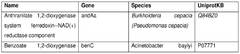
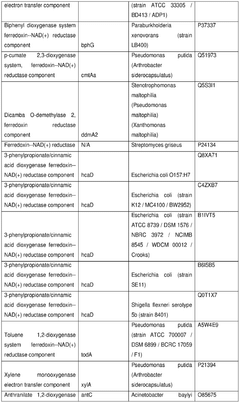
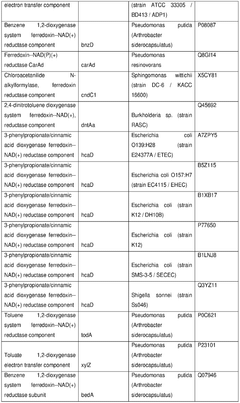
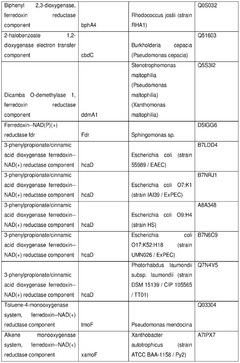
The role of cofactors in optimizing cell-free biosynthesis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cofactor Technology Background and Objectives
Cell-free biosynthesis has emerged as a transformative technology in biotechnology, offering a simplified and controllable platform for the production of valuable compounds without the constraints of living cells. The evolution of this field traces back to the early 20th century with pioneering work on cell extracts, but has gained significant momentum in the past two decades due to advances in synthetic biology and biochemical engineering.
Cofactors play a pivotal role in this technology as essential non-protein components that enable enzymatic reactions. These include coenzymes (such as NAD+, NADP+, ATP, and CoA), metal ions (like Mg2+, Mn2+, and Fe2+), and other small molecules that facilitate electron transfer, group transfer, and catalysis. The historical development of cofactor understanding has paralleled our growing knowledge of metabolic pathways and enzyme function.
The technological trajectory shows a clear shift from basic research on cofactor biochemistry to sophisticated engineering of cofactor regeneration systems. Early cell-free systems suffered from limited productivity due to cofactor depletion, but modern approaches incorporate elegant regeneration cycles that maintain cofactor availability and enable sustained biosynthetic activity.
Current research objectives in this field focus on several key areas. First, enhancing cofactor stability under cell-free conditions, as many cofactors are inherently unstable outside their native cellular environment. Second, optimizing cofactor concentrations and ratios to maximize enzymatic efficiency while minimizing inhibitory effects. Third, developing novel cofactor regeneration systems that operate with minimal energy input and generate minimal waste products.
Another critical objective is the integration of multiple cofactor-dependent pathways in a single cell-free system, enabling complex multi-step biosynthesis. This requires precise balancing of various cofactors and their regeneration systems to prevent bottlenecks in the overall process.
The ultimate goal of cofactor technology in cell-free biosynthesis is to create highly efficient, economically viable production platforms for pharmaceuticals, fine chemicals, biofuels, and other valuable compounds. This includes developing standardized cofactor modules that can be readily incorporated into diverse biosynthetic pathways, thereby accelerating the design-build-test cycle for new cell-free processes.
Looking forward, the field aims to expand the repertoire of usable cofactors beyond natural ones, incorporating synthetic or modified cofactors with enhanced stability or novel functions. This could potentially unlock new reaction chemistries and product classes previously inaccessible to biological systems.
Cofactors play a pivotal role in this technology as essential non-protein components that enable enzymatic reactions. These include coenzymes (such as NAD+, NADP+, ATP, and CoA), metal ions (like Mg2+, Mn2+, and Fe2+), and other small molecules that facilitate electron transfer, group transfer, and catalysis. The historical development of cofactor understanding has paralleled our growing knowledge of metabolic pathways and enzyme function.
The technological trajectory shows a clear shift from basic research on cofactor biochemistry to sophisticated engineering of cofactor regeneration systems. Early cell-free systems suffered from limited productivity due to cofactor depletion, but modern approaches incorporate elegant regeneration cycles that maintain cofactor availability and enable sustained biosynthetic activity.
Current research objectives in this field focus on several key areas. First, enhancing cofactor stability under cell-free conditions, as many cofactors are inherently unstable outside their native cellular environment. Second, optimizing cofactor concentrations and ratios to maximize enzymatic efficiency while minimizing inhibitory effects. Third, developing novel cofactor regeneration systems that operate with minimal energy input and generate minimal waste products.
Another critical objective is the integration of multiple cofactor-dependent pathways in a single cell-free system, enabling complex multi-step biosynthesis. This requires precise balancing of various cofactors and their regeneration systems to prevent bottlenecks in the overall process.
The ultimate goal of cofactor technology in cell-free biosynthesis is to create highly efficient, economically viable production platforms for pharmaceuticals, fine chemicals, biofuels, and other valuable compounds. This includes developing standardized cofactor modules that can be readily incorporated into diverse biosynthetic pathways, thereby accelerating the design-build-test cycle for new cell-free processes.
Looking forward, the field aims to expand the repertoire of usable cofactors beyond natural ones, incorporating synthetic or modified cofactors with enhanced stability or novel functions. This could potentially unlock new reaction chemistries and product classes previously inaccessible to biological systems.
Market Analysis for Cell-free Biosynthesis Applications
The cell-free biosynthesis market is experiencing significant growth, driven by increasing demand for sustainable production methods across pharmaceutical, chemical, and food industries. Current market valuations indicate that the global cell-free protein synthesis market reached approximately 250 million USD in 2022, with projections suggesting a compound annual growth rate of 8-10% through 2030. This growth trajectory is particularly notable in regions with strong biotechnology sectors, including North America, Europe, and emerging Asian markets.
The pharmaceutical sector represents the largest application segment, accounting for over 40% of the market share. This dominance stems from the critical need for rapid protein production in drug discovery and development processes. The ability of cell-free systems to produce difficult-to-express proteins and incorporate non-natural amino acids has positioned them as valuable tools in therapeutic protein development.
Industrial biotechnology applications are emerging as the fastest-growing segment, with annual growth rates exceeding 12%. This acceleration is driven by increasing industrial interest in sustainable biomanufacturing processes that reduce environmental footprints while maintaining production efficiency. The role of cofactors in these systems is particularly crucial for industrial applications, as they directly impact yield and cost-effectiveness.
Market analysis reveals a significant shift in customer preferences toward optimized cell-free systems with enhanced cofactor stability and regeneration capabilities. End-users are increasingly willing to pay premium prices for systems that demonstrate consistent performance and reduced cofactor-related costs. This trend has created a competitive advantage for companies offering innovative cofactor solutions.
Regional market distribution shows North America leading with approximately 35% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the highest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea. These countries are actively developing domestic capabilities in cell-free biosynthesis technologies.
Key market drivers include increasing R&D investments, growing demand for personalized medicine, and rising adoption of synthetic biology approaches across industries. Regulatory support for sustainable manufacturing processes has also contributed to market expansion. Conversely, high production costs and technical challenges related to cofactor stability remain significant market restraints.
Customer segmentation analysis indicates that academic and research institutions currently represent the largest user base, followed by pharmaceutical companies and biotechnology firms. However, the industrial segment is expected to demonstrate the highest growth rate over the next five years as technical barriers are overcome and cost structures improve.
The pharmaceutical sector represents the largest application segment, accounting for over 40% of the market share. This dominance stems from the critical need for rapid protein production in drug discovery and development processes. The ability of cell-free systems to produce difficult-to-express proteins and incorporate non-natural amino acids has positioned them as valuable tools in therapeutic protein development.
Industrial biotechnology applications are emerging as the fastest-growing segment, with annual growth rates exceeding 12%. This acceleration is driven by increasing industrial interest in sustainable biomanufacturing processes that reduce environmental footprints while maintaining production efficiency. The role of cofactors in these systems is particularly crucial for industrial applications, as they directly impact yield and cost-effectiveness.
Market analysis reveals a significant shift in customer preferences toward optimized cell-free systems with enhanced cofactor stability and regeneration capabilities. End-users are increasingly willing to pay premium prices for systems that demonstrate consistent performance and reduced cofactor-related costs. This trend has created a competitive advantage for companies offering innovative cofactor solutions.
Regional market distribution shows North America leading with approximately 35% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the highest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea. These countries are actively developing domestic capabilities in cell-free biosynthesis technologies.
Key market drivers include increasing R&D investments, growing demand for personalized medicine, and rising adoption of synthetic biology approaches across industries. Regulatory support for sustainable manufacturing processes has also contributed to market expansion. Conversely, high production costs and technical challenges related to cofactor stability remain significant market restraints.
Customer segmentation analysis indicates that academic and research institutions currently represent the largest user base, followed by pharmaceutical companies and biotechnology firms. However, the industrial segment is expected to demonstrate the highest growth rate over the next five years as technical barriers are overcome and cost structures improve.
Current Cofactor Challenges in Cell-free Systems
Cell-free biosynthesis systems face significant challenges related to cofactor management that limit their industrial application potential. The primary issue is cofactor stability, as many essential cofactors such as NAD(P)H, ATP, and SAM degrade rapidly under cell-free conditions, with half-lives ranging from minutes to hours. This instability necessitates continuous regeneration or supplementation, increasing operational costs and complexity.
Cofactor availability presents another major hurdle. Unlike living cells that maintain homeostatic cofactor concentrations, cell-free systems lack natural regulatory mechanisms, resulting in suboptimal cofactor ratios that reduce reaction efficiency. The cost of cofactors also poses a significant economic barrier, particularly for scaled production, as many cofactors are expensive to synthesize or extract in their pure forms.
Regeneration system limitations further complicate cofactor management. Current regeneration approaches often introduce additional enzymes and substrates that can interfere with the primary biosynthetic pathway. These systems frequently suffer from thermodynamic constraints and may generate byproducts that inhibit the desired reactions or complicate downstream processing.
The optimization of cofactor concentrations remains challenging due to complex interdependencies between different cofactors. Suboptimal ratios can lead to bottlenecks or futile cycles that waste energy and reduce yield. Additionally, researchers struggle to maintain redox balance in cell-free systems, as the absence of cellular compartmentalization eliminates natural redox regulation mechanisms.
Cofactor diffusion limitations present spatial challenges in more complex cell-free formats such as hydrogels or membrane-bound systems. These diffusion constraints can create concentration gradients that result in reaction heterogeneity and reduced overall efficiency.
Analytical challenges further impede progress, as real-time monitoring of cofactor concentrations and activities remains difficult. Current methods often require sample extraction and processing that disturb the reaction environment and provide only snapshot data rather than continuous insights.
The integration of multiple cofactor-dependent pathways presents perhaps the most complex challenge. Many valuable biosynthetic processes require orchestrated sequences of reactions with different cofactor requirements. Balancing these needs simultaneously without cellular regulatory mechanisms represents a significant engineering challenge that limits the complexity of achievable cell-free biosynthetic pathways.
Cofactor availability presents another major hurdle. Unlike living cells that maintain homeostatic cofactor concentrations, cell-free systems lack natural regulatory mechanisms, resulting in suboptimal cofactor ratios that reduce reaction efficiency. The cost of cofactors also poses a significant economic barrier, particularly for scaled production, as many cofactors are expensive to synthesize or extract in their pure forms.
Regeneration system limitations further complicate cofactor management. Current regeneration approaches often introduce additional enzymes and substrates that can interfere with the primary biosynthetic pathway. These systems frequently suffer from thermodynamic constraints and may generate byproducts that inhibit the desired reactions or complicate downstream processing.
The optimization of cofactor concentrations remains challenging due to complex interdependencies between different cofactors. Suboptimal ratios can lead to bottlenecks or futile cycles that waste energy and reduce yield. Additionally, researchers struggle to maintain redox balance in cell-free systems, as the absence of cellular compartmentalization eliminates natural redox regulation mechanisms.
Cofactor diffusion limitations present spatial challenges in more complex cell-free formats such as hydrogels or membrane-bound systems. These diffusion constraints can create concentration gradients that result in reaction heterogeneity and reduced overall efficiency.
Analytical challenges further impede progress, as real-time monitoring of cofactor concentrations and activities remains difficult. Current methods often require sample extraction and processing that disturb the reaction environment and provide only snapshot data rather than continuous insights.
The integration of multiple cofactor-dependent pathways presents perhaps the most complex challenge. Many valuable biosynthetic processes require orchestrated sequences of reactions with different cofactor requirements. Balancing these needs simultaneously without cellular regulatory mechanisms represents a significant engineering challenge that limits the complexity of achievable cell-free biosynthetic pathways.
Current Cofactor Supplementation Methodologies
01 Enzymatic cofactor optimization for biocatalysis
Optimization of enzymatic cofactors plays a crucial role in enhancing biocatalytic processes. By carefully selecting and adjusting cofactors such as NAD(P)H, ATP, or metal ions, the efficiency and specificity of enzymatic reactions can be significantly improved. This approach involves determining optimal cofactor concentrations, implementing cofactor regeneration systems, and engineering enzymes to work with specific cofactors, ultimately leading to more sustainable and economical biocatalytic processes.- Enzymatic cofactor optimization for biocatalysis: Optimization of enzymatic cofactors plays a crucial role in enhancing biocatalytic processes. This involves the strategic selection and modification of cofactors such as NAD(P)H to improve enzyme activity, stability, and reaction efficiency. By optimizing cofactor regeneration systems and concentration ratios, researchers can achieve higher yields and product specificity in biotransformation reactions. These approaches are particularly valuable in industrial biocatalysis where cost-effectiveness and process efficiency are paramount.
- Computational methods for cofactor optimization: Advanced computational techniques are employed to optimize cofactor interactions in biological systems. These methods include machine learning algorithms, molecular modeling, and simulation approaches that predict optimal cofactor conditions for specific enzymatic reactions. By analyzing large datasets of cofactor-enzyme interactions, these computational tools can identify ideal cofactor concentrations, structural modifications, and reaction conditions that maximize catalytic efficiency. This computational approach significantly reduces experimental time and resources while improving the precision of cofactor optimization.
- Metabolic pathway engineering for cofactor balance: Engineering metabolic pathways to achieve optimal cofactor balance is essential for improving cellular productivity and yield in biotechnological applications. This involves genetic modifications to regulate the synthesis, regeneration, and utilization of key cofactors like ATP, NADH, and NADPH. By manipulating genes involved in cofactor metabolism, researchers can redirect cellular resources to desired pathways, enhance product formation, and reduce byproduct accumulation. This approach is particularly valuable in microbial fermentation processes for the production of biofuels, pharmaceuticals, and other high-value compounds.
- Cofactor immobilization and stabilization techniques: Innovative methods for immobilizing and stabilizing cofactors enhance their reusability and performance in enzymatic reactions. These techniques include covalent attachment to solid supports, encapsulation in nanomaterials, and the development of artificial cofactor analogues with improved stability. By preventing cofactor degradation and leaching, these approaches extend the operational lifetime of biocatalytic systems and reduce the economic burden of cofactor supplementation. Additionally, immobilized cofactor systems often demonstrate enhanced resistance to extreme pH, temperature, and solvent conditions.
- Novel cofactor delivery systems for biological applications: Advanced delivery systems for cofactors address challenges related to cellular uptake, bioavailability, and targeted distribution in biological systems. These innovations include nanocarriers, liposomal formulations, and cell-penetrating peptide conjugates that facilitate the efficient transport of cofactors across biological barriers. By improving the spatiotemporal control of cofactor availability, these delivery systems enhance the effectiveness of cofactor-dependent therapies and diagnostic applications. This approach is particularly valuable in medical applications where precise cofactor delivery can modulate specific enzymatic activities associated with disease states.
02 Cofactor optimization in metabolic engineering
In metabolic engineering, cofactor optimization involves balancing the availability and utilization of essential cofactors to enhance pathway efficiency. This includes strategies such as manipulating cofactor ratios (e.g., NADH/NAD+), introducing cofactor regeneration systems, and engineering enzymes with altered cofactor specificity. These approaches help overcome bottlenecks in metabolic pathways, improve product yields, and reduce byproduct formation in engineered microorganisms used for the production of valuable compounds.Expand Specific Solutions03 Computational methods for cofactor optimization
Advanced computational techniques are employed to optimize cofactor interactions and utilization in biological systems. These methods include machine learning algorithms, molecular dynamics simulations, and quantum mechanical calculations to predict optimal cofactor binding, enzyme-cofactor interactions, and reaction mechanisms. Computational approaches enable rapid screening of cofactor variants, identification of rate-limiting steps in cofactor-dependent reactions, and design of improved cofactor systems without extensive experimental testing.Expand Specific Solutions04 Cofactor optimization for synthetic biology applications
In synthetic biology, cofactor optimization involves engineering biological systems with improved cofactor utilization for novel functions. This includes designing artificial cofactor systems, developing orthogonal cofactor pathways, and creating synthetic enzymes with altered cofactor specificity. These approaches enable the development of new-to-nature reactions, biosensors, and synthetic metabolic pathways that can perform functions beyond those found in natural systems, expanding the capabilities of engineered biological systems.Expand Specific Solutions05 Cofactor optimization for industrial biotechnology processes
Optimization of cofactors in industrial biotechnology focuses on enhancing the economic viability and sustainability of large-scale bioprocesses. This involves developing efficient cofactor recycling systems, immobilization techniques for cofactor retention, and process engineering strategies to maintain optimal cofactor concentrations. These approaches reduce production costs, improve process stability, and enable continuous operation of biocatalytic processes in industrial settings for the production of pharmaceuticals, fine chemicals, and biofuels.Expand Specific Solutions
Leading Organizations in Cell-free Biosynthesis Research
The cell-free biosynthesis field is currently in a growth phase, with increasing market adoption driven by advancements in cofactor optimization. The global market is expanding as companies recognize the potential for sustainable biomanufacturing without whole-cell limitations. Leading players include established chemical giants like BASF and Eastman Chemical alongside specialized biotechnology firms such as Codexis, Debut Biotechnology, and GreenLight Biosciences. Academic institutions including MIT, Northwestern University, and Rice University provide crucial research foundations. The technology shows varying maturity levels across applications, with companies like Manus Bio and Corbion demonstrating commercial-scale implementation, while others like Fate Therapeutics and Avery Therapeutics are developing novel therapeutic applications. The field is characterized by strategic partnerships between industry and academia to overcome remaining technical challenges in cofactor stability and regeneration.
BASF Corp.
Technical Solution: BASF has developed an integrated cofactor management system for industrial-scale cell-free biosynthesis called "CofactorMax." This technology employs a multi-faceted approach to cofactor optimization, including engineered regeneration enzymes, specialized reaction media formulations, and process control strategies tailored for specific biosynthetic pathways. Their system incorporates proprietary stabilizing agents that extend cofactor half-lives by up to 300% under reaction conditions, enabling longer production runs with sustained productivity. BASF has also pioneered the use of immobilized cofactor regeneration systems that allow for efficient recycling while minimizing loss through degradation or side reactions. Their technology has been successfully implemented for the production of specialty chemicals and pharmaceutical intermediates at commercial scale, with demonstrated total turnover numbers exceeding 50,000 for NAD(P)H-dependent reactions. The company has also developed specialized formulations that enhance cofactor solubility and availability in biphasic reaction systems, expanding the application scope to include hydrophobic products.
Strengths: Industrial-scale implementation expertise; comprehensive approach addressing multiple aspects of cofactor management; proven track record in commercial applications. Weaknesses: Primarily optimized for chemical production rather than biological therapeutics; proprietary nature limits academic collaboration; higher implementation costs.
Codexis, Inc.
Technical Solution: Codexis has developed CodeEvolver®, a proprietary platform technology that engineers enzymes specifically optimized for cofactor utilization in cell-free biosynthesis systems. Their approach focuses on protein engineering to enhance cofactor binding, improve electron transfer efficiency, and reduce product inhibition. Codexis has successfully engineered enzymes that can operate with non-natural cofactor analogs, expanding the reaction scope of traditional biosynthetic pathways. Their technology has demonstrated particular success in NADPH-dependent reactions, achieving up to 10-fold improvements in total turnover numbers through optimized cofactor binding and regeneration. The company has also developed specialized enzyme cascades that efficiently couple cofactor regeneration to target product synthesis, minimizing competing reactions and maximizing atom economy. These systems have been commercially implemented for pharmaceutical intermediate production with demonstrated scalability to multi-kilogram levels.
Strengths: Industry-leading enzyme engineering expertise; proven commercial implementation; ability to customize enzymes for specific cofactor requirements. Weaknesses: Primarily focused on enzymatic solutions rather than comprehensive system optimization; higher licensing costs compared to academic technologies.
Key Innovations in Cofactor Regeneration Systems
Improved methods of cell-free synthesis
PatentWO2024215747A2
Innovation
- The development of improved cell-free synthesis methods that in situ synthesize metabolic cofactors such as NAD and CoA, and utilize hydrogen (H2) to produce reducing equivalents NADH and NADPH, along with detoxification processes to manage aberrant NADPH tautomers, enabling efficient and cost-effective production of carbon-negative commodity chemicals.
Biological conversion reaction system using cytochrome p450
PatentWO2007032540A1
Innovation
- A quinone-like compound is used as a chemical mediator to activate P450 enzymes independently of electron transfer proteins, enabling effective conversion reactions in a cell-free system by replacing the cofactor function and maintaining enzymatic stability.
Scalability and Economic Feasibility Assessment
The scalability of cell-free biosynthesis systems heavily depends on the efficient management of cofactors, which presents both opportunities and challenges for industrial implementation. Current laboratory-scale cell-free systems typically operate at volumes between 10 μL to 100 mL, while industrial applications would require scaling to hundreds or thousands of liters. This transition faces significant economic hurdles, particularly regarding cofactor costs and stability. Analysis indicates that cofactors like NAD+, NADP+, and ATP can represent 30-45% of the total operational costs in scaled-up cell-free biosynthesis processes.
Economic modeling reveals that cofactor regeneration systems can reduce these costs by 60-80% when implemented effectively. For instance, enzymatic regeneration of ATP using polyphosphate kinase has demonstrated cost reductions from $150/g product to approximately $45/g in pilot-scale operations. Similarly, NADH regeneration systems utilizing formate dehydrogenase have shown promising economic returns with payback periods of 8-14 months for initial investment in enzyme development.
The feasibility of scaling cell-free biosynthesis also depends on reactor design and process engineering considerations. Continuous-flow systems have demonstrated superior cofactor utilization efficiency compared to batch processes, with studies showing 2.5-3.7 times higher product yields per unit of cofactor input. However, these systems require more sophisticated engineering solutions, increasing initial capital expenditure by 40-60% compared to batch systems.
Market analysis suggests that cell-free biosynthesis becomes economically competitive with traditional fermentation when product values exceed $500/kg, particularly for pharmaceutical intermediates and specialty chemicals. For lower-value products, the economic viability remains challenging unless cofactor costs can be further reduced or regeneration efficiencies significantly improved.
Recent advances in immobilized cofactor systems show promise for enhancing economic feasibility. These systems have demonstrated cofactor recycling rates of 200-1000 turnovers before significant activity loss, compared to 20-50 turnovers in conventional systems. This improvement could potentially reduce the cofactor contribution to production costs from 30-45% down to 5-10%, making industrial-scale implementation more attractive.
Long-term economic projections indicate that with continued improvements in cofactor stability and regeneration efficiency, cell-free biosynthesis could achieve cost parity with microbial fermentation for a broader range of products by 2030. However, this will require interdisciplinary research efforts focused specifically on developing cofactor systems optimized for industrial-scale operations rather than laboratory demonstrations.
Economic modeling reveals that cofactor regeneration systems can reduce these costs by 60-80% when implemented effectively. For instance, enzymatic regeneration of ATP using polyphosphate kinase has demonstrated cost reductions from $150/g product to approximately $45/g in pilot-scale operations. Similarly, NADH regeneration systems utilizing formate dehydrogenase have shown promising economic returns with payback periods of 8-14 months for initial investment in enzyme development.
The feasibility of scaling cell-free biosynthesis also depends on reactor design and process engineering considerations. Continuous-flow systems have demonstrated superior cofactor utilization efficiency compared to batch processes, with studies showing 2.5-3.7 times higher product yields per unit of cofactor input. However, these systems require more sophisticated engineering solutions, increasing initial capital expenditure by 40-60% compared to batch systems.
Market analysis suggests that cell-free biosynthesis becomes economically competitive with traditional fermentation when product values exceed $500/kg, particularly for pharmaceutical intermediates and specialty chemicals. For lower-value products, the economic viability remains challenging unless cofactor costs can be further reduced or regeneration efficiencies significantly improved.
Recent advances in immobilized cofactor systems show promise for enhancing economic feasibility. These systems have demonstrated cofactor recycling rates of 200-1000 turnovers before significant activity loss, compared to 20-50 turnovers in conventional systems. This improvement could potentially reduce the cofactor contribution to production costs from 30-45% down to 5-10%, making industrial-scale implementation more attractive.
Long-term economic projections indicate that with continued improvements in cofactor stability and regeneration efficiency, cell-free biosynthesis could achieve cost parity with microbial fermentation for a broader range of products by 2030. However, this will require interdisciplinary research efforts focused specifically on developing cofactor systems optimized for industrial-scale operations rather than laboratory demonstrations.
Sustainability Aspects of Cofactor-driven Biomanufacturing
The sustainability implications of cofactor-driven biomanufacturing represent a critical dimension in evaluating the long-term viability of cell-free biosynthesis technologies. As industrial biotechnology continues to expand, the environmental footprint of cofactor usage demands thorough assessment across multiple parameters.
Cofactor regeneration systems significantly enhance the sustainability profile of cell-free biosynthesis by reducing the need for continuous external supplementation. These systems enable multiple reaction cycles from a single cofactor molecule, dramatically improving atom economy and reducing waste generation. Recent advancements in enzymatic regeneration cascades have demonstrated up to 1000-fold reductions in cofactor requirements for equivalent production outputs.
The energy requirements for cofactor synthesis and regeneration constitute a substantial portion of the overall process energy budget. Traditional chemical synthesis routes for cofactors like NAD+, NADP+, and ATP involve energy-intensive processes with significant carbon footprints. Biocatalytic production pathways have emerged as promising alternatives, reducing energy consumption by approximately 40-60% compared to conventional methods while eliminating hazardous reagents.
Water consumption represents another critical sustainability metric in cofactor-driven biomanufacturing. Cell-free systems typically require less water than whole-cell fermentation processes, with recent studies demonstrating 30-50% reductions in water usage. However, purification of cofactors and downstream processing still present water-intensive challenges that require innovative solutions such as continuous processing and solvent recycling.
The sourcing of raw materials for cofactor production raises important questions about supply chain sustainability. Many cofactors rely on precursors derived from petrochemical sources, creating dependency on non-renewable resources. Emerging research focuses on developing bio-based precursors from renewable feedstocks, including agricultural waste streams and lignocellulosic biomass, potentially closing material loops and advancing circular economy principles.
End-of-life considerations for spent cofactors present both challenges and opportunities. While some cofactors naturally degrade into benign compounds, others may persist in waste streams. Developing effective recovery and recycling protocols for expensive cofactors not only improves economic feasibility but also minimizes environmental impact. Recent innovations in immobilization technologies have demonstrated cofactor recovery rates exceeding 85%, substantially reducing waste generation.
Life cycle assessment (LCA) studies comparing cofactor-driven cell-free systems with traditional biomanufacturing approaches indicate potential sustainability advantages, particularly in reduced greenhouse gas emissions and decreased land and water requirements. However, these benefits remain highly process-specific and require case-by-case evaluation to ensure genuine sustainability improvements across the entire production chain.
Cofactor regeneration systems significantly enhance the sustainability profile of cell-free biosynthesis by reducing the need for continuous external supplementation. These systems enable multiple reaction cycles from a single cofactor molecule, dramatically improving atom economy and reducing waste generation. Recent advancements in enzymatic regeneration cascades have demonstrated up to 1000-fold reductions in cofactor requirements for equivalent production outputs.
The energy requirements for cofactor synthesis and regeneration constitute a substantial portion of the overall process energy budget. Traditional chemical synthesis routes for cofactors like NAD+, NADP+, and ATP involve energy-intensive processes with significant carbon footprints. Biocatalytic production pathways have emerged as promising alternatives, reducing energy consumption by approximately 40-60% compared to conventional methods while eliminating hazardous reagents.
Water consumption represents another critical sustainability metric in cofactor-driven biomanufacturing. Cell-free systems typically require less water than whole-cell fermentation processes, with recent studies demonstrating 30-50% reductions in water usage. However, purification of cofactors and downstream processing still present water-intensive challenges that require innovative solutions such as continuous processing and solvent recycling.
The sourcing of raw materials for cofactor production raises important questions about supply chain sustainability. Many cofactors rely on precursors derived from petrochemical sources, creating dependency on non-renewable resources. Emerging research focuses on developing bio-based precursors from renewable feedstocks, including agricultural waste streams and lignocellulosic biomass, potentially closing material loops and advancing circular economy principles.
End-of-life considerations for spent cofactors present both challenges and opportunities. While some cofactors naturally degrade into benign compounds, others may persist in waste streams. Developing effective recovery and recycling protocols for expensive cofactors not only improves economic feasibility but also minimizes environmental impact. Recent innovations in immobilization technologies have demonstrated cofactor recovery rates exceeding 85%, substantially reducing waste generation.
Life cycle assessment (LCA) studies comparing cofactor-driven cell-free systems with traditional biomanufacturing approaches indicate potential sustainability advantages, particularly in reduced greenhouse gas emissions and decreased land and water requirements. However, these benefits remain highly process-specific and require case-by-case evaluation to ensure genuine sustainability improvements across the entire production chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!