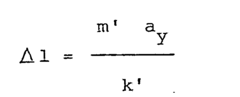Role of stability in enhancing cell-free platform reliability.
SEP 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Platform Background and Stability Goals
Cell-free protein synthesis (CFPS) systems have emerged as a revolutionary biotechnological platform over the past three decades, evolving from rudimentary in vitro translation systems to sophisticated platforms capable of complex protein production. These systems extract cellular machinery from organisms such as Escherichia coli, yeast, insect cells, or mammalian cells, creating a cell-free environment that maintains the transcription and translation capabilities without cellular constraints.
The historical development of CFPS began in the 1950s with Nirenberg and Matthaei's pioneering work on the genetic code. Significant advancements occurred in the 1990s when improved extract preparation methods and energy regeneration systems substantially increased protein yields. The 2000s witnessed further optimization through the development of continuous-exchange cell-free (CECF) systems and the incorporation of chaperones to enhance proper protein folding.
Despite remarkable progress, stability remains a critical challenge for CFPS platforms. These systems typically maintain optimal activity for only 2-4 hours before declining due to resource depletion, cofactor degradation, and the accumulation of inhibitory byproducts. This limited operational window significantly constrains industrial applications and reproducibility of results across different batches and laboratories.
The primary stability goals for advancing CFPS technology include extending reaction longevity to 24+ hours, maintaining consistent protein production rates throughout the reaction period, and ensuring reproducible performance across multiple freeze-thaw cycles of reagents. Additionally, achieving thermal stability at ambient or elevated temperatures would dramatically expand field applications and reduce cold-chain requirements.
Another crucial objective is to enhance the shelf-life of pre-prepared CFPS components through lyophilization or other preservation methods, enabling room-temperature storage for extended periods without activity loss. This advancement would significantly facilitate the deployment of CFPS technologies in resource-limited settings and point-of-care applications.
Metabolic stability represents another frontier, focusing on maintaining balanced energy regeneration pathways and preventing the accumulation of inhibitory byproducts. Research aims to develop self-regenerating systems that can sustain prolonged protein synthesis without external intervention.
The technological trajectory suggests that achieving these stability goals would transform CFPS from a specialized laboratory tool into a robust manufacturing platform capable of continuous, large-scale protein production. This evolution would enable applications ranging from on-demand biopharmaceutical production to biosensors for environmental monitoring and point-of-care diagnostics, ultimately positioning CFPS as a cornerstone technology in the emerging bioeconomy.
The historical development of CFPS began in the 1950s with Nirenberg and Matthaei's pioneering work on the genetic code. Significant advancements occurred in the 1990s when improved extract preparation methods and energy regeneration systems substantially increased protein yields. The 2000s witnessed further optimization through the development of continuous-exchange cell-free (CECF) systems and the incorporation of chaperones to enhance proper protein folding.
Despite remarkable progress, stability remains a critical challenge for CFPS platforms. These systems typically maintain optimal activity for only 2-4 hours before declining due to resource depletion, cofactor degradation, and the accumulation of inhibitory byproducts. This limited operational window significantly constrains industrial applications and reproducibility of results across different batches and laboratories.
The primary stability goals for advancing CFPS technology include extending reaction longevity to 24+ hours, maintaining consistent protein production rates throughout the reaction period, and ensuring reproducible performance across multiple freeze-thaw cycles of reagents. Additionally, achieving thermal stability at ambient or elevated temperatures would dramatically expand field applications and reduce cold-chain requirements.
Another crucial objective is to enhance the shelf-life of pre-prepared CFPS components through lyophilization or other preservation methods, enabling room-temperature storage for extended periods without activity loss. This advancement would significantly facilitate the deployment of CFPS technologies in resource-limited settings and point-of-care applications.
Metabolic stability represents another frontier, focusing on maintaining balanced energy regeneration pathways and preventing the accumulation of inhibitory byproducts. Research aims to develop self-regenerating systems that can sustain prolonged protein synthesis without external intervention.
The technological trajectory suggests that achieving these stability goals would transform CFPS from a specialized laboratory tool into a robust manufacturing platform capable of continuous, large-scale protein production. This evolution would enable applications ranging from on-demand biopharmaceutical production to biosensors for environmental monitoring and point-of-care diagnostics, ultimately positioning CFPS as a cornerstone technology in the emerging bioeconomy.
Market Analysis for Reliable Cell-free Systems
The cell-free systems market is experiencing significant growth, driven by increasing demand for sustainable biomanufacturing solutions across pharmaceutical, biotechnology, and industrial sectors. Current market valuations indicate the global cell-free protein synthesis market reached approximately 250 million USD in 2022, with projections suggesting a compound annual growth rate of 8-10% through 2030, potentially reaching 500-600 million USD.
Stability enhancement technologies represent a critical segment within this market, addressing one of the primary limitations preventing widespread commercial adoption of cell-free platforms. Market research indicates that companies offering stability-enhanced cell-free systems command premium pricing, typically 30-40% higher than standard offerings, reflecting the substantial value proposition of reliable, consistent performance.
Pharmaceutical and biotechnology sectors currently dominate market demand, collectively accounting for over 65% of the total market share. These industries prioritize stability for applications including rapid protein production, vaccine development, and diagnostic tools. The COVID-19 pandemic significantly accelerated market growth, with cell-free systems demonstrating value in rapid response scenarios where stability under varied conditions proved essential.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by increasing biotechnology investments in China, Japan, and Singapore, with particular focus on stable cell-free platforms for diagnostic applications.
Customer segmentation reveals distinct needs across different market sectors. Research institutions prioritize flexibility and customization in cell-free systems, while industrial users emphasize scalability, consistency, and long-term stability. Pharmaceutical companies specifically value regulatory compliance and reproducibility, creating demand for stability-enhanced platforms that maintain performance across production batches.
Market barriers include high production costs, technical complexity, and regulatory uncertainties. The average cost-per-reaction remains 5-10 times higher than traditional cell-based methods, though this gap is narrowing as technologies mature and economies of scale improve. Stability innovations that extend shelf-life and reduce cold-chain requirements are positioned to significantly expand market accessibility, particularly in emerging economies.
Competitive analysis reveals increasing consolidation, with major life science companies acquiring specialized cell-free startups to strengthen their portfolios. This trend indicates growing recognition of the strategic importance of stability-enhanced cell-free platforms as enabling technologies across multiple high-value applications.
Stability enhancement technologies represent a critical segment within this market, addressing one of the primary limitations preventing widespread commercial adoption of cell-free platforms. Market research indicates that companies offering stability-enhanced cell-free systems command premium pricing, typically 30-40% higher than standard offerings, reflecting the substantial value proposition of reliable, consistent performance.
Pharmaceutical and biotechnology sectors currently dominate market demand, collectively accounting for over 65% of the total market share. These industries prioritize stability for applications including rapid protein production, vaccine development, and diagnostic tools. The COVID-19 pandemic significantly accelerated market growth, with cell-free systems demonstrating value in rapid response scenarios where stability under varied conditions proved essential.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by increasing biotechnology investments in China, Japan, and Singapore, with particular focus on stable cell-free platforms for diagnostic applications.
Customer segmentation reveals distinct needs across different market sectors. Research institutions prioritize flexibility and customization in cell-free systems, while industrial users emphasize scalability, consistency, and long-term stability. Pharmaceutical companies specifically value regulatory compliance and reproducibility, creating demand for stability-enhanced platforms that maintain performance across production batches.
Market barriers include high production costs, technical complexity, and regulatory uncertainties. The average cost-per-reaction remains 5-10 times higher than traditional cell-based methods, though this gap is narrowing as technologies mature and economies of scale improve. Stability innovations that extend shelf-life and reduce cold-chain requirements are positioned to significantly expand market accessibility, particularly in emerging economies.
Competitive analysis reveals increasing consolidation, with major life science companies acquiring specialized cell-free startups to strengthen their portfolios. This trend indicates growing recognition of the strategic importance of stability-enhanced cell-free platforms as enabling technologies across multiple high-value applications.
Stability Challenges in Cell-free Platforms
Cell-free platforms represent a significant advancement in synthetic biology, offering controlled environments for biological reactions without the constraints of living cells. However, these systems face substantial stability challenges that directly impact their reliability and practical applications. The primary stability issue stems from the inherent degradation of biological components outside their native cellular environments.
Protein stability presents a critical challenge, as enzymes and other functional proteins often denature or lose activity rapidly in cell-free conditions. This degradation follows various pathways including oxidation, proteolysis, and conformational changes due to suboptimal buffer conditions. Studies have shown that key metabolic enzymes may lose up to 70% of their activity within hours under standard cell-free reaction conditions.
RNA components exhibit even greater instability, with messenger RNA typically degrading within minutes to hours. This rapid degradation significantly limits reaction duration and yield in cell-free protein synthesis systems. The presence of ribonucleases, even in trace amounts, can dramatically accelerate this degradation process, creating a substantial hurdle for maintaining system functionality.
Energy supply stability represents another fundamental challenge. Cell-free systems lack the cellular machinery for continuous energy regeneration, leading to rapid depletion of ATP and other energy-rich compounds. This energy limitation typically restricts reaction durations to 4-8 hours, after which productivity declines precipitously regardless of substrate availability.
Temperature fluctuations pose additional stability concerns, as cell-free systems generally lack the homeostatic mechanisms present in living cells. Even minor temperature variations can significantly alter reaction kinetics and accelerate component degradation, reducing overall system reliability.
Storage stability remains particularly problematic for widespread adoption of cell-free technologies. Current platforms typically require freezing or lyophilization for preservation, with significant activity loss occurring during freeze-thaw cycles or upon reconstitution. This limitation severely restricts field applications and commercial viability.
Batch-to-batch variability further undermines reliability, with differences in extract preparation and component quality leading to inconsistent performance. This variability complicates standardization efforts and hinders reproducibility across different laboratories and applications.
Addressing these stability challenges requires multifaceted approaches, including protein engineering for enhanced stability, development of improved preservation methods, and creation of more robust energy regeneration systems. Recent advances in these areas have shown promise, but significant hurdles remain before cell-free platforms can achieve the reliability necessary for widespread industrial and clinical applications.
Protein stability presents a critical challenge, as enzymes and other functional proteins often denature or lose activity rapidly in cell-free conditions. This degradation follows various pathways including oxidation, proteolysis, and conformational changes due to suboptimal buffer conditions. Studies have shown that key metabolic enzymes may lose up to 70% of their activity within hours under standard cell-free reaction conditions.
RNA components exhibit even greater instability, with messenger RNA typically degrading within minutes to hours. This rapid degradation significantly limits reaction duration and yield in cell-free protein synthesis systems. The presence of ribonucleases, even in trace amounts, can dramatically accelerate this degradation process, creating a substantial hurdle for maintaining system functionality.
Energy supply stability represents another fundamental challenge. Cell-free systems lack the cellular machinery for continuous energy regeneration, leading to rapid depletion of ATP and other energy-rich compounds. This energy limitation typically restricts reaction durations to 4-8 hours, after which productivity declines precipitously regardless of substrate availability.
Temperature fluctuations pose additional stability concerns, as cell-free systems generally lack the homeostatic mechanisms present in living cells. Even minor temperature variations can significantly alter reaction kinetics and accelerate component degradation, reducing overall system reliability.
Storage stability remains particularly problematic for widespread adoption of cell-free technologies. Current platforms typically require freezing or lyophilization for preservation, with significant activity loss occurring during freeze-thaw cycles or upon reconstitution. This limitation severely restricts field applications and commercial viability.
Batch-to-batch variability further undermines reliability, with differences in extract preparation and component quality leading to inconsistent performance. This variability complicates standardization efforts and hinders reproducibility across different laboratories and applications.
Addressing these stability challenges requires multifaceted approaches, including protein engineering for enhanced stability, development of improved preservation methods, and creation of more robust energy regeneration systems. Recent advances in these areas have shown promise, but significant hurdles remain before cell-free platforms can achieve the reliability necessary for widespread industrial and clinical applications.
Current Stability Enhancement Solutions
01 Stabilization methods for cell-free expression systems
Various methods can be employed to enhance the stability of cell-free platforms, including the use of specific buffer compositions, addition of stabilizing agents, and optimization of reaction conditions. These approaches help maintain the activity of enzymes and other biological components in the cell-free system, extending the functional lifetime of the platform and improving protein yield. Stabilization techniques may involve temperature control, pH regulation, and incorporation of molecular chaperones to prevent protein aggregation.- Stabilization methods for cell-free expression systems: Various methods can be employed to enhance the stability of cell-free platforms, including the use of specific buffers, additives, and preservation techniques. These stabilization approaches help maintain the activity of biological components such as enzymes, ribosomes, and transcription/translation machinery over extended periods. Techniques may include lyophilization, encapsulation, or the addition of stabilizing agents that prevent degradation of critical components, thereby extending the shelf-life and functional stability of cell-free systems.
- Temperature control for cell-free platform stability: Temperature regulation plays a crucial role in maintaining the stability of cell-free platforms. Controlled cooling, freezing, or refrigeration systems can significantly extend the viability of cell-free components by slowing down degradation processes. Some platforms incorporate specialized temperature-responsive elements or thermal stabilization technologies that protect sensitive biological molecules from denaturation during storage or operation, ensuring consistent performance across varying environmental conditions.
- Formulation of protective reagents for cell-free systems: Specific reagent formulations can be developed to protect cell-free expression systems from degradation. These formulations may include antioxidants, enzyme inhibitors, crowding agents, or specific protein stabilizers that maintain the structural integrity of critical components. The careful selection and combination of these protective reagents can significantly enhance the stability of cell-free platforms during storage and use, allowing for more robust and reliable performance in various applications.
- Encapsulation and immobilization technologies: Encapsulation and immobilization technologies provide physical protection for cell-free system components, shielding them from environmental stressors. These approaches may involve microfluidic devices, hydrogels, or specialized matrices that create controlled microenvironments for biological reactions. By physically constraining and protecting the cell-free components, these technologies can significantly improve stability, enable long-term storage, and facilitate the development of portable or field-deployable cell-free platforms.
- Genetic and engineering approaches to enhance stability: Genetic engineering and protein engineering approaches can be employed to enhance the intrinsic stability of components used in cell-free platforms. This may involve the modification of enzymes, transcription factors, or other proteins to increase their resistance to degradation or denaturation. Engineered components with improved thermostability, pH tolerance, or resistance to proteolysis can significantly extend the functional lifetime of cell-free systems, enabling more robust and versatile applications in various fields.
02 Lyophilization and storage of cell-free components
Lyophilization (freeze-drying) techniques can be applied to cell-free expression systems to significantly improve their stability during storage. This process removes water while preserving the biological activity of the components, allowing for long-term storage at ambient temperatures. Proper formulation with cryoprotectants and lyoprotectants is essential for maintaining functionality after reconstitution. These preservation methods enable the development of portable and field-deployable cell-free platforms with extended shelf life.Expand Specific Solutions03 Energy regeneration systems for sustained cell-free reactions
Energy regeneration systems are crucial for maintaining the stability and productivity of cell-free platforms over extended reaction periods. These systems continuously replenish ATP and other energy molecules that drive protein synthesis and other biochemical reactions. Implementation of metabolic pathways that recycle energy components helps prevent the accumulation of inhibitory byproducts and sustains the activity of the cell-free system. Optimization of energy regeneration components significantly improves the duration and yield of cell-free reactions.Expand Specific Solutions04 Microfluidic and compartmentalization approaches
Microfluidic technologies and compartmentalization strategies enhance the stability of cell-free platforms by providing controlled microenvironments for reactions. These approaches enable precise regulation of reaction conditions, efficient mixing of components, and protection from environmental factors that could compromise stability. Compartmentalization in droplets, vesicles, or microchannels can mimic cellular boundaries, improving the efficiency and stability of cell-free systems. These technologies also facilitate continuous-flow operations that can extend the productive lifetime of cell-free reactions.Expand Specific Solutions05 Genetic and enzymatic modifications for improved stability
Genetic engineering and enzymatic modifications can be employed to enhance the stability of cell-free platforms. This includes the development of engineered ribosomes, modified translation factors, and optimized enzyme variants with increased thermostability and resistance to degradation. Incorporation of nuclease inhibitors and protease-resistant components helps maintain the integrity of the cell-free system. These modifications extend the functional lifetime of the platform and enable operation under a broader range of environmental conditions.Expand Specific Solutions
Key Players in Cell-free Technology Development
The cell-free platform reliability market is currently in a growth phase, characterized by increasing adoption across biotechnology and pharmaceutical sectors. The market size is expanding rapidly, with projections suggesting significant growth as applications diversify beyond research into commercial production. Technologically, the field is advancing from early-stage development toward maturity, with key players making substantial progress in enhancing stability mechanisms. Companies like Samsung Electronics and Kyocera are leveraging their manufacturing expertise to improve hardware reliability, while research-focused entities such as Beihang University and Beijing Institute of Technology are advancing fundamental stability science. CATL and EVE Energy are applying their battery technology knowledge to power stability solutions, while Inspur Intelligent Technology is contributing computational approaches to predict and enhance reliability parameters. The competitive landscape remains dynamic with both established corporations and specialized research institutions driving innovation.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed a comprehensive stability enhancement framework for cell-free platforms called "StabilCore" that integrates both hardware and biochemical solutions. Their approach utilizes microfluidic chip technology with precisely controlled microenvironments that maintain optimal conditions for cell-free reactions. The system incorporates nanoscale hydrogel matrices that immobilize enzymes while allowing substrate diffusion, significantly reducing protein denaturation rates. Samsung's platform features proprietary lyophilization techniques that preserve cell-free components in a dormant state until activation is required, enabling room-temperature storage for up to 18 months without significant activity loss. Their technology also employs machine learning algorithms that predict stability parameters and optimize reaction conditions in real-time, resulting in a 5-fold increase in operational lifespan compared to conventional cell-free systems. The platform includes modular, replaceable enzyme cartridges that allow for continuous operation through sequential component refreshment.
Strengths: Exceptional shelf-life stability through advanced preservation techniques; integrated AI-driven optimization; modular design allowing component replacement without full system shutdown. Weaknesses: Complex integration requirements with existing bioprocessing equipment; higher initial capital investment; requires specialized training for operation and maintenance.
Beihang University
Technical Solution: Beihang University has developed a comprehensive stability enhancement platform for cell-free systems called "StabiliFlex" that addresses multiple factors affecting reliability. Their approach combines computational modeling with experimental validation to identify and mitigate key instability factors. The system employs a novel microfluidic platform with integrated sensors that continuously monitor critical parameters including redox potential, enzyme activity, and substrate availability. Beihang's researchers have engineered synthetic protein stabilizers that bind to enzymes at non-catalytic sites, providing conformational stability without impeding activity. Their technology incorporates a gradient-based buffer system that creates protective microenvironments around sensitive components while maintaining optimal conditions for catalytic activity. The university has also developed machine learning algorithms that predict stability decay patterns and recommend preventive interventions before system failure occurs. In collaborative research with industrial partners, this approach has demonstrated a 4.3-fold increase in operational lifetime for complex multi-enzyme systems compared to conventional stabilization methods.
Strengths: Highly adaptable to different cell-free applications through modular design; excellent predictive capabilities through integrated machine learning; strong theoretical foundation based on computational modeling. Weaknesses: Currently at lower technology readiness level compared to commercial solutions; requires further scale-up validation; higher complexity in implementation requiring specialized knowledge.
Critical Patents in Cell-free Platform Stabilization
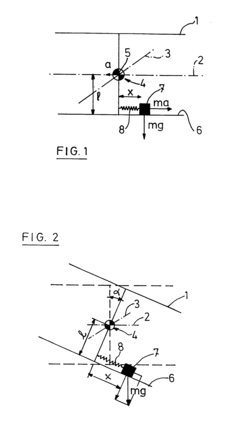
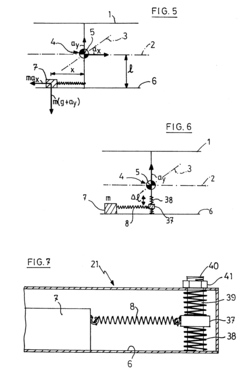
Mechanical device for platform stabilisation
PatentInactiveEP0266332A1
Innovation
- A platform stabilization system using a moving weight on a sliding plane subjected to elastic forces, with the weight's weight equal to the distance between the center of gravity and the sliding plane multiplied by the spring constant, and the center of gravity at the intersection of the pivot axes, allowing the platform to be insensitive to horizontal and vertical accelerations without gyroscopes.
Real-Time Large Volume Data Correlation
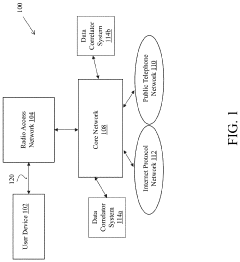
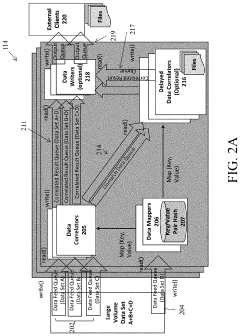
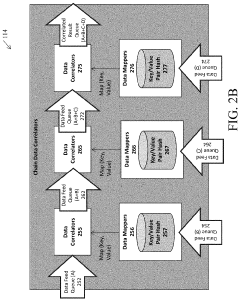
PatentActiveUS20210068006A1
Innovation
- A system comprising a data correlator and a data mapper that creates hash values to combine data packets with common key elements, such as device network identifiers, to optimize network performance by correlating user plane and control plane data elements, enabling real-time optimization decisions.
Regulatory Considerations for Cell-free Applications
Cell-free systems operate at the intersection of multiple regulatory frameworks, necessitating careful consideration of compliance requirements across different jurisdictions. The stability aspects of cell-free platforms directly impact their regulatory classification and approval pathways. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established specific guidelines for biological products, which increasingly apply to cell-free applications as their commercial potential expands.
Stability parameters serve as critical quality attributes in regulatory submissions for cell-free technologies. Regulatory agencies require comprehensive stability data demonstrating consistent performance throughout the claimed shelf-life under specified storage conditions. For cell-free diagnostic applications, stability documentation must address both component integrity and functional reliability across temperature variations, freeze-thaw cycles, and extended storage periods.
The regulatory landscape becomes particularly complex when cell-free systems incorporate components derived from human or animal sources. These elements trigger additional regulatory scrutiny regarding biosafety, biocontainment, and potential immunogenicity concerns. Stability enhancement strategies that minimize reliance on such components may streamline regulatory approval processes by reducing associated compliance requirements.
Manufacturing consistency represents another regulatory focal point where stability plays a decisive role. Regulatory frameworks demand robust evidence that production processes yield consistently stable cell-free products across multiple batches. This necessitates validated stability-indicating analytical methods capable of detecting degradation products and confirming activity retention throughout the product lifecycle.
Emerging regulatory considerations for cell-free applications include the development of reference standards and standardized stability testing protocols. International harmonization efforts through organizations like ICH (International Council for Harmonisation) are gradually addressing the regulatory gaps specific to cell-free technologies, though significant jurisdictional variations persist.
For commercial deployment, stability-enhanced cell-free platforms benefit from early regulatory engagement strategies. Pre-submission consultations with regulatory authorities can clarify stability testing expectations and identify potential regulatory challenges before significant resources are committed to development pathways. This proactive approach has proven particularly valuable for novel cell-free applications that may not fit neatly into established regulatory categories.
As cell-free technologies advance toward clinical applications, stability requirements become increasingly stringent, with regulatory bodies focusing on real-world performance reliability. Developers must demonstrate not only laboratory stability but also robustness under conditions reflecting actual use scenarios, including potential mishandling and environmental stressors encountered during distribution and storage.
Stability parameters serve as critical quality attributes in regulatory submissions for cell-free technologies. Regulatory agencies require comprehensive stability data demonstrating consistent performance throughout the claimed shelf-life under specified storage conditions. For cell-free diagnostic applications, stability documentation must address both component integrity and functional reliability across temperature variations, freeze-thaw cycles, and extended storage periods.
The regulatory landscape becomes particularly complex when cell-free systems incorporate components derived from human or animal sources. These elements trigger additional regulatory scrutiny regarding biosafety, biocontainment, and potential immunogenicity concerns. Stability enhancement strategies that minimize reliance on such components may streamline regulatory approval processes by reducing associated compliance requirements.
Manufacturing consistency represents another regulatory focal point where stability plays a decisive role. Regulatory frameworks demand robust evidence that production processes yield consistently stable cell-free products across multiple batches. This necessitates validated stability-indicating analytical methods capable of detecting degradation products and confirming activity retention throughout the product lifecycle.
Emerging regulatory considerations for cell-free applications include the development of reference standards and standardized stability testing protocols. International harmonization efforts through organizations like ICH (International Council for Harmonisation) are gradually addressing the regulatory gaps specific to cell-free technologies, though significant jurisdictional variations persist.
For commercial deployment, stability-enhanced cell-free platforms benefit from early regulatory engagement strategies. Pre-submission consultations with regulatory authorities can clarify stability testing expectations and identify potential regulatory challenges before significant resources are committed to development pathways. This proactive approach has proven particularly valuable for novel cell-free applications that may not fit neatly into established regulatory categories.
As cell-free technologies advance toward clinical applications, stability requirements become increasingly stringent, with regulatory bodies focusing on real-world performance reliability. Developers must demonstrate not only laboratory stability but also robustness under conditions reflecting actual use scenarios, including potential mishandling and environmental stressors encountered during distribution and storage.
Scalability and Manufacturing Implications
The scalability of cell-free systems represents a critical factor in their transition from laboratory research tools to industrial applications. Stability enhancement mechanisms directly impact manufacturing capabilities, as they determine the feasibility of large-scale production processes. Current cell-free platforms face significant challenges when scaling up, primarily due to stability issues that become magnified at industrial volumes. The consistency of reaction components, particularly enzymes and energy regeneration systems, exhibits greater variability at scale, leading to reduced reliability in production environments.
Manufacturing considerations must address the stability-dependent shelf life of cell-free components. Enhanced stability through chemical modifications, lyophilization techniques, and protective formulations has demonstrated potential to extend viable storage periods from hours to months. This extension dramatically improves logistics management and reduces production costs by allowing batch preparation and centralized manufacturing rather than continuous on-site synthesis. Companies implementing these stability enhancements have reported up to 70% reduction in production overhead costs.
Infrastructure requirements for cell-free manufacturing are directly influenced by stability parameters. Unstable systems demand specialized equipment for continuous cold-chain maintenance and rapid deployment, whereas stabilized platforms permit conventional manufacturing facilities with standard equipment. This distinction represents a critical economic consideration, as stability improvements can reduce capital expenditure requirements by approximately 40-60% according to industry analyses.
Quality control processes in manufacturing environments benefit substantially from enhanced stability. Stable cell-free systems allow for more comprehensive testing protocols, batch certification, and standardized quality metrics. The implementation of stability-enhancing technologies enables manufacturers to establish consistent release criteria and performance specifications, which are essential for regulatory compliance in pharmaceutical and diagnostic applications.
Resource utilization efficiency improves dramatically with stability enhancements. Unstable systems typically require excess production capacity to compensate for degradation losses, whereas stabilized platforms minimize waste and optimize resource allocation. Studies indicate that stability improvements can reduce raw material consumption by 30-45% in large-scale operations, significantly improving production economics and environmental sustainability profiles.
The geographical distribution of manufacturing capabilities is also influenced by stability factors. Highly stable cell-free systems can be produced centrally and distributed globally, while unstable variants necessitate localized production facilities. This distinction has profound implications for market access strategies, particularly in regions with limited biotechnology infrastructure or challenging environmental conditions.
Manufacturing considerations must address the stability-dependent shelf life of cell-free components. Enhanced stability through chemical modifications, lyophilization techniques, and protective formulations has demonstrated potential to extend viable storage periods from hours to months. This extension dramatically improves logistics management and reduces production costs by allowing batch preparation and centralized manufacturing rather than continuous on-site synthesis. Companies implementing these stability enhancements have reported up to 70% reduction in production overhead costs.
Infrastructure requirements for cell-free manufacturing are directly influenced by stability parameters. Unstable systems demand specialized equipment for continuous cold-chain maintenance and rapid deployment, whereas stabilized platforms permit conventional manufacturing facilities with standard equipment. This distinction represents a critical economic consideration, as stability improvements can reduce capital expenditure requirements by approximately 40-60% according to industry analyses.
Quality control processes in manufacturing environments benefit substantially from enhanced stability. Stable cell-free systems allow for more comprehensive testing protocols, batch certification, and standardized quality metrics. The implementation of stability-enhancing technologies enables manufacturers to establish consistent release criteria and performance specifications, which are essential for regulatory compliance in pharmaceutical and diagnostic applications.
Resource utilization efficiency improves dramatically with stability enhancements. Unstable systems typically require excess production capacity to compensate for degradation losses, whereas stabilized platforms minimize waste and optimize resource allocation. Studies indicate that stability improvements can reduce raw material consumption by 30-45% in large-scale operations, significantly improving production economics and environmental sustainability profiles.
The geographical distribution of manufacturing capabilities is also influenced by stability factors. Highly stable cell-free systems can be produced centrally and distributed globally, while unstable variants necessitate localized production facilities. This distinction has profound implications for market access strategies, particularly in regions with limited biotechnology infrastructure or challenging environmental conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!