Application of cell-free systems in personalized medicine.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Systems Background and Objectives
Cell-free systems represent a revolutionary approach in biotechnology that has evolved significantly over the past decades. Initially developed as research tools to study fundamental biological processes, these systems have now emerged as powerful platforms for various applications, including diagnostics, therapeutics, and personalized medicine. Cell-free systems essentially consist of cellular machinery extracted from cells, allowing biochemical reactions to occur without the constraints of cell walls and cellular metabolism.
The evolution of cell-free systems can be traced back to the 1950s with the groundbreaking work on in vitro protein synthesis. However, significant advancements in the field occurred in the 1990s and early 2000s with improved extraction methods and the development of more stable and efficient cell-free protein synthesis (CFPS) systems. Recent technological breakthroughs in synthetic biology, microfluidics, and high-throughput screening have further accelerated the development and application of these systems.
The current technological trajectory indicates a shift towards more sophisticated, customizable, and scalable cell-free platforms. These systems are increasingly being designed to accommodate complex biochemical pathways and to produce a wider range of biomolecules, including proteins, nucleic acids, and metabolites. The integration of cell-free systems with other emerging technologies, such as CRISPR-Cas systems and artificial intelligence, is opening new avenues for innovation in personalized medicine.
In the context of personalized medicine, cell-free systems aim to enable rapid, cost-effective, and patient-specific diagnostic and therapeutic solutions. The primary objectives include developing point-of-care diagnostic devices capable of detecting biomarkers with high sensitivity and specificity, creating platforms for on-demand synthesis of personalized therapeutics, and establishing systems for real-time monitoring of patient responses to treatments.
Another critical goal is to overcome the current limitations of cell-free systems, such as reduced efficiency compared to in vivo systems, limited scalability, and challenges in maintaining long-term stability. Addressing these challenges will be essential for the widespread adoption of cell-free technologies in clinical settings and for realizing their full potential in personalized medicine.
The ultimate vision for cell-free systems in personalized medicine is to create a paradigm shift in healthcare delivery, moving from standardized treatments to highly individualized approaches that consider each patient's unique genetic makeup, environmental factors, and disease characteristics. This transition promises to improve treatment outcomes, reduce adverse effects, and ultimately enhance patient quality of life.
The evolution of cell-free systems can be traced back to the 1950s with the groundbreaking work on in vitro protein synthesis. However, significant advancements in the field occurred in the 1990s and early 2000s with improved extraction methods and the development of more stable and efficient cell-free protein synthesis (CFPS) systems. Recent technological breakthroughs in synthetic biology, microfluidics, and high-throughput screening have further accelerated the development and application of these systems.
The current technological trajectory indicates a shift towards more sophisticated, customizable, and scalable cell-free platforms. These systems are increasingly being designed to accommodate complex biochemical pathways and to produce a wider range of biomolecules, including proteins, nucleic acids, and metabolites. The integration of cell-free systems with other emerging technologies, such as CRISPR-Cas systems and artificial intelligence, is opening new avenues for innovation in personalized medicine.
In the context of personalized medicine, cell-free systems aim to enable rapid, cost-effective, and patient-specific diagnostic and therapeutic solutions. The primary objectives include developing point-of-care diagnostic devices capable of detecting biomarkers with high sensitivity and specificity, creating platforms for on-demand synthesis of personalized therapeutics, and establishing systems for real-time monitoring of patient responses to treatments.
Another critical goal is to overcome the current limitations of cell-free systems, such as reduced efficiency compared to in vivo systems, limited scalability, and challenges in maintaining long-term stability. Addressing these challenges will be essential for the widespread adoption of cell-free technologies in clinical settings and for realizing their full potential in personalized medicine.
The ultimate vision for cell-free systems in personalized medicine is to create a paradigm shift in healthcare delivery, moving from standardized treatments to highly individualized approaches that consider each patient's unique genetic makeup, environmental factors, and disease characteristics. This transition promises to improve treatment outcomes, reduce adverse effects, and ultimately enhance patient quality of life.
Market Analysis for Cell-Free Diagnostics
The cell-free diagnostics market is experiencing significant growth, driven by increasing demand for rapid, accurate, and personalized diagnostic solutions. Current market valuations place the global cell-free diagnostics sector at approximately $3.2 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 11.7% through 2030. This growth trajectory is supported by substantial investments in research and development, with venture capital funding exceeding $850 million in the past two years alone.
The market segmentation reveals distinct application areas, with oncology representing the largest share at 42% of the total market. This is followed by prenatal testing (28%), infectious disease diagnostics (18%), and other applications including transplant medicine and autoimmune disorders (12%). Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
Consumer demand for personalized medicine solutions is reshaping the diagnostic landscape, with patients increasingly seeking tailored treatment approaches based on their unique genetic profiles. This shift is evidenced by the 34% annual increase in direct-to-consumer genetic testing services over the past three years. Healthcare providers are responding to this trend, with 67% of surveyed institutions reporting plans to expand their personalized medicine offerings within the next five years.
Key market drivers include technological advancements in cell-free DNA/RNA detection methods, decreasing costs of sequencing technologies, growing prevalence of chronic diseases requiring early and accurate diagnosis, and increasing healthcare expenditure worldwide. The integration of artificial intelligence and machine learning algorithms with cell-free diagnostic platforms is creating new market opportunities, enhancing test sensitivity and specificity while reducing turnaround times.
Market challenges persist, including regulatory hurdles for novel diagnostic approaches, reimbursement issues affecting adoption rates, and technical limitations in detecting low-abundance biomarkers. Additionally, standardization across different testing platforms remains problematic, with inter-laboratory variability affecting result consistency and clinical interpretation.
The competitive landscape features established diagnostic companies expanding their cell-free testing portfolios alongside innovative startups developing disruptive technologies. Recent market consolidation through mergers and acquisitions indicates industry maturation, with larger companies acquiring specialized technologies to strengthen their personalized medicine offerings.
Consumer accessibility remains a critical factor for market growth, with current pricing models limiting widespread adoption. However, emerging subscription-based models and increasing insurance coverage for validated tests are gradually addressing affordability concerns, potentially expanding the addressable market by an estimated 30% over the next decade.
The market segmentation reveals distinct application areas, with oncology representing the largest share at 42% of the total market. This is followed by prenatal testing (28%), infectious disease diagnostics (18%), and other applications including transplant medicine and autoimmune disorders (12%). Geographically, North America leads with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
Consumer demand for personalized medicine solutions is reshaping the diagnostic landscape, with patients increasingly seeking tailored treatment approaches based on their unique genetic profiles. This shift is evidenced by the 34% annual increase in direct-to-consumer genetic testing services over the past three years. Healthcare providers are responding to this trend, with 67% of surveyed institutions reporting plans to expand their personalized medicine offerings within the next five years.
Key market drivers include technological advancements in cell-free DNA/RNA detection methods, decreasing costs of sequencing technologies, growing prevalence of chronic diseases requiring early and accurate diagnosis, and increasing healthcare expenditure worldwide. The integration of artificial intelligence and machine learning algorithms with cell-free diagnostic platforms is creating new market opportunities, enhancing test sensitivity and specificity while reducing turnaround times.
Market challenges persist, including regulatory hurdles for novel diagnostic approaches, reimbursement issues affecting adoption rates, and technical limitations in detecting low-abundance biomarkers. Additionally, standardization across different testing platforms remains problematic, with inter-laboratory variability affecting result consistency and clinical interpretation.
The competitive landscape features established diagnostic companies expanding their cell-free testing portfolios alongside innovative startups developing disruptive technologies. Recent market consolidation through mergers and acquisitions indicates industry maturation, with larger companies acquiring specialized technologies to strengthen their personalized medicine offerings.
Consumer accessibility remains a critical factor for market growth, with current pricing models limiting widespread adoption. However, emerging subscription-based models and increasing insurance coverage for validated tests are gradually addressing affordability concerns, potentially expanding the addressable market by an estimated 30% over the next decade.
Technical Challenges in Cell-Free Personalized Medicine
Despite significant advancements in cell-free systems for personalized medicine, several technical challenges continue to impede widespread clinical implementation. One primary obstacle is the standardization of cell-free extract preparation. Current protocols exhibit considerable batch-to-batch variability, affecting reproducibility and reliability of diagnostic and therapeutic applications. This inconsistency stems from differences in source cells, lysis methods, and post-lysis processing techniques, creating significant hurdles for regulatory approval and clinical adoption.
Stability limitations represent another critical challenge. Cell-free systems typically maintain optimal activity for only 4-8 hours at physiological temperatures, restricting their practical utility in point-of-care settings. While freeze-drying techniques have shown promise in extending shelf-life, they often result in reduced functionality upon rehydration, particularly for complex diagnostic applications requiring multiple enzymatic reactions.
Scalability concerns also persist in the production of cell-free systems. Current laboratory-scale preparation methods do not readily translate to industrial-scale manufacturing without significant loss of efficiency or increased costs. The complex nature of extract preparation, involving precise cell growth conditions, mechanical disruption, and careful fractionation, creates bottlenecks in scaling production to meet potential clinical demand.
Sensitivity and specificity issues present additional technical barriers. While cell-free systems offer impressive detection capabilities for certain biomarkers, they still struggle with complex biological samples containing inhibitors, degradative enzymes, or competing analytes. This is particularly problematic when analyzing clinical specimens like blood, saliva, or tissue extracts, where matrix effects can significantly impair performance.
Integration with existing clinical workflows poses another substantial challenge. Cell-free diagnostic platforms often require specialized equipment or technical expertise not readily available in standard clinical laboratories. The development of user-friendly interfaces and automated systems that seamlessly connect with established healthcare infrastructure remains underdeveloped.
Regulatory uncertainties further complicate advancement in this field. Cell-free systems represent a novel technology class that doesn't neatly fit into existing regulatory frameworks. Questions regarding appropriate validation metrics, quality control standards, and performance benchmarks remain largely unresolved, creating significant barriers to clinical translation and commercialization.
Cost considerations also limit widespread adoption. Current cell-free applications typically require expensive reagents, specialized equipment, and skilled personnel, making them prohibitively expensive for routine clinical use, particularly in resource-limited settings where personalized medicine approaches could provide significant benefits.
Stability limitations represent another critical challenge. Cell-free systems typically maintain optimal activity for only 4-8 hours at physiological temperatures, restricting their practical utility in point-of-care settings. While freeze-drying techniques have shown promise in extending shelf-life, they often result in reduced functionality upon rehydration, particularly for complex diagnostic applications requiring multiple enzymatic reactions.
Scalability concerns also persist in the production of cell-free systems. Current laboratory-scale preparation methods do not readily translate to industrial-scale manufacturing without significant loss of efficiency or increased costs. The complex nature of extract preparation, involving precise cell growth conditions, mechanical disruption, and careful fractionation, creates bottlenecks in scaling production to meet potential clinical demand.
Sensitivity and specificity issues present additional technical barriers. While cell-free systems offer impressive detection capabilities for certain biomarkers, they still struggle with complex biological samples containing inhibitors, degradative enzymes, or competing analytes. This is particularly problematic when analyzing clinical specimens like blood, saliva, or tissue extracts, where matrix effects can significantly impair performance.
Integration with existing clinical workflows poses another substantial challenge. Cell-free diagnostic platforms often require specialized equipment or technical expertise not readily available in standard clinical laboratories. The development of user-friendly interfaces and automated systems that seamlessly connect with established healthcare infrastructure remains underdeveloped.
Regulatory uncertainties further complicate advancement in this field. Cell-free systems represent a novel technology class that doesn't neatly fit into existing regulatory frameworks. Questions regarding appropriate validation metrics, quality control standards, and performance benchmarks remain largely unresolved, creating significant barriers to clinical translation and commercialization.
Cost considerations also limit widespread adoption. Current cell-free applications typically require expensive reagents, specialized equipment, and skilled personnel, making them prohibitively expensive for routine clinical use, particularly in resource-limited settings where personalized medicine approaches could provide significant benefits.
Current Cell-Free Diagnostic Solutions
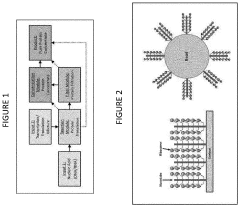
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems allow for the production of proteins outside of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, nucleotides, and amino acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells.- Cell-free protein synthesis systems: Cell-free protein synthesis systems allow for the production of proteins outside of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, nucleotides, and amino acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells.
- Cell-free diagnostics and biosensors: Cell-free systems are utilized in diagnostic applications and biosensors for detecting various analytes including pathogens, toxins, and biomarkers. These diagnostic platforms leverage cell-free transcription-translation reactions coupled with reporter systems to provide rapid and sensitive detection. The elimination of whole cells simplifies storage, transportation, and use in resource-limited settings while maintaining high specificity and sensitivity for point-of-care applications.
- Cell-free metabolic engineering: Cell-free metabolic engineering involves the design and optimization of biochemical pathways outside of living cells. This approach allows for the production of valuable compounds including biofuels, pharmaceuticals, and fine chemicals without cellular growth constraints. The open nature of these systems facilitates direct monitoring and manipulation of reaction conditions, enabling rapid prototyping of pathways and enhanced production yields compared to traditional cell-based methods.
- Cell-free synthetic genomics: Cell-free synthetic genomics focuses on the synthesis, assembly, and manipulation of genetic material outside of living cells. These systems enable the construction of artificial genomes, gene circuits, and genetic elements without cellular constraints. The technology facilitates rapid prototyping of genetic designs, testing of gene function, and creation of novel genetic systems that might be difficult to implement in living organisms due to toxicity or complexity issues.
- Cell-free therapeutic production: Cell-free systems are employed for the production of therapeutic proteins, vaccines, and other biopharmaceuticals. These systems offer advantages including rapid production timelines, elimination of cellular contaminants, and simplified purification processes. The open nature of cell-free reactions allows for the incorporation of non-natural amino acids and direct manipulation of reaction conditions to optimize product yield and quality, making them valuable for personalized medicine applications and rapid response to emerging health threats.
02 Cell-free diagnostics and biosensors
Cell-free systems are utilized for diagnostic applications and biosensors. These systems can detect specific biomarkers, pathogens, or molecules of interest without requiring intact cells. They often incorporate synthetic gene circuits or enzymes that produce detectable signals in response to target analytes. Cell-free diagnostics offer advantages including rapid results, portability, and stability at room temperature, making them suitable for point-of-care testing and field applications.Expand Specific Solutions03 Cell-free metabolic engineering
Cell-free metabolic engineering involves the design and manipulation of metabolic pathways outside of living cells. These systems allow researchers to study and optimize biochemical reactions without cellular constraints. By eliminating cell walls and other cellular components, substrates and products can be more easily added or removed from the reaction environment. This approach enables the production of valuable compounds, biofuels, and chemicals through enzymatic cascades in a controlled setting.Expand Specific Solutions04 Cell-free synthetic biology platforms
Cell-free synthetic biology platforms provide a framework for designing and testing genetic circuits and biological systems without using intact cells. These platforms typically contain the machinery necessary for gene expression along with customizable genetic elements. They allow for rapid prototyping of genetic designs, characterization of biological parts, and testing of synthetic gene networks. The open nature of these systems facilitates direct manipulation and monitoring of reactions in real-time.Expand Specific Solutions05 Cell-free therapeutic production systems
Cell-free systems are employed for the production of therapeutic proteins, vaccines, and other biopharmaceuticals. These systems can rapidly produce medical countermeasures in response to emerging threats or pandemics. By eliminating the need for cell culture, they reduce production time and can be deployed in resource-limited settings. Cell-free therapeutic production systems can be freeze-dried for long-term storage and reconstituted when needed, enabling on-demand manufacturing of critical medicines.Expand Specific Solutions
Key Industry Players and Stakeholders
The cell-free systems market in personalized medicine is in an early growth phase, characterized by increasing research activities and emerging commercial applications. The market size is expanding rapidly, driven by advancements in synthetic biology and precision medicine, with projections suggesting significant growth over the next decade. Technologically, the field shows varying maturity levels across applications, with companies like Factor Bioscience pioneering regenerative medicine approaches, Xilis developing patient-derived models for precision oncology, and National Resilience building advanced biomanufacturing platforms. Established players such as Janssen Biotech and Roche are integrating cell-free technologies into their R&D pipelines, while research institutions like Duke University and Moffitt Cancer Center are advancing fundamental innovations that bridge laboratory discoveries with clinical applications.
Janssen Biotech, Inc.
Technical Solution: Janssen Biotech has developed a sophisticated cell-free diagnostic platform called "J-CFMED" (Janssen Cell-Free Molecular Evaluation and Diagnostics) for personalized medicine applications. This system utilizes cell-free nucleic acids extracted from patient blood samples to detect disease biomarkers and monitor treatment response. Their technology combines digital droplet PCR with proprietary machine learning algorithms to achieve high sensitivity detection of rare mutations, enabling early disease detection and recurrence monitoring. The platform has been particularly advanced in oncology applications, where it can detect tumor-specific mutations at concentrations as low as 0.1% variant allele frequency. Janssen has integrated this technology with their therapeutic development pipeline, creating companion diagnostics that help identify patients most likely to benefit from specific treatments. Their system includes automated sample processing modules that standardize pre-analytical variables, improving reproducibility across different clinical settings and facilitating widespread adoption in precision medicine programs.
Strengths: High sensitivity detection capabilities for rare genetic variants; seamless integration with therapeutic development pipeline; automated workflow reducing technical variability. Weaknesses: Limited to detection of known genetic alterations; requires specialized equipment and trained personnel; higher cost compared to conventional diagnostic methods.
Xilis, Inc.
Technical Solution: Xilis has developed a revolutionary cell-free approach to personalized cancer treatment called MicroOrganoSphere (MOS) technology. This platform combines patient-derived micro-organoids with cell-free analytical systems to rapidly assess drug efficacy on individual patient tumors. Their technology preserves the native tumor microenvironment while enabling high-throughput screening of multiple therapeutic options simultaneously. The MOS platform can generate functional drug response data in just 7-10 days, significantly faster than traditional patient-derived xenograft models that typically require months. Xilis employs advanced imaging and AI-based analysis to quantify treatment responses at both cellular and molecular levels. Their system incorporates cell-free transcriptomic and proteomic analysis of treatment-exposed micro-organoids, providing mechanistic insights into drug sensitivity and resistance patterns. This comprehensive approach enables oncologists to make data-driven treatment decisions based on the actual behavior of a patient's tumor, rather than relying solely on genetic biomarkers that may not fully predict functional response.
Strengths: Rapid turnaround time enabling clinical decision-making within actionable timeframes; preservation of tumor heterogeneity and microenvironment; functional assessment beyond genetic profiling. Weaknesses: Requires viable tumor tissue which may be challenging to obtain for certain cancer types; complex workflow requiring specialized expertise; higher cost compared to standard genetic testing approaches.
Core Technologies in Cell-Free Systems
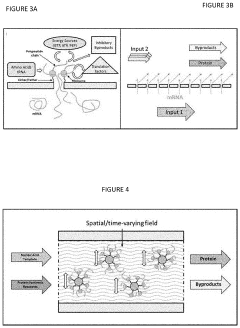
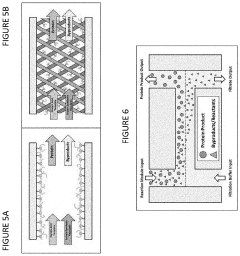
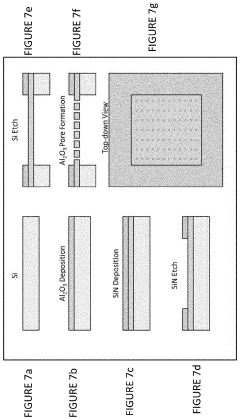
Cell-free protein synthesis systems
PatentActiveUS20200255881A1
Innovation
- A cell-free protein synthesis (CFPS) system with localized ribosomes attached to structures, allowing for repeated use in in vitro translation reactions, maximizing surface area for biomolecular interactions and enabling continuous operation with efficient product collection and replacement, thereby enhancing protein production rates.
Computer systems and methods for providing health care
PatentWO2005116890A1
Innovation
- A system and method that combine molecular profiles from patient specimens with clinical characterization to select personalized treatment regimens, allowing for continuous refinement of treatment protocols based on patient data and outcomes, and enabling remote molecular profiling in affiliate facilities.
Regulatory Framework for Cell-Free Diagnostics
The regulatory landscape for cell-free diagnostic systems presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies most cell-free diagnostic tools as in vitro diagnostic devices (IVDs), requiring premarket approval or 510(k) clearance depending on their risk classification. Cell-free DNA testing for cancer detection and monitoring, for instance, typically falls under Class II or III devices, necessitating substantial clinical validation data.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, most cell-free diagnostic applications are classified as Class C or D devices, requiring notified body involvement and comprehensive technical documentation.
Regulatory challenges specific to cell-free systems include standardization issues, as different platforms may yield varying results for the same biomarker. The FDA has addressed this through initiatives like the Precision FDA platform, which aims to establish reference standards for next-generation sequencing and cell-free applications.
Privacy regulations present another critical dimension, as cell-free diagnostics often involve processing sensitive genetic information. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on data handling, consent procedures, and security measures for patient information derived from cell-free testing.
Reimbursement pathways remain underdeveloped for many cell-free diagnostic applications, creating market access barriers despite technological readiness. The Centers for Medicare & Medicaid Services (CMS) has established coverage determinations for certain cell-free DNA tests, particularly in prenatal and oncology applications, but many emerging applications lack clear reimbursement codes.
Laboratory-developed tests (LDTs) using cell-free technologies currently operate under the Clinical Laboratory Improvement Amendments (CLIA) in the US, though the FDA has signaled intentions to increase oversight in this area. This regulatory uncertainty affects investment and development strategies for companies working on novel cell-free diagnostic platforms.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches across major markets, potentially streamlining approval processes for cell-free diagnostic technologies in the future.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, most cell-free diagnostic applications are classified as Class C or D devices, requiring notified body involvement and comprehensive technical documentation.
Regulatory challenges specific to cell-free systems include standardization issues, as different platforms may yield varying results for the same biomarker. The FDA has addressed this through initiatives like the Precision FDA platform, which aims to establish reference standards for next-generation sequencing and cell-free applications.
Privacy regulations present another critical dimension, as cell-free diagnostics often involve processing sensitive genetic information. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on data handling, consent procedures, and security measures for patient information derived from cell-free testing.
Reimbursement pathways remain underdeveloped for many cell-free diagnostic applications, creating market access barriers despite technological readiness. The Centers for Medicare & Medicaid Services (CMS) has established coverage determinations for certain cell-free DNA tests, particularly in prenatal and oncology applications, but many emerging applications lack clear reimbursement codes.
Laboratory-developed tests (LDTs) using cell-free technologies currently operate under the Clinical Laboratory Improvement Amendments (CLIA) in the US, though the FDA has signaled intentions to increase oversight in this area. This regulatory uncertainty affects investment and development strategies for companies working on novel cell-free diagnostic platforms.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches across major markets, potentially streamlining approval processes for cell-free diagnostic technologies in the future.
Clinical Integration Strategies
The integration of cell-free systems into clinical practice represents a critical challenge for realizing their potential in personalized medicine. Healthcare institutions must develop comprehensive strategies that address regulatory, operational, and technical aspects of implementation. Currently, most cell-free diagnostic applications remain confined to research settings, with limited translation to point-of-care environments where their impact would be greatest.
Successful clinical integration requires establishing standardized protocols for sample collection, processing, and analysis. These protocols must be robust enough to function reliably in diverse healthcare settings while maintaining sensitivity and specificity. Several pioneering medical centers have begun developing workflow models that incorporate cell-free diagnostics into existing clinical pathways, particularly in oncology and infectious disease management.
Infrastructure adaptation presents another significant consideration. Healthcare facilities need dedicated laboratory spaces with appropriate biosafety measures and specialized equipment for cell-free system operations. The development of compact, automated platforms that can be operated by clinical staff with minimal specialized training will accelerate adoption across different healthcare settings.
Data management systems must evolve to handle the complex information generated by cell-free diagnostics. Integration with electronic health records (EHRs) and clinical decision support systems is essential for physicians to effectively utilize results in treatment planning. Several healthcare technology companies are developing specialized software solutions that can interpret cell-free system outputs and present actionable recommendations to clinicians.
Clinician education represents a critical component of successful integration. Medical professionals require training not only in the technical aspects of cell-free systems but also in result interpretation and clinical application. Multidisciplinary teams including molecular biologists, physicians, and bioinformaticians will be necessary to maximize the utility of these technologies in personalized treatment planning.
Reimbursement pathways must be established to ensure economic sustainability. Current healthcare payment models often fail to account for the value of personalized diagnostic approaches. Demonstrating cost-effectiveness through reduced hospitalization, avoided adverse events, and improved outcomes will be essential for securing coverage from insurers and healthcare systems.
Patient engagement strategies should not be overlooked. Effective communication about the capabilities and limitations of cell-free diagnostics will help manage expectations and improve acceptance. Patient-centered approaches that emphasize shared decision-making will be particularly important as these technologies enable increasingly personalized treatment options.
Successful clinical integration requires establishing standardized protocols for sample collection, processing, and analysis. These protocols must be robust enough to function reliably in diverse healthcare settings while maintaining sensitivity and specificity. Several pioneering medical centers have begun developing workflow models that incorporate cell-free diagnostics into existing clinical pathways, particularly in oncology and infectious disease management.
Infrastructure adaptation presents another significant consideration. Healthcare facilities need dedicated laboratory spaces with appropriate biosafety measures and specialized equipment for cell-free system operations. The development of compact, automated platforms that can be operated by clinical staff with minimal specialized training will accelerate adoption across different healthcare settings.
Data management systems must evolve to handle the complex information generated by cell-free diagnostics. Integration with electronic health records (EHRs) and clinical decision support systems is essential for physicians to effectively utilize results in treatment planning. Several healthcare technology companies are developing specialized software solutions that can interpret cell-free system outputs and present actionable recommendations to clinicians.
Clinician education represents a critical component of successful integration. Medical professionals require training not only in the technical aspects of cell-free systems but also in result interpretation and clinical application. Multidisciplinary teams including molecular biologists, physicians, and bioinformaticians will be necessary to maximize the utility of these technologies in personalized treatment planning.
Reimbursement pathways must be established to ensure economic sustainability. Current healthcare payment models often fail to account for the value of personalized diagnostic approaches. Demonstrating cost-effectiveness through reduced hospitalization, avoided adverse events, and improved outcomes will be essential for securing coverage from insurers and healthcare systems.
Patient engagement strategies should not be overlooked. Effective communication about the capabilities and limitations of cell-free diagnostics will help manage expectations and improve acceptance. Patient-centered approaches that emphasize shared decision-making will be particularly important as these technologies enable increasingly personalized treatment options.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!