fermentation approaches, particularly regarding containment and biosafety levels. However, this advantage is counterbalanced by increased scrutiny on process validation and product consistency.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance for biocatalyst production. Manufacturers must demonstrate thorough understanding of critical quality attributes and process parameters that influence enzyme functionality, stability, and purity. For rare enzymes produced in cell-free systems, establishing appropriate reference standards presents a particular challenge due to limited availability of comparator materials.
Intellectual property considerations intersect with regulatory frameworks, especially for novel cell-free expression systems. Patent protection strategies must account for regulatory timelines, as extended approval processes may significantly reduce effective market exclusivity periods. Cross-licensing agreements between technology platform providers and enzyme developers have emerged as common strategies to navigate this complex landscape.
Environmental regulations also impact cell-free enzyme production, with increasing focus on sustainable manufacturing practices. While cell-free systems generally have reduced environmental footprints compared to whole-cell fermentation, waste management protocols for reaction components and byproducts remain subject to strict oversight. Recent regulatory trends indicate movement toward lifecycle assessment approaches that consider environmental impacts from raw material sourcing through product disposal.
International harmonization efforts, such as those through the International Council for Harmonisation (ICH), are gradually addressing regulatory divergences across markets. However, significant regional differences persist, necessitating tailored regulatory strategies for global commercialization of rare enzymes produced via cell-free systems. Regulatory intelligence and early engagement with authorities have become essential components of successful biocatalyst development programs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!