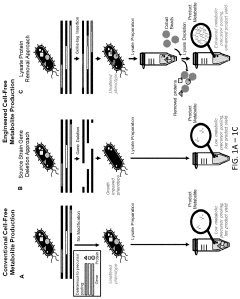
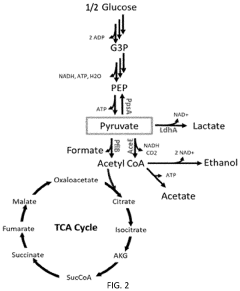
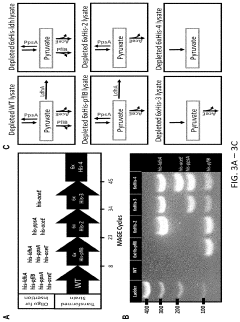
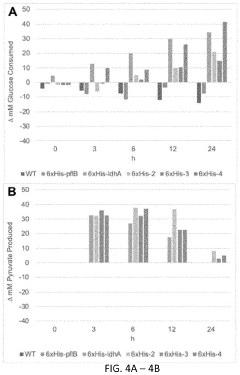
Metabolic pathway reconstruction in cell-free systems.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Metabolic Engineering Background and Objectives
Cell-free metabolic engineering represents a paradigm shift in biotechnology, emerging from the convergence of synthetic biology and biochemical engineering. This approach involves the reconstruction of metabolic pathways outside living cells, utilizing cellular extracts or purified enzymes to create controlled reaction environments. The field traces its origins to the pioneering work on cell-free protein synthesis in the 1960s, which demonstrated that biological processes could function in vitro. Over subsequent decades, researchers expanded these principles to encompass more complex metabolic transformations.
The evolution of cell-free systems has accelerated dramatically in recent years, driven by advances in enzyme engineering, analytical techniques, and computational modeling. This progression has transformed cell-free systems from simple demonstration platforms to sophisticated tools capable of producing valuable compounds at increasingly practical scales. The integration of high-throughput screening methodologies and machine learning approaches has further enhanced the precision and efficiency of pathway design and optimization.
The primary objective of metabolic pathway reconstruction in cell-free systems is to establish simplified, controllable environments for studying and manipulating biochemical reactions without cellular complexity. This approach offers unprecedented access to reaction components, allowing researchers to directly monitor metabolite concentrations, enzyme activities, and flux distributions without interference from competing cellular processes or regulatory mechanisms. Such transparency facilitates rapid iteration and optimization of pathway performance.
Beyond fundamental research applications, cell-free metabolic engineering aims to develop sustainable biomanufacturing platforms for producing pharmaceuticals, fine chemicals, and biofuels. The technology promises shorter development cycles compared to whole-cell approaches, as it circumvents challenges related to cell growth, genetic stability, and product toxicity. Additionally, cell-free systems offer potential advantages in terms of reaction specificity, yield, and process control.
Current technical objectives include enhancing system longevity through improved energy regeneration mechanisms, developing standardized components for modular pathway assembly, and scaling production to industrially relevant levels. Researchers are also focused on expanding the repertoire of compatible enzymes and reactions, particularly those involving membrane-associated processes or requiring specialized cofactors. The field is increasingly moving toward integrated approaches that combine cell-free metabolic engineering with other emerging technologies such as microfluidics, biosensors, and artificial cells.
The evolution of cell-free systems has accelerated dramatically in recent years, driven by advances in enzyme engineering, analytical techniques, and computational modeling. This progression has transformed cell-free systems from simple demonstration platforms to sophisticated tools capable of producing valuable compounds at increasingly practical scales. The integration of high-throughput screening methodologies and machine learning approaches has further enhanced the precision and efficiency of pathway design and optimization.
The primary objective of metabolic pathway reconstruction in cell-free systems is to establish simplified, controllable environments for studying and manipulating biochemical reactions without cellular complexity. This approach offers unprecedented access to reaction components, allowing researchers to directly monitor metabolite concentrations, enzyme activities, and flux distributions without interference from competing cellular processes or regulatory mechanisms. Such transparency facilitates rapid iteration and optimization of pathway performance.
Beyond fundamental research applications, cell-free metabolic engineering aims to develop sustainable biomanufacturing platforms for producing pharmaceuticals, fine chemicals, and biofuels. The technology promises shorter development cycles compared to whole-cell approaches, as it circumvents challenges related to cell growth, genetic stability, and product toxicity. Additionally, cell-free systems offer potential advantages in terms of reaction specificity, yield, and process control.
Current technical objectives include enhancing system longevity through improved energy regeneration mechanisms, developing standardized components for modular pathway assembly, and scaling production to industrially relevant levels. Researchers are also focused on expanding the repertoire of compatible enzymes and reactions, particularly those involving membrane-associated processes or requiring specialized cofactors. The field is increasingly moving toward integrated approaches that combine cell-free metabolic engineering with other emerging technologies such as microfluidics, biosensors, and artificial cells.
Market Analysis for Cell-Free Biosynthesis Applications
The cell-free biosynthesis market is experiencing significant growth, driven by increasing demand for sustainable production methods across pharmaceutical, chemical, and food industries. Current market valuations indicate that the global cell-free protein synthesis market reached approximately $250 million in 2022 and is projected to grow at a compound annual growth rate of 8-10% through 2030, potentially exceeding $500 million by the end of the decade.
Pharmaceutical applications currently dominate the market landscape, accounting for roughly 60% of the total market share. This segment is primarily focused on the production of therapeutic proteins, vaccines, and antibodies. The ability of cell-free systems to rapidly produce complex proteins without concerns about cell viability makes them particularly valuable for drug discovery and development processes.
Industrial biotechnology represents the fastest-growing segment, with an estimated growth rate of 12-15% annually. Companies are increasingly adopting cell-free systems for the production of enzymes, fine chemicals, and biofuels. The elimination of cellular constraints allows for the synthesis of products that might be toxic to living cells, expanding the range of possible applications.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Key market drivers include the rising demand for personalized medicine, advancements in synthetic biology tools, and increasing pressure for sustainable manufacturing processes. The ability of cell-free systems to enable rapid prototyping and optimization of metabolic pathways provides significant advantages in terms of development time and cost efficiency compared to traditional cell-based methods.
Market challenges primarily revolve around scalability issues, high production costs, and regulatory uncertainties. The cost of cell extract preparation and reaction components remains a significant barrier to widespread commercial adoption, particularly for bulk chemical production. Additionally, standardization of protocols and quality control measures are needed to ensure consistent performance across different applications.
Emerging opportunities include the development of portable, freeze-dried cell-free systems for point-of-care diagnostics and on-demand biomanufacturing, particularly in resource-limited settings. The integration of cell-free systems with artificial intelligence for pathway design and optimization also presents significant market potential, potentially revolutionizing how metabolic engineering is approached.
Pharmaceutical applications currently dominate the market landscape, accounting for roughly 60% of the total market share. This segment is primarily focused on the production of therapeutic proteins, vaccines, and antibodies. The ability of cell-free systems to rapidly produce complex proteins without concerns about cell viability makes them particularly valuable for drug discovery and development processes.
Industrial biotechnology represents the fastest-growing segment, with an estimated growth rate of 12-15% annually. Companies are increasingly adopting cell-free systems for the production of enzymes, fine chemicals, and biofuels. The elimination of cellular constraints allows for the synthesis of products that might be toxic to living cells, expanding the range of possible applications.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Key market drivers include the rising demand for personalized medicine, advancements in synthetic biology tools, and increasing pressure for sustainable manufacturing processes. The ability of cell-free systems to enable rapid prototyping and optimization of metabolic pathways provides significant advantages in terms of development time and cost efficiency compared to traditional cell-based methods.
Market challenges primarily revolve around scalability issues, high production costs, and regulatory uncertainties. The cost of cell extract preparation and reaction components remains a significant barrier to widespread commercial adoption, particularly for bulk chemical production. Additionally, standardization of protocols and quality control measures are needed to ensure consistent performance across different applications.
Emerging opportunities include the development of portable, freeze-dried cell-free systems for point-of-care diagnostics and on-demand biomanufacturing, particularly in resource-limited settings. The integration of cell-free systems with artificial intelligence for pathway design and optimization also presents significant market potential, potentially revolutionizing how metabolic engineering is approached.
Current Challenges in Metabolic Pathway Reconstruction
Despite significant advancements in cell-free metabolic engineering, several critical challenges continue to impede the efficient reconstruction of metabolic pathways in cell-free systems. One fundamental obstacle is the limited stability of enzymes and cofactors outside their native cellular environment. Many enzymes exhibit reduced activity or accelerated degradation when extracted from cells, necessitating continuous optimization of buffer conditions and stabilization strategies to maintain functionality over extended periods.
The regeneration and maintenance of cofactors presents another significant hurdle. Essential cofactors such as ATP, NAD(P)H, and CoA are continuously consumed during pathway operation, requiring efficient regeneration systems. Current regeneration approaches often suffer from imbalances that lead to pathway inefficiencies, particularly in multi-step reactions where cofactor requirements vary between enzymatic steps.
Scale-up challenges represent a major limitation for industrial applications. While small-scale demonstrations have shown promising results, transitioning to larger volumes introduces issues related to oxygen transfer limitations, mixing inefficiencies, and increased costs of enzyme production. The economic viability of large-scale cell-free systems remains questionable without significant improvements in enzyme production costs and stability.
Pathway optimization in cell-free systems faces unique difficulties compared to in vivo approaches. The absence of cellular regulatory mechanisms requires careful balancing of enzyme concentrations to prevent bottlenecks and accumulation of potentially inhibitory intermediates. Current optimization strategies often rely on trial-and-error approaches rather than systematic methodologies, limiting the efficiency of pathway design.
Analytical limitations further complicate pathway reconstruction efforts. Real-time monitoring of metabolic intermediates and products remains challenging, particularly for complex pathways with multiple intermediates. This hampers rapid iteration and optimization of pathway designs, as researchers must often rely on endpoint measurements rather than dynamic pathway analysis.
Integration of multiple pathways represents perhaps the most ambitious challenge. While individual pathways have been successfully reconstructed, connecting multiple pathways to create complex metabolic networks encounters difficulties in maintaining balanced flux and preventing cross-pathway interference. The absence of cellular compartmentalization that naturally separates competing reactions in vivo requires innovative solutions for pathway isolation and control in cell-free systems.
Addressing these challenges will require interdisciplinary approaches combining enzyme engineering, analytical chemistry, systems biology, and process engineering to develop more robust, scalable, and economically viable cell-free metabolic pathway reconstruction technologies.
The regeneration and maintenance of cofactors presents another significant hurdle. Essential cofactors such as ATP, NAD(P)H, and CoA are continuously consumed during pathway operation, requiring efficient regeneration systems. Current regeneration approaches often suffer from imbalances that lead to pathway inefficiencies, particularly in multi-step reactions where cofactor requirements vary between enzymatic steps.
Scale-up challenges represent a major limitation for industrial applications. While small-scale demonstrations have shown promising results, transitioning to larger volumes introduces issues related to oxygen transfer limitations, mixing inefficiencies, and increased costs of enzyme production. The economic viability of large-scale cell-free systems remains questionable without significant improvements in enzyme production costs and stability.
Pathway optimization in cell-free systems faces unique difficulties compared to in vivo approaches. The absence of cellular regulatory mechanisms requires careful balancing of enzyme concentrations to prevent bottlenecks and accumulation of potentially inhibitory intermediates. Current optimization strategies often rely on trial-and-error approaches rather than systematic methodologies, limiting the efficiency of pathway design.
Analytical limitations further complicate pathway reconstruction efforts. Real-time monitoring of metabolic intermediates and products remains challenging, particularly for complex pathways with multiple intermediates. This hampers rapid iteration and optimization of pathway designs, as researchers must often rely on endpoint measurements rather than dynamic pathway analysis.
Integration of multiple pathways represents perhaps the most ambitious challenge. While individual pathways have been successfully reconstructed, connecting multiple pathways to create complex metabolic networks encounters difficulties in maintaining balanced flux and preventing cross-pathway interference. The absence of cellular compartmentalization that naturally separates competing reactions in vivo requires innovative solutions for pathway isolation and control in cell-free systems.
Addressing these challenges will require interdisciplinary approaches combining enzyme engineering, analytical chemistry, systems biology, and process engineering to develop more robust, scalable, and economically viable cell-free metabolic pathway reconstruction technologies.
Existing Methodologies for Pathway Assembly and Optimization
01 Computational methods for metabolic pathway reconstruction
Advanced computational algorithms and bioinformatics tools are used to reconstruct metabolic pathways from genomic and proteomic data. These methods involve analyzing gene sequences, identifying enzymes, and predicting biochemical reactions to create comprehensive metabolic network models. Machine learning approaches can be employed to improve the accuracy of pathway predictions and to identify missing reactions or enzymes in existing pathway models.- Computational methods for metabolic pathway reconstruction: Advanced computational algorithms and bioinformatics tools are used to reconstruct metabolic pathways from genomic and proteomic data. These methods involve analyzing gene sequences, identifying enzymes, and predicting biochemical reactions to build comprehensive metabolic networks. Machine learning approaches can improve the accuracy of pathway predictions by integrating multiple data sources and identifying patterns in metabolic interactions.
- Genome-scale metabolic network reconstruction: Techniques for reconstructing complete metabolic networks at the genome scale involve systematic identification of all metabolic reactions in an organism. This approach integrates genomic annotations, biochemical knowledge, and experimental data to create comprehensive models of cellular metabolism. These reconstructions can be used to predict metabolic capabilities, identify essential genes, and understand the metabolic basis of various phenotypes.
- Experimental validation of metabolic pathways: Methods for experimentally validating reconstructed metabolic pathways include metabolic flux analysis, isotope labeling experiments, and high-throughput screening techniques. These approaches provide empirical evidence for predicted metabolic reactions and help refine computational models. Integration of experimental data with in silico predictions improves the accuracy and reliability of metabolic pathway reconstructions.
- Metabolic engineering applications: Metabolic pathway reconstruction enables targeted engineering of cellular metabolism for biotechnological applications. By understanding and manipulating metabolic networks, researchers can optimize production of valuable compounds, develop microbial cell factories, and create synthetic metabolic pathways. These applications include production of biofuels, pharmaceuticals, and other high-value chemicals through redirecting metabolic flux.
- Integration of multi-omics data for pathway reconstruction: Advanced approaches for metabolic pathway reconstruction integrate multiple types of omics data, including genomics, transcriptomics, proteomics, and metabolomics. This multi-omics integration provides a more comprehensive view of cellular metabolism and improves the accuracy of pathway predictions. Machine learning and artificial intelligence techniques are increasingly used to process and interpret these complex, heterogeneous datasets for more accurate metabolic network reconstruction.
02 Genome-scale metabolic network reconstruction
Genome-scale metabolic network reconstruction involves creating comprehensive models of an organism's metabolism based on genomic information. This approach integrates gene annotations, protein functions, and biochemical reaction data to build detailed metabolic maps. These reconstructions can be used to predict metabolic capabilities, identify essential genes, and understand the metabolic response to environmental changes, providing valuable insights for metabolic engineering and therapeutic interventions.Expand Specific Solutions03 Experimental validation of reconstructed metabolic pathways
Experimental techniques are essential for validating computationally reconstructed metabolic pathways. These methods include metabolomics, fluxomics, and isotope labeling experiments to track metabolite flow through pathways. High-throughput screening approaches can identify enzyme activities and substrate specificities, while gene knockout studies help verify the functional roles of enzymes in metabolic networks. Integration of experimental data with computational models improves the accuracy and reliability of pathway reconstructions.Expand Specific Solutions04 Metabolic pathway reconstruction for therapeutic applications
Reconstructing metabolic pathways has significant implications for developing therapeutic interventions. By understanding disease-specific metabolic alterations, researchers can identify potential drug targets and biomarkers. Metabolic pathway reconstruction enables the design of targeted therapies that modulate specific metabolic reactions or enzymes. This approach is particularly valuable for addressing metabolic disorders, cancer, and infectious diseases where metabolic reprogramming plays a crucial role in pathogenesis.Expand Specific Solutions05 Synthetic biology applications of metabolic pathway reconstruction
Metabolic pathway reconstruction is fundamental to synthetic biology applications, enabling the design and engineering of novel metabolic pathways in organisms. This approach allows for the production of valuable compounds such as biofuels, pharmaceuticals, and specialty chemicals through microbial cell factories. By reconstructing and optimizing metabolic pathways, researchers can enhance production yields, reduce byproduct formation, and create sustainable bioprocesses for industrial applications.Expand Specific Solutions
Leading Research Groups and Companies in Cell-Free Technology
Metabolic pathway reconstruction in cell-free systems is currently in a growth phase, with an expanding market driven by applications in synthetic biology and biomanufacturing. The global market size is estimated to reach significant value as industries recognize the potential for sustainable chemical production. Technologically, academic institutions like MIT, Northwestern University, and Tsinghua University are leading fundamental research, while companies such as GreenLight Biosciences, Evonik Operations, and Lonza are advancing commercial applications. The field shows varying maturity levels across applications, with pharmaceutical and fine chemical production systems more developed than complex metabolic networks. Industry-academic partnerships between entities like BASF and research universities are accelerating technology transfer, positioning cell-free systems as a transformative platform for next-generation biotechnology.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free protein synthesis platform specifically optimized for metabolic pathway reconstruction. Their technology utilizes purified cellular components to create an open, controllable environment for biochemical reactions. The company's proprietary cell-free system enables rapid prototyping of metabolic pathways by allowing direct manipulation of enzyme concentrations, cofactor levels, and reaction conditions without cellular constraints. GreenLight's platform incorporates advanced RNA technology to express multiple enzymes simultaneously with precise control over expression levels, facilitating the optimization of complex metabolic pathways. Their cell-free systems are designed with enhanced stability and scalability, supporting both small-scale screening and larger production applications in biomanufacturing.
Strengths: Rapid prototyping capability allows faster pathway optimization compared to in vivo systems; precise control over reaction components enables better understanding of pathway kinetics and bottlenecks. Weaknesses: Higher production costs compared to cell-based systems; potential challenges in maintaining long-term stability of cell-free reactions for industrial-scale applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered innovative approaches to cell-free metabolic pathway reconstruction through their CFME (Cell-Free Metabolic Engineering) platform. Their technology integrates computational modeling with experimental design to create optimized cell-free systems for diverse biochemical pathways. MIT researchers have developed methods for enhancing cofactor regeneration systems critical for sustained metabolic activity in cell-free environments, significantly extending reaction lifetimes. Their approach includes the development of lyophilized cell-free systems that maintain stability during storage while preserving metabolic activity upon rehydration. MIT's platform incorporates modular design principles, allowing rapid assembly and testing of different pathway configurations through a plug-and-play approach. The institute has demonstrated successful implementation of complex multi-enzyme cascades for the production of high-value chemicals and pharmaceuticals using their cell-free systems.
Strengths: Strong integration of computational modeling with experimental approaches enables rational design of complex pathways; advanced cofactor regeneration systems support extended reaction times. Weaknesses: Higher technical complexity requires specialized expertise; scaling challenges when transitioning from laboratory demonstrations to industrial applications.
Key Enzymes and Cofactor Regeneration Strategies
Cell-free metabolic pathway optimization through removal of select proteins
PatentInactiveUS20210324425A1
Innovation
- Genetic engineering of cells to link affinity tags to specific enzymes affecting metabolite production, allowing for their removal through affinity purification, thereby creating a cell-free extract with directed metabolic flux towards a metabolite of interest.
Methods for control of FLUX in metabolic pathways through enzyme relocation
PatentWO2011140516A2
Innovation
- The method involves genetically modifying cells to relocate key enzymes in a metabolic pathway to the periplasmic space during the cell growth phase, and then combining the enzyme with cytoplasmic enzymes in a cell-free system during the production phase to control metabolic flux and increase product yield.
Scalability and Industrial Implementation Considerations
The transition from laboratory-scale cell-free metabolic pathway reconstruction to industrial implementation presents significant challenges that must be addressed for commercial viability. Current cell-free systems typically operate at milliliter volumes in research settings, while industrial applications require scaling to hundreds or thousands of liters. This scale-up introduces complexities in maintaining reaction homogeneity, temperature control, and efficient mixing without damaging delicate biological components. Additionally, the cost structure changes dramatically at industrial scale, necessitating optimization of enzyme production, purification methods, and substrate utilization efficiency.
Continuous processing represents a promising approach for industrial implementation, allowing for sustained production rather than batch operations. Several pioneering companies have developed flow-based cell-free systems that maintain productivity over extended periods by continuously supplying substrates and removing products and inhibitory byproducts. These systems have demonstrated improved yields and reduced enzyme requirements compared to batch processes, though challenges in maintaining long-term stability remain.
Raw material sourcing becomes increasingly critical at industrial scale. The production of purified enzymes, nucleotides, and other cofactors represents a substantial portion of operational costs. Recent advances in enzyme expression systems, including cell-free protein synthesis for enzyme production, offer potential cost reductions. Alternative approaches utilizing crude cell lysates rather than purified components have shown promise for reducing production costs, though often with trade-offs in reaction control and reproducibility.
Standardization and modularization of cell-free systems represent essential considerations for industrial implementation. The development of standardized reaction components, predictable expression cassettes, and modular pathway elements would facilitate rapid process development and optimization. Several research groups have proposed standardized cell-free toolkits that could accelerate industrial adoption by reducing development timelines and improving reproducibility across production facilities.
Regulatory considerations also impact industrial implementation strategies. Cell-free systems offer potential advantages in regulatory approval processes compared to whole-cell systems, as they contain no viable organisms. However, the novel nature of these production platforms may require new regulatory frameworks. Early engagement with regulatory authorities and development of industry standards for quality control and product characterization will be essential for commercial deployment of cell-free metabolic pathway technologies.
Continuous processing represents a promising approach for industrial implementation, allowing for sustained production rather than batch operations. Several pioneering companies have developed flow-based cell-free systems that maintain productivity over extended periods by continuously supplying substrates and removing products and inhibitory byproducts. These systems have demonstrated improved yields and reduced enzyme requirements compared to batch processes, though challenges in maintaining long-term stability remain.
Raw material sourcing becomes increasingly critical at industrial scale. The production of purified enzymes, nucleotides, and other cofactors represents a substantial portion of operational costs. Recent advances in enzyme expression systems, including cell-free protein synthesis for enzyme production, offer potential cost reductions. Alternative approaches utilizing crude cell lysates rather than purified components have shown promise for reducing production costs, though often with trade-offs in reaction control and reproducibility.
Standardization and modularization of cell-free systems represent essential considerations for industrial implementation. The development of standardized reaction components, predictable expression cassettes, and modular pathway elements would facilitate rapid process development and optimization. Several research groups have proposed standardized cell-free toolkits that could accelerate industrial adoption by reducing development timelines and improving reproducibility across production facilities.
Regulatory considerations also impact industrial implementation strategies. Cell-free systems offer potential advantages in regulatory approval processes compared to whole-cell systems, as they contain no viable organisms. However, the novel nature of these production platforms may require new regulatory frameworks. Early engagement with regulatory authorities and development of industry standards for quality control and product characterization will be essential for commercial deployment of cell-free metabolic pathway technologies.
Biosafety and Regulatory Framework for Cell-Free Products
Cell-free systems represent a unique regulatory challenge as they operate at the intersection of traditional biotechnology and emerging synthetic biology. The biosafety considerations for cell-free products differ significantly from whole-cell systems, primarily due to the absence of living organisms capable of replication and environmental spread. However, this does not exempt them from rigorous safety assessment and regulatory oversight.
Current regulatory frameworks worldwide are still evolving to address cell-free metabolic pathway products specifically. In the United States, the FDA, EPA, and USDA share jurisdiction depending on the intended use of the cell-free product. The FDA typically regulates cell-free products intended for therapeutic applications under the biologics framework, while those for food applications may fall under GRAS (Generally Recognized As Safe) considerations.
The European Union applies a more precautionary approach through the Directive 2001/18/EC on deliberate release of genetically modified organisms, which may extend to certain cell-free products containing genetic material. However, many cell-free systems fall into regulatory gray areas, necessitating case-by-case evaluation.
Biosafety considerations for cell-free metabolic pathway products include potential toxicity of synthesized compounds, allergenicity, and environmental impact if released. Of particular concern is the potential for horizontal gene transfer if nucleic acids are present in the final product. Risk assessment protocols typically evaluate the stability of the cell-free system, degradation pathways, and potential for interaction with environmental organisms.
Containment strategies for cell-free systems are generally less stringent than for living organisms but still require careful consideration. Physical containment through proper packaging and disposal protocols remains essential, particularly for systems containing complete genetic elements.
Industry self-regulation has emerged as an important complement to formal regulatory frameworks. Organizations like the International Gene Synthesis Consortium (IGSC) have developed screening protocols for DNA synthesis orders to prevent misuse, which applies to components used in cell-free systems.
Looking forward, regulatory harmonization across jurisdictions will be crucial for the commercial viability of cell-free products. The development of standardized risk assessment methodologies specifically designed for cell-free systems would significantly benefit both regulators and developers, ensuring safety while enabling innovation in this promising field.
Current regulatory frameworks worldwide are still evolving to address cell-free metabolic pathway products specifically. In the United States, the FDA, EPA, and USDA share jurisdiction depending on the intended use of the cell-free product. The FDA typically regulates cell-free products intended for therapeutic applications under the biologics framework, while those for food applications may fall under GRAS (Generally Recognized As Safe) considerations.
The European Union applies a more precautionary approach through the Directive 2001/18/EC on deliberate release of genetically modified organisms, which may extend to certain cell-free products containing genetic material. However, many cell-free systems fall into regulatory gray areas, necessitating case-by-case evaluation.
Biosafety considerations for cell-free metabolic pathway products include potential toxicity of synthesized compounds, allergenicity, and environmental impact if released. Of particular concern is the potential for horizontal gene transfer if nucleic acids are present in the final product. Risk assessment protocols typically evaluate the stability of the cell-free system, degradation pathways, and potential for interaction with environmental organisms.
Containment strategies for cell-free systems are generally less stringent than for living organisms but still require careful consideration. Physical containment through proper packaging and disposal protocols remains essential, particularly for systems containing complete genetic elements.
Industry self-regulation has emerged as an important complement to formal regulatory frameworks. Organizations like the International Gene Synthesis Consortium (IGSC) have developed screening protocols for DNA synthesis orders to prevent misuse, which applies to components used in cell-free systems.
Looking forward, regulatory harmonization across jurisdictions will be crucial for the commercial viability of cell-free products. The development of standardized risk assessment methodologies specifically designed for cell-free systems would significantly benefit both regulators and developers, ensuring safety while enabling innovation in this promising field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!