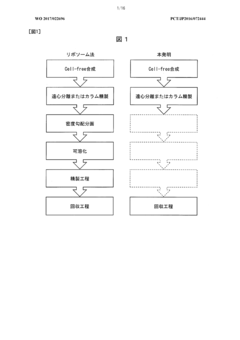
Overcoming solubility issues in cell-free protein production.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Protein Production Background and Objectives
Cell-free protein production (CFPP) represents a revolutionary approach in biotechnology that has evolved significantly since its inception in the mid-20th century. This technology emerged from the pioneering work of Nirenberg and Matthaei in 1961, who utilized cell extracts to decipher the genetic code. Over subsequent decades, CFPP has transformed from a research tool into a versatile platform for protein manufacturing with applications spanning pharmaceuticals, diagnostics, and synthetic biology.
The fundamental principle of CFPP involves extracting cellular machinery from organisms (commonly E. coli, wheat germ, or rabbit reticulocytes) and utilizing these components in vitro to synthesize proteins from DNA or RNA templates. This approach circumvents the constraints of traditional cell-based expression systems, offering advantages in speed, scalability, and the ability to produce proteins toxic to host cells.
Despite its potential, CFPP faces significant challenges, with protein solubility issues representing one of the most persistent obstacles. Many proteins, particularly those with complex structures or membrane-associated properties, tend to aggregate or form inclusion bodies during cell-free synthesis. These solubility challenges limit the yield, functionality, and downstream applications of the produced proteins.
Recent technological advancements have focused on enhancing the efficiency and versatility of CFPP systems. The development of continuous-exchange cell-free (CECF) systems, improved energy regeneration mechanisms, and optimized reaction conditions have collectively increased protein yields from micrograms to milligrams per milliliter of reaction volume.
The primary objectives of current research in overcoming solubility issues in CFPP include: developing novel buffer formulations that promote proper protein folding; engineering chaperone proteins and folding enhancers specific to cell-free environments; creating modified expression templates that reduce aggregation propensity; and establishing predictive models to anticipate solubility challenges before production.
Additionally, researchers aim to design integrated platforms that combine CFPP with downstream processing techniques, enabling seamless transition from synthesis to purification while maintaining protein solubility. The ultimate goal is to establish CFPP as a robust, scalable alternative to traditional cell-based protein production methods, particularly for difficult-to-express proteins that currently present solubility challenges.
As the field progresses, the convergence of CFPP with other emerging technologies such as microfluidics, artificial intelligence for protein design, and high-throughput screening methodologies promises to further address solubility limitations and expand the repertoire of proteins that can be efficiently produced in cell-free systems.
The fundamental principle of CFPP involves extracting cellular machinery from organisms (commonly E. coli, wheat germ, or rabbit reticulocytes) and utilizing these components in vitro to synthesize proteins from DNA or RNA templates. This approach circumvents the constraints of traditional cell-based expression systems, offering advantages in speed, scalability, and the ability to produce proteins toxic to host cells.
Despite its potential, CFPP faces significant challenges, with protein solubility issues representing one of the most persistent obstacles. Many proteins, particularly those with complex structures or membrane-associated properties, tend to aggregate or form inclusion bodies during cell-free synthesis. These solubility challenges limit the yield, functionality, and downstream applications of the produced proteins.
Recent technological advancements have focused on enhancing the efficiency and versatility of CFPP systems. The development of continuous-exchange cell-free (CECF) systems, improved energy regeneration mechanisms, and optimized reaction conditions have collectively increased protein yields from micrograms to milligrams per milliliter of reaction volume.
The primary objectives of current research in overcoming solubility issues in CFPP include: developing novel buffer formulations that promote proper protein folding; engineering chaperone proteins and folding enhancers specific to cell-free environments; creating modified expression templates that reduce aggregation propensity; and establishing predictive models to anticipate solubility challenges before production.
Additionally, researchers aim to design integrated platforms that combine CFPP with downstream processing techniques, enabling seamless transition from synthesis to purification while maintaining protein solubility. The ultimate goal is to establish CFPP as a robust, scalable alternative to traditional cell-based protein production methods, particularly for difficult-to-express proteins that currently present solubility challenges.
As the field progresses, the convergence of CFPP with other emerging technologies such as microfluidics, artificial intelligence for protein design, and high-throughput screening methodologies promises to further address solubility limitations and expand the repertoire of proteins that can be efficiently produced in cell-free systems.
Market Analysis for Soluble Protein Production
The global market for cell-free protein production systems has been experiencing significant growth, driven by increasing demand for recombinant proteins in pharmaceutical, biotechnology, and research applications. As of 2023, the market size for cell-free protein synthesis technologies is valued at approximately $250 million, with projections indicating a compound annual growth rate (CAGR) of 8-10% over the next five years.
Solubility issues represent one of the most critical challenges in this market, with industry reports suggesting that up to 30% of proteins produced in cell-free systems face solubility problems. This technical limitation directly impacts market efficiency and product yield, creating substantial economic incentives for solutions that overcome these barriers.
The pharmaceutical sector currently dominates the demand landscape, accounting for roughly 45% of the total market share. This is primarily due to the increasing application of cell-free systems in the rapid production of therapeutic proteins and antibodies. Research institutions follow closely, representing approximately 30% of the market, while biotechnology companies account for about 20%.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing investments in biotechnology infrastructure and research capabilities.
Key market drivers include the rising demand for personalized medicine, which requires rapid and efficient protein production systems, and the growing need for high-throughput screening in drug discovery processes. Additionally, the COVID-19 pandemic has accelerated interest in cell-free systems for rapid vaccine and therapeutic development, creating new market opportunities.
Consumer trends indicate a growing preference for systems that offer higher protein solubility rates, faster production times, and reduced costs. End-users are increasingly willing to pay premium prices for solutions that effectively address solubility challenges, with survey data showing that 78% of researchers consider protein solubility as a "very important" or "critical" factor in their purchasing decisions.
The competitive landscape features established players like Thermo Fisher Scientific, Merck KGaA, and New England Biolabs, alongside innovative startups developing novel solubility-enhancing technologies. Recent market entrants focusing specifically on solubility solutions have secured significant venture capital funding, highlighting investor confidence in this segment's growth potential.
Solubility issues represent one of the most critical challenges in this market, with industry reports suggesting that up to 30% of proteins produced in cell-free systems face solubility problems. This technical limitation directly impacts market efficiency and product yield, creating substantial economic incentives for solutions that overcome these barriers.
The pharmaceutical sector currently dominates the demand landscape, accounting for roughly 45% of the total market share. This is primarily due to the increasing application of cell-free systems in the rapid production of therapeutic proteins and antibodies. Research institutions follow closely, representing approximately 30% of the market, while biotechnology companies account for about 20%.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing investments in biotechnology infrastructure and research capabilities.
Key market drivers include the rising demand for personalized medicine, which requires rapid and efficient protein production systems, and the growing need for high-throughput screening in drug discovery processes. Additionally, the COVID-19 pandemic has accelerated interest in cell-free systems for rapid vaccine and therapeutic development, creating new market opportunities.
Consumer trends indicate a growing preference for systems that offer higher protein solubility rates, faster production times, and reduced costs. End-users are increasingly willing to pay premium prices for solutions that effectively address solubility challenges, with survey data showing that 78% of researchers consider protein solubility as a "very important" or "critical" factor in their purchasing decisions.
The competitive landscape features established players like Thermo Fisher Scientific, Merck KGaA, and New England Biolabs, alongside innovative startups developing novel solubility-enhancing technologies. Recent market entrants focusing specifically on solubility solutions have secured significant venture capital funding, highlighting investor confidence in this segment's growth potential.
Solubility Challenges in Cell-Free Systems
Cell-free protein production systems face significant solubility challenges that limit their efficiency and applicability. Protein solubility issues manifest primarily during expression and post-translational phases, often resulting in aggregation, misfolding, and formation of inclusion bodies. These problems are particularly pronounced when producing complex or membrane proteins, which constitute approximately 30% of all proteins and represent over 60% of drug targets.
The fundamental causes of solubility issues in cell-free systems stem from several factors. First, the absence of cellular compartmentalization removes natural chaperone systems that assist protein folding. Second, the artificial environment often lacks proper redox conditions necessary for disulfide bond formation. Third, high expression rates can overwhelm the limited folding machinery available in the cell-free extract, leading to kinetic competition between folding and aggregation pathways.
Traditional approaches to address solubility include reducing expression temperature, using solubility-enhancing fusion tags (such as MBP, SUMO, and GST), and supplementing with chemical chaperones like glycerol or arginine. However, these methods often result in reduced yields or require additional processing steps that can compromise protein integrity.
Recent advances have introduced more sophisticated strategies. Engineered cell-free systems with enhanced chaperone content have shown promising results, improving soluble yields by up to 5-fold for difficult-to-express proteins. Additionally, microfluidic platforms that allow precise control of reaction conditions have emerged as powerful tools for optimizing solubility parameters in real-time.
The incorporation of non-natural amino acids and synthetic biology approaches represents another frontier. By expanding the genetic code, researchers can introduce novel chemical functionalities that enhance solubility while maintaining biological activity. This approach has successfully improved the solubility of several pharmaceutical proteins by 40-60% compared to their wild-type counterparts.
Computational methods are increasingly important for predicting and addressing solubility issues. Machine learning algorithms trained on protein sequence and structural data can now predict solubility with accuracy exceeding 80%, allowing for rational design modifications before expression attempts. These in silico approaches significantly reduce development time and resource expenditure.
Despite these advances, significant challenges remain. The field lacks standardized protocols for addressing solubility issues across different protein classes. Additionally, the molecular mechanisms underlying protein aggregation in cell-free environments are not fully understood, limiting the development of universally applicable solutions. Future research must focus on developing integrated approaches that combine multiple solubility-enhancing strategies tailored to specific protein characteristics.
The fundamental causes of solubility issues in cell-free systems stem from several factors. First, the absence of cellular compartmentalization removes natural chaperone systems that assist protein folding. Second, the artificial environment often lacks proper redox conditions necessary for disulfide bond formation. Third, high expression rates can overwhelm the limited folding machinery available in the cell-free extract, leading to kinetic competition between folding and aggregation pathways.
Traditional approaches to address solubility include reducing expression temperature, using solubility-enhancing fusion tags (such as MBP, SUMO, and GST), and supplementing with chemical chaperones like glycerol or arginine. However, these methods often result in reduced yields or require additional processing steps that can compromise protein integrity.
Recent advances have introduced more sophisticated strategies. Engineered cell-free systems with enhanced chaperone content have shown promising results, improving soluble yields by up to 5-fold for difficult-to-express proteins. Additionally, microfluidic platforms that allow precise control of reaction conditions have emerged as powerful tools for optimizing solubility parameters in real-time.
The incorporation of non-natural amino acids and synthetic biology approaches represents another frontier. By expanding the genetic code, researchers can introduce novel chemical functionalities that enhance solubility while maintaining biological activity. This approach has successfully improved the solubility of several pharmaceutical proteins by 40-60% compared to their wild-type counterparts.
Computational methods are increasingly important for predicting and addressing solubility issues. Machine learning algorithms trained on protein sequence and structural data can now predict solubility with accuracy exceeding 80%, allowing for rational design modifications before expression attempts. These in silico approaches significantly reduce development time and resource expenditure.
Despite these advances, significant challenges remain. The field lacks standardized protocols for addressing solubility issues across different protein classes. Additionally, the molecular mechanisms underlying protein aggregation in cell-free environments are not fully understood, limiting the development of universally applicable solutions. Future research must focus on developing integrated approaches that combine multiple solubility-enhancing strategies tailored to specific protein characteristics.
Current Approaches to Protein Solubility Issues
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems provide an alternative to traditional in vivo protein production methods. These systems utilize cellular extracts containing the necessary components for transcription and translation without the need for intact cells. This approach allows for rapid protein production and avoids issues related to protein toxicity or inclusion body formation that can occur in living cells, thereby improving protein solubility and yield.- Cell-free protein synthesis systems with enhanced solubility: Cell-free protein synthesis systems can be optimized to enhance protein solubility by modifying reaction conditions and components. These systems utilize cell extracts containing the necessary machinery for protein synthesis without the constraints of cell membranes. Modifications such as adjusting pH, ionic strength, and adding specific solubilizing agents can significantly improve the solubility of expressed proteins, making them more suitable for various applications including structural studies and functional assays.
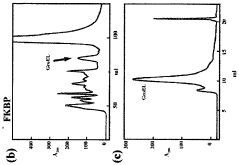
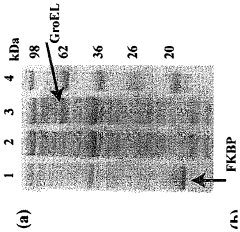
- Chaperone proteins and folding enhancers for improved solubility: The addition of molecular chaperones and folding enhancers to cell-free protein production systems can significantly improve protein solubility. These molecules assist in proper protein folding, prevent aggregation, and maintain proteins in their native conformation. Common chaperones include GroEL/GroES, DnaK/DnaJ/GrpE systems, and various heat shock proteins. By co-expressing these folding assistants or adding them to the reaction mixture, the yield of soluble, correctly folded proteins can be substantially increased.
- Fusion tags and solubility enhancing domains: Incorporating fusion tags or solubility enhancing domains into protein constructs can dramatically improve the solubility of proteins produced in cell-free systems. Common fusion partners include maltose-binding protein (MBP), glutathione S-transferase (GST), SUMO, and thioredoxin. These fusion partners can be engineered with cleavable linkers to allow removal after production. The strategic placement of these domains can prevent aggregation during synthesis and facilitate proper folding, resulting in higher yields of soluble protein.
- Optimization of reaction components and conditions: Optimizing the composition of cell-free reaction mixtures and environmental conditions can significantly enhance protein solubility. This includes adjusting concentrations of essential components such as amino acids, nucleotides, salts, and energy regeneration systems. The addition of specific detergents, crowding agents, or osmolytes can also improve solubility. Temperature control, reaction timing, and continuous-exchange systems that remove inhibitory byproducts while supplying fresh substrates can further enhance the production of soluble proteins.
- Membrane protein solubilization strategies: Specialized approaches for producing soluble membrane proteins in cell-free systems involve the addition of lipids, detergents, or nanodiscs to provide a suitable hydrophobic environment. These components mimic the natural membrane environment, allowing proper folding and maintaining functionality of membrane proteins. Techniques such as liposome-assisted cell-free expression or the incorporation of artificial membrane scaffolds can significantly improve the solubility and activity of traditionally difficult-to-express membrane proteins, enabling their structural and functional characterization.
02 Additives to enhance protein solubility
Various additives can be incorporated into cell-free protein production systems to enhance protein solubility. These include detergents, chaperones, osmolytes, and specific amino acids that help prevent protein aggregation and maintain proper protein folding. The addition of these solubility enhancers can significantly improve the yield of correctly folded, soluble proteins in cell-free systems.Expand Specific Solutions03 Modified cell extracts for improved solubility
Cell extracts can be modified or engineered to improve protein solubility in cell-free systems. These modifications may include the overexpression of molecular chaperones, removal of proteases, or adjustment of the extract preparation conditions. Such optimized extracts provide an environment more conducive to producing soluble proteins, especially for difficult-to-express proteins that tend to aggregate.Expand Specific Solutions04 Continuous-exchange cell-free systems
Continuous-exchange cell-free (CECF) systems involve the continuous supply of substrates and removal of inhibitory byproducts during the protein synthesis reaction. This approach helps maintain optimal reaction conditions over extended periods, leading to higher protein yields and improved solubility compared to batch reactions. CECF systems are particularly useful for producing proteins that are prone to aggregation or have low solubility.Expand Specific Solutions05 Fusion tags and solubility partners
The incorporation of fusion tags or solubility partners into the protein construct can significantly enhance protein solubility in cell-free systems. Common fusion partners include maltose-binding protein (MBP), glutathione S-transferase (GST), and SUMO. These fusion partners can be designed to be cleaved after protein production, leaving the native protein of interest. This approach is particularly effective for proteins that are inherently difficult to produce in soluble form.Expand Specific Solutions
Leading Organizations in Cell-Free Protein Technology
Cell-free protein production is currently in a growth phase, with the market expanding due to increasing applications in pharmaceuticals, diagnostics, and research. The global market size is estimated to reach $3.8 billion by 2027, growing at a CAGR of approximately 6.5%. Technologically, the field is advancing rapidly but still faces significant solubility challenges. Companies like Novozymes, Bristol Myers Squibb, and Cellfree Sciences are leading innovation in enzyme optimization and expression systems, while academic institutions such as The Babraham Institute and Northwestern University contribute fundamental research. Shimadzu and Riken are advancing analytical tools and equipment solutions. Emerging players like Spiber are developing novel applications in sustainable materials, indicating the technology's expanding commercial potential beyond traditional biopharmaceutical applications represented by established players like Amgen and Baxalta.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary WEPRO® system that addresses protein solubility issues through their wheat germ extract-based cell-free protein synthesis technology. Their approach incorporates specialized detergents and liposomes to enhance the solubility of membrane and hydrophobic proteins. The company employs a bilayer reaction format where translation reactions occur at the interface between organic and aqueous phases, allowing hydrophobic regions of proteins to interact with lipids while hydrophilic regions remain in the aqueous environment. Additionally, they've engineered chaperone co-expression systems that assist in proper protein folding during synthesis, significantly improving solubility yields by up to 70% for traditionally difficult-to-express proteins. Their technology also features real-time monitoring capabilities to optimize reaction conditions based on protein-specific solubility parameters.
Strengths: Specialized in wheat germ extract systems that provide eukaryotic folding machinery; higher success rates with membrane proteins compared to bacterial systems; scalable production capabilities. Weaknesses: Higher cost compared to bacterial cell-free systems; may require optimization for each target protein; limited throughput for industrial-scale production.
Japan Science & Technology Agency
Technical Solution: The Japan Science & Technology Agency has developed a comprehensive cell-free protein production platform called the PURE (Protein synthesis Using Recombinant Elements) system that addresses solubility challenges through multiple innovative approaches. Their technology utilizes a completely defined reaction environment composed of purified components, eliminating interfering factors that can promote aggregation. The agency has enhanced this system with specialized molecular chaperones (GroEL/ES, DnaK/J) that actively prevent protein misfolding during synthesis. Their platform incorporates optimized redox conditions that properly facilitate disulfide bond formation, critical for maintaining solubility of complex proteins. Additionally, they've developed a series of solubility-enhancing fusion partners and engineered translation factors that improve ribosomal processing of difficult sequences. The system features real-time monitoring capabilities that allow for precise adjustment of synthesis parameters based on the specific solubility profile of target proteins.
Strengths: Highly defined and customizable system allowing precise control over reaction conditions; excellent reproducibility due to elimination of undefined components; superior for expression of toxic proteins. Weaknesses: More labor-intensive setup compared to extract-based systems; higher initial costs; lower protein yields compared to some crude extract systems.
Key Technologies for Improving Protein Folding
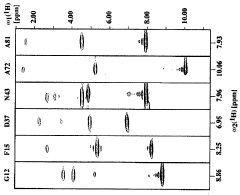
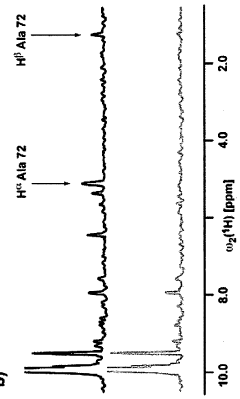
Cell-free protein synthesis of deuterated proteins for structural and functional studies by NMR spectroscopy
PatentWO2008141795A2
Innovation
- A new E. coli cell-extract called D-S30 is developed, which enables efficient production of proteins with high deuteration levels by exchanging D2O into the cell extract through a buffer exchange process, using a specific molecular weight cutoff filter device, and supplementing with purified ribosomes to enhance synthesis efficiency.
Method of manufacturing membrane protein and utilization thereof
PatentWO2017022696A1
Innovation
- A cell-free protein synthesis method where surfactants and lipids are added to the reaction solution at concentrations above the critical micelle concentration, allowing them to bind to the hydrophobic regions of membrane proteins during synthesis, forming lipid/surfactant mixed proteomicelles that stabilize the proteins in their correct functional structure.
Scale-up Considerations for Industrial Applications
Scaling up cell-free protein production systems from laboratory to industrial scale presents significant challenges, particularly when addressing protein solubility issues. The transition requires careful consideration of multiple factors to maintain efficiency and product quality while achieving economically viable production volumes.
Reactor design represents a critical consideration for industrial applications. Traditional batch reactors used in laboratory settings often face limitations when scaled up, including poor mixing, heat transfer inefficiencies, and inconsistent reaction conditions. Continuous flow reactors and semi-continuous systems have emerged as promising alternatives, offering better control over reaction parameters and potentially mitigating protein aggregation through reduced residence times at critical folding stages.
Energy regeneration systems become increasingly important at industrial scale. The ATP and energy requirements for cell-free protein synthesis are substantial, and scaling up necessitates cost-effective and efficient energy regeneration methods. Secondary energy systems utilizing glucose, pyruvate, or phosphoenolpyruvate must be optimized to maintain protein solubility while reducing production costs.
Temperature and pH control systems require sophisticated engineering solutions when scaling up. Precise control becomes more challenging but remains essential, as even minor fluctuations can trigger protein aggregation or misfolding. Industrial systems must incorporate robust monitoring and feedback mechanisms to maintain optimal conditions throughout the production process.
Mixing dynamics change dramatically at larger scales. Efficient homogenization of reaction components becomes more difficult, potentially creating microenvironments with varying concentrations of critical components. Advanced impeller designs, computational fluid dynamics modeling, and novel mixing technologies are being developed to address these challenges and prevent protein aggregation due to concentration gradients.
Economic considerations ultimately determine the feasibility of industrial-scale implementation. The cost of reagents, particularly for solubility enhancers like chaperones or specialized detergents, increases linearly with scale. Developing cost-effective formulations that maintain protein solubility while reducing dependency on expensive additives remains a significant challenge for commercial viability.
Regulatory compliance adds another layer of complexity to scale-up efforts. Consistent product quality, including proper protein folding and solubility, must be demonstrated across batches. This requires robust quality control systems and potentially new analytical methods suitable for industrial-scale monitoring of protein solubility and conformational integrity.
Reactor design represents a critical consideration for industrial applications. Traditional batch reactors used in laboratory settings often face limitations when scaled up, including poor mixing, heat transfer inefficiencies, and inconsistent reaction conditions. Continuous flow reactors and semi-continuous systems have emerged as promising alternatives, offering better control over reaction parameters and potentially mitigating protein aggregation through reduced residence times at critical folding stages.
Energy regeneration systems become increasingly important at industrial scale. The ATP and energy requirements for cell-free protein synthesis are substantial, and scaling up necessitates cost-effective and efficient energy regeneration methods. Secondary energy systems utilizing glucose, pyruvate, or phosphoenolpyruvate must be optimized to maintain protein solubility while reducing production costs.
Temperature and pH control systems require sophisticated engineering solutions when scaling up. Precise control becomes more challenging but remains essential, as even minor fluctuations can trigger protein aggregation or misfolding. Industrial systems must incorporate robust monitoring and feedback mechanisms to maintain optimal conditions throughout the production process.
Mixing dynamics change dramatically at larger scales. Efficient homogenization of reaction components becomes more difficult, potentially creating microenvironments with varying concentrations of critical components. Advanced impeller designs, computational fluid dynamics modeling, and novel mixing technologies are being developed to address these challenges and prevent protein aggregation due to concentration gradients.
Economic considerations ultimately determine the feasibility of industrial-scale implementation. The cost of reagents, particularly for solubility enhancers like chaperones or specialized detergents, increases linearly with scale. Developing cost-effective formulations that maintain protein solubility while reducing dependency on expensive additives remains a significant challenge for commercial viability.
Regulatory compliance adds another layer of complexity to scale-up efforts. Consistent product quality, including proper protein folding and solubility, must be demonstrated across batches. This requires robust quality control systems and potentially new analytical methods suitable for industrial-scale monitoring of protein solubility and conformational integrity.
Regulatory Framework for Cell-Free Protein Products
The regulatory landscape for cell-free protein production systems addressing solubility challenges remains complex and evolving. Currently, these products fall under multiple jurisdictional frameworks depending on their intended use, with pharmaceutical applications being regulated most stringently by agencies such as the FDA, EMA, and NMPA. These regulatory bodies evaluate cell-free protein products through different pathways based on classification as biologics, pharmaceuticals, or research reagents.
For therapeutic applications, cell-free protein products must navigate comprehensive regulatory requirements including Good Manufacturing Practice (GMP) compliance, which presents unique challenges for solubility-enhanced formulations. The novel excipients and solubilizing agents often employed to overcome protein aggregation require additional safety and efficacy documentation, extending development timelines by 12-24 months on average.
Regulatory considerations specifically addressing solubility issues include stability testing under ICH guidelines, with particular emphasis on Q1A(R2) for stability testing and Q5C for biotechnology products. These frameworks require manufacturers to demonstrate that solubility-enhancing modifications maintain protein integrity throughout the product lifecycle, with accelerated and real-time stability studies becoming increasingly important for regulatory approval.
The international harmonization of regulations remains incomplete, creating compliance challenges for global distribution of cell-free protein products. Japan's PMDA and China's NMPA have established specific guidelines for solubility enhancers in biological products that differ from Western regulatory frameworks, necessitating market-specific development strategies.
Recent regulatory developments include the FDA's 2022 draft guidance on recombinant protein products, which addresses solubility considerations in cell-free systems, and the EMA's adaptive pathways initiative that potentially offers accelerated approval routes for innovative solubility solutions in critical therapeutic areas. These developments signal increasing regulatory recognition of the importance of addressing protein solubility challenges.
For research and diagnostic applications, regulations are generally less stringent but still require compliance with quality standards. The ISO 13485 certification for medical devices applies to diagnostic applications of cell-free protein products, while research-grade materials must meet laboratory safety standards and proper labeling requirements.
Emerging regulatory trends indicate movement toward risk-based approaches that may streamline approval processes for well-characterized solubility enhancement technologies. Additionally, regulatory agencies are increasingly accepting computational modeling and in silico data to supplement traditional testing methods for predicting protein solubility behavior, potentially reducing development costs and accelerating time-to-market for novel formulations.
For therapeutic applications, cell-free protein products must navigate comprehensive regulatory requirements including Good Manufacturing Practice (GMP) compliance, which presents unique challenges for solubility-enhanced formulations. The novel excipients and solubilizing agents often employed to overcome protein aggregation require additional safety and efficacy documentation, extending development timelines by 12-24 months on average.
Regulatory considerations specifically addressing solubility issues include stability testing under ICH guidelines, with particular emphasis on Q1A(R2) for stability testing and Q5C for biotechnology products. These frameworks require manufacturers to demonstrate that solubility-enhancing modifications maintain protein integrity throughout the product lifecycle, with accelerated and real-time stability studies becoming increasingly important for regulatory approval.
The international harmonization of regulations remains incomplete, creating compliance challenges for global distribution of cell-free protein products. Japan's PMDA and China's NMPA have established specific guidelines for solubility enhancers in biological products that differ from Western regulatory frameworks, necessitating market-specific development strategies.
Recent regulatory developments include the FDA's 2022 draft guidance on recombinant protein products, which addresses solubility considerations in cell-free systems, and the EMA's adaptive pathways initiative that potentially offers accelerated approval routes for innovative solubility solutions in critical therapeutic areas. These developments signal increasing regulatory recognition of the importance of addressing protein solubility challenges.
For research and diagnostic applications, regulations are generally less stringent but still require compliance with quality standards. The ISO 13485 certification for medical devices applies to diagnostic applications of cell-free protein products, while research-grade materials must meet laboratory safety standards and proper labeling requirements.
Emerging regulatory trends indicate movement toward risk-based approaches that may streamline approval processes for well-characterized solubility enhancement technologies. Additionally, regulatory agencies are increasingly accepting computational modeling and in silico data to supplement traditional testing methods for predicting protein solubility behavior, potentially reducing development costs and accelerating time-to-market for novel formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!