Interdisciplinary approaches to advancing cell-free biomanufacturing.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Biomanufacturing Background and Objectives
Cell-free biomanufacturing represents a paradigm shift in biotechnology, emerging from the convergence of synthetic biology, biochemistry, and engineering principles. This approach harnesses biological machinery outside the constraints of living cells, offering unprecedented flexibility and control over biological processes. The evolution of this technology can be traced back to the mid-20th century with the groundbreaking work on cell-free protein synthesis, but has gained significant momentum in the past decade due to advances in molecular biology techniques and analytical tools.
The trajectory of cell-free systems has evolved from simple protein production platforms to sophisticated biomanufacturing systems capable of producing complex molecules, biofuels, and pharmaceuticals. Recent technological breakthroughs in cell lysate preparation, energy regeneration systems, and reaction optimization have dramatically improved the efficiency and scalability of these systems, positioning them as viable alternatives to traditional cell-based manufacturing.
The primary objective of advancing interdisciplinary approaches to cell-free biomanufacturing is to overcome current limitations in yield, stability, and scalability. By integrating expertise from diverse fields such as microfluidics, materials science, computational modeling, and process engineering, researchers aim to develop robust platforms that can operate continuously with enhanced productivity and reduced costs.
Another critical goal is to expand the repertoire of molecules that can be synthesized using cell-free systems. This includes complex proteins with post-translational modifications, novel biopolymers, and specialized metabolites that are challenging to produce in conventional cellular systems due to toxicity or metabolic burden constraints.
Sustainability represents a key objective in the advancement of cell-free biomanufacturing. The technology offers potential advantages in resource efficiency, reduced waste generation, and lower environmental footprint compared to traditional manufacturing processes. Developing circular bioeconomy approaches that integrate renewable feedstocks and recyclable components aligns with global sustainability goals.
Standardization and automation constitute essential objectives for the maturation of cell-free technologies. Establishing reproducible protocols, quality control metrics, and automated production systems will facilitate broader adoption across industries and research settings. The development of integrated, user-friendly platforms that require minimal specialized expertise would democratize access to this powerful technology.
The ultimate aim is to position cell-free biomanufacturing as a transformative technology platform that enables rapid prototyping, distributed manufacturing, and on-demand production of critical biomolecules. This could revolutionize sectors ranging from healthcare and agriculture to materials science and environmental remediation, offering solutions to some of society's most pressing challenges.
The trajectory of cell-free systems has evolved from simple protein production platforms to sophisticated biomanufacturing systems capable of producing complex molecules, biofuels, and pharmaceuticals. Recent technological breakthroughs in cell lysate preparation, energy regeneration systems, and reaction optimization have dramatically improved the efficiency and scalability of these systems, positioning them as viable alternatives to traditional cell-based manufacturing.
The primary objective of advancing interdisciplinary approaches to cell-free biomanufacturing is to overcome current limitations in yield, stability, and scalability. By integrating expertise from diverse fields such as microfluidics, materials science, computational modeling, and process engineering, researchers aim to develop robust platforms that can operate continuously with enhanced productivity and reduced costs.
Another critical goal is to expand the repertoire of molecules that can be synthesized using cell-free systems. This includes complex proteins with post-translational modifications, novel biopolymers, and specialized metabolites that are challenging to produce in conventional cellular systems due to toxicity or metabolic burden constraints.
Sustainability represents a key objective in the advancement of cell-free biomanufacturing. The technology offers potential advantages in resource efficiency, reduced waste generation, and lower environmental footprint compared to traditional manufacturing processes. Developing circular bioeconomy approaches that integrate renewable feedstocks and recyclable components aligns with global sustainability goals.
Standardization and automation constitute essential objectives for the maturation of cell-free technologies. Establishing reproducible protocols, quality control metrics, and automated production systems will facilitate broader adoption across industries and research settings. The development of integrated, user-friendly platforms that require minimal specialized expertise would democratize access to this powerful technology.
The ultimate aim is to position cell-free biomanufacturing as a transformative technology platform that enables rapid prototyping, distributed manufacturing, and on-demand production of critical biomolecules. This could revolutionize sectors ranging from healthcare and agriculture to materials science and environmental remediation, offering solutions to some of society's most pressing challenges.
Market Analysis for Cell-Free Biological Products
The cell-free biological products market is experiencing significant growth, driven by increasing demand for sustainable biomanufacturing solutions across pharmaceutical, agricultural, and industrial sectors. Current market valuations indicate that cell-free protein synthesis technologies alone represent a market segment of approximately 3 billion USD, with projections suggesting a compound annual growth rate of 10-12% through 2030.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 60% of cell-free product applications. This is primarily due to the advantages cell-free systems offer in rapid vaccine development, therapeutic protein production, and diagnostic tool manufacturing. The COVID-19 pandemic served as a catalyst, demonstrating the value of cell-free systems in responding quickly to global health emergencies.
Industrial biotechnology represents the fastest-growing segment, with annual growth rates exceeding 15%. Companies are increasingly adopting cell-free approaches for enzyme production, biofuel development, and specialty chemical synthesis. This shift is largely attributed to the reduced capital expenditure requirements compared to traditional cell-based manufacturing facilities and the elimination of concerns related to genetically modified organisms.
Consumer demand for sustainable products is creating new market opportunities in food technology and biomaterials. Cell-free systems are being explored for alternative protein production, flavor compound synthesis, and biodegradable material development. Market research indicates consumer willingness to pay premium prices for products with improved environmental profiles, creating favorable conditions for cell-free derived consumer goods.
Regional analysis reveals North America currently leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to increasing biotechnology investments in China, Japan, and Singapore, coupled with favorable regulatory environments for novel biomanufacturing approaches.
Key market challenges include scaling limitations, high reagent costs, and regulatory uncertainties. Despite these challenges, venture capital investment in cell-free biotechnology startups has tripled over the past five years, indicating strong investor confidence in the sector's commercial potential.
Market forecasts suggest that as interdisciplinary approaches advance cell-free biomanufacturing capabilities, new application areas will emerge, particularly in personalized medicine, environmental remediation, and space-based biomanufacturing. These emerging applications could potentially double the total addressable market by 2035, representing a significant opportunity for early market entrants and technology developers.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 60% of cell-free product applications. This is primarily due to the advantages cell-free systems offer in rapid vaccine development, therapeutic protein production, and diagnostic tool manufacturing. The COVID-19 pandemic served as a catalyst, demonstrating the value of cell-free systems in responding quickly to global health emergencies.
Industrial biotechnology represents the fastest-growing segment, with annual growth rates exceeding 15%. Companies are increasingly adopting cell-free approaches for enzyme production, biofuel development, and specialty chemical synthesis. This shift is largely attributed to the reduced capital expenditure requirements compared to traditional cell-based manufacturing facilities and the elimination of concerns related to genetically modified organisms.
Consumer demand for sustainable products is creating new market opportunities in food technology and biomaterials. Cell-free systems are being explored for alternative protein production, flavor compound synthesis, and biodegradable material development. Market research indicates consumer willingness to pay premium prices for products with improved environmental profiles, creating favorable conditions for cell-free derived consumer goods.
Regional analysis reveals North America currently leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to increasing biotechnology investments in China, Japan, and Singapore, coupled with favorable regulatory environments for novel biomanufacturing approaches.
Key market challenges include scaling limitations, high reagent costs, and regulatory uncertainties. Despite these challenges, venture capital investment in cell-free biotechnology startups has tripled over the past five years, indicating strong investor confidence in the sector's commercial potential.
Market forecasts suggest that as interdisciplinary approaches advance cell-free biomanufacturing capabilities, new application areas will emerge, particularly in personalized medicine, environmental remediation, and space-based biomanufacturing. These emerging applications could potentially double the total addressable market by 2035, representing a significant opportunity for early market entrants and technology developers.
Current Challenges in Cell-Free Systems
Despite the promising potential of cell-free biomanufacturing systems, several significant challenges currently impede their widespread industrial adoption and commercial viability. One primary limitation is the relatively short reaction lifetime of cell-free systems, typically ranging from hours to days, which restricts their application in continuous manufacturing processes. This constraint stems from the gradual depletion of energy sources, accumulation of inhibitory byproducts, and degradation of essential components over time.
Scalability presents another formidable challenge, as most cell-free systems have been optimized at laboratory scales (microliter to milliliter volumes), with limited success in industrial-scale implementations. The transition to larger volumes introduces complications in maintaining homogeneity, efficient mass transfer, and consistent performance across batches, significantly impacting production economics.
Cost considerations remain a substantial barrier, particularly regarding the preparation of cell extracts and the supply of expensive cofactors and energy sources. Current extraction methods are labor-intensive and yield relatively small quantities of active lysate, driving up production costs. Additionally, the continuous supplementation of ATP and other energy-rich compounds further increases operational expenses, making cell-free systems less economically competitive compared to traditional cell-based processes.
Standardization and reproducibility issues persist across different laboratories and production facilities. The performance of cell-free systems can vary significantly depending on extract preparation methods, strain backgrounds, and reaction conditions. This variability complicates technology transfer and scale-up efforts, hindering industrial implementation.
Regulatory uncertainties surrounding cell-free products represent another challenge, particularly for applications in pharmaceuticals and food production. The novel nature of these systems means that regulatory frameworks are still evolving, creating potential delays and additional costs for companies seeking approval for cell-free manufactured products.
The limited repertoire of characterized genetic parts optimized specifically for cell-free expression also constrains system capabilities. While many genetic elements function well in living cells, their performance can differ substantially in cell-free environments due to the absence of cellular homeostasis mechanisms and different biochemical conditions.
Finally, interdisciplinary integration challenges exist between biological engineering, chemical engineering, materials science, and process engineering. Effective advancement of cell-free biomanufacturing requires seamless collaboration across these disciplines to address complex technical issues, but organizational and communication barriers often impede such integration.
Scalability presents another formidable challenge, as most cell-free systems have been optimized at laboratory scales (microliter to milliliter volumes), with limited success in industrial-scale implementations. The transition to larger volumes introduces complications in maintaining homogeneity, efficient mass transfer, and consistent performance across batches, significantly impacting production economics.
Cost considerations remain a substantial barrier, particularly regarding the preparation of cell extracts and the supply of expensive cofactors and energy sources. Current extraction methods are labor-intensive and yield relatively small quantities of active lysate, driving up production costs. Additionally, the continuous supplementation of ATP and other energy-rich compounds further increases operational expenses, making cell-free systems less economically competitive compared to traditional cell-based processes.
Standardization and reproducibility issues persist across different laboratories and production facilities. The performance of cell-free systems can vary significantly depending on extract preparation methods, strain backgrounds, and reaction conditions. This variability complicates technology transfer and scale-up efforts, hindering industrial implementation.
Regulatory uncertainties surrounding cell-free products represent another challenge, particularly for applications in pharmaceuticals and food production. The novel nature of these systems means that regulatory frameworks are still evolving, creating potential delays and additional costs for companies seeking approval for cell-free manufactured products.
The limited repertoire of characterized genetic parts optimized specifically for cell-free expression also constrains system capabilities. While many genetic elements function well in living cells, their performance can differ substantially in cell-free environments due to the absence of cellular homeostasis mechanisms and different biochemical conditions.
Finally, interdisciplinary integration challenges exist between biological engineering, chemical engineering, materials science, and process engineering. Effective advancement of cell-free biomanufacturing requires seamless collaboration across these disciplines to address complex technical issues, but organizational and communication barriers often impede such integration.
Interdisciplinary Solutions in Cell-Free Platforms
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems enable the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, and substrates. By eliminating the constraints of cell viability, these systems allow for the production of proteins that might be toxic to living cells and enable rapid protein expression. They can be derived from various organisms and optimized for specific applications in biomanufacturing.- Cell-free protein synthesis systems: Cell-free protein synthesis systems enable the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, and substrates. By eliminating the constraints of cell viability, these systems allow for the rapid production of proteins that might be toxic to living cells, and enable easier manipulation of reaction conditions for optimized protein production.
- Cell-free metabolic engineering: Cell-free metabolic engineering involves the design and optimization of biochemical pathways outside of living cells. This approach allows for the direct manipulation of enzymatic reactions to produce desired compounds without cellular constraints. By removing cellular barriers, researchers can achieve higher yields, faster reaction rates, and produce compounds that might be toxic to living organisms. This technology enables the production of various chemicals, biofuels, and pharmaceuticals in a more controlled environment.
- Cell-free biosensors and diagnostics: Cell-free systems can be engineered to function as biosensors for detecting specific molecules or pathogens. These biosensors utilize biological components such as enzymes, antibodies, or nucleic acids to recognize target analytes and produce detectable signals. The cell-free format allows for improved stability, portability, and rapid response times compared to whole-cell biosensors. Applications include point-of-care diagnostics, environmental monitoring, and food safety testing.
- Cell-free biomanufacturing platforms and technologies: Advanced platforms and technologies have been developed to enhance cell-free biomanufacturing processes. These include specialized reaction vessels, continuous-flow systems, and immobilized enzyme reactors that improve efficiency and scalability. Additionally, computational tools and artificial intelligence approaches are being integrated to optimize reaction conditions and predict product yields. These technological advancements are making cell-free biomanufacturing more commercially viable for industrial applications.
- Cell-free synthetic biology applications: Cell-free synthetic biology extends beyond protein production to include the synthesis of complex biological structures and systems. This includes the assembly of artificial cells, production of nucleic acids, and creation of biomaterials. By working outside the constraints of living cells, researchers can design and build biological components with novel functions that would not be possible in traditional cellular systems. These applications have potential in medicine, materials science, and environmental remediation.
02 Cell-free metabolic engineering
Cell-free metabolic engineering involves the design and manipulation of metabolic pathways outside of living cells. This approach allows for the production of valuable compounds through enzymatic reactions in a controlled environment. By removing cellular constraints, researchers can optimize reaction conditions, increase yields, and produce compounds that might be toxic to living cells. This technology enables the synthesis of various chemicals, biofuels, and pharmaceuticals through carefully designed enzymatic cascades.Expand Specific Solutions03 Cell-free biosensors and diagnostics
Cell-free systems can be engineered to function as biosensors for detecting specific molecules or pathogens. These diagnostic tools utilize cell-free transcription and translation machinery coupled with reporter systems to provide rapid and sensitive detection. The absence of living cells increases stability and shelf-life while reducing biosafety concerns. These biosensors can be freeze-dried for storage and rapidly rehydrated when needed, making them suitable for point-of-care diagnostics and environmental monitoring applications.Expand Specific Solutions04 Cell-free biomanufacturing platforms and scale-up
Cell-free biomanufacturing platforms focus on the development of scalable and efficient systems for industrial applications. These platforms address challenges related to reaction longevity, component stability, and cost-effectiveness. Innovations include continuous-flow systems, immobilized enzyme reactors, and regenerative energy systems that extend reaction lifetimes. Scale-up strategies involve optimizing reaction conditions, developing cost-effective extract preparation methods, and implementing process control systems to maintain productivity at larger scales.Expand Specific Solutions05 Cell-free synthetic biology applications
Cell-free synthetic biology applies engineering principles to design and construct biological systems outside of living cells. This approach enables the rapid prototyping of genetic circuits, testing of synthetic pathways, and production of novel biomolecules. By working in a cell-free environment, researchers can bypass genetic stability issues and cellular regulation constraints. Applications include the synthesis of artificial proteins with non-natural amino acids, development of minimal cell systems, and creation of biomaterials with programmable properties.Expand Specific Solutions
Key Industry Players and Academic Contributors
Cell-free biomanufacturing is emerging as a transformative approach at the intersection of synthetic biology, biochemistry, and engineering. The market is in its early growth phase, with significant research momentum but limited commercial maturity. Key players represent diverse sectors: academic institutions (MIT, Tsinghua University, Northwestern University) driving fundamental research; established biopharmaceutical companies (Amgen, Regeneron, Janssen Biotech) exploring applications; and specialized biotechnology firms (Cellino Biotech, Cellfree Sciences) developing platform technologies. The competitive landscape features both traditional cell-based manufacturing incumbents and innovative startups focused on cell-free systems. Technical challenges remain in scaling production, optimizing yields, and ensuring regulatory compliance, but collaborative interdisciplinary approaches between academia and industry are accelerating development toward commercial viability in therapeutic protein production, diagnostics, and sustainable biomanufacturing.
Tsinghua University
Technical Solution: Tsinghua University has developed an integrated cell-free biomanufacturing platform that combines synthetic biology principles with advanced bioprocess engineering. Their approach focuses on creating highly efficient cell-free systems optimized for the production of complex biomolecules including therapeutic proteins, vaccines, and specialty chemicals. Researchers at Tsinghua have engineered enhanced cell extracts from various organisms (E. coli, yeast, and CHO cells) with improved energy regeneration systems that significantly extend reaction lifetimes and increase product yields. A distinctive feature of their technology is the development of continuous-flow cell-free bioreactors that overcome traditional batch reaction limitations, allowing for sustained production over extended periods. Their platform incorporates microfluidic devices for precise control of reaction conditions and real-time monitoring of product formation. Additionally, Tsinghua researchers have pioneered the integration of artificial intelligence algorithms to optimize reaction parameters and predict protein expression outcomes, significantly reducing development timelines for new products.
Strengths: Strong integration of engineering principles with biological systems; innovative continuous production systems; advanced computational tools for process optimization; extensive experience with diverse target molecules. Weaknesses: Technology transfer to industrial settings still in progress; higher complexity of continuous systems may present operational challenges; energy supply remains a limiting factor for some applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered transformative approaches to cell-free biomanufacturing through their Synthetic Biology Center and other research initiatives. Their technology platform centers on highly optimized cell-free protein synthesis (CFPS) systems derived from E. coli and other organisms, engineered for maximum productivity and versatility. MIT researchers have developed methods to lyophilize (freeze-dry) these systems, creating shelf-stable reagents that can be activated by simple rehydration, enabling point-of-use manufacturing even in resource-limited settings. They've also created innovative "on-demand" biosensors using cell-free systems that can detect pathogens, environmental contaminants, and medical biomarkers. A key innovation is their development of cell-free metabolic engineering approaches that allow for the synthesis of complex biomolecules and natural products through the integration of multiple enzymatic pathways. MIT's interdisciplinary approach combines computational modeling with experimental validation to optimize reaction conditions and pathway design, significantly improving yields and reducing costs compared to traditional methods.
Strengths: World-leading expertise in synthetic biology and metabolic engineering; innovative approaches to system stabilization and portability; strong focus on practical applications and commercialization pathways. Weaknesses: Some technologies still at early research stage; scaling production to industrial levels remains challenging; regulatory pathway for novel cell-free therapeutics still evolving.
Critical Patents and Breakthroughs
A cell-free BIO-manufacturing platform for production of fatty acids and cannabinoids
PatentWO2022099255A1
Innovation
- A cell-free bio-manufacturing platform is developed for the synthesis of fatty acids and cannabinoids from glucose, utilizing an optimized reverse beta-oxidation pathway and biosynthetic enzymes to produce hexanoyl-CoA, olivetolic acid, and cannabigerolic acid, with the use of cerulenin to increase yields and prevent depletion of malonyl-CoA pools.
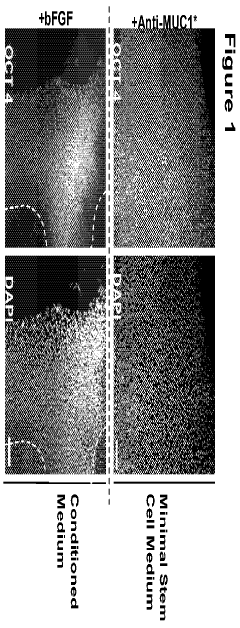
Methods for culturing stem and progenitor cells
PatentWO2010144887A1
Innovation
- A method involving attaching stem cells to a surface through a ligand that binds to specific cell surface proteins, using recombinant or synthetic agents like antibodies and growth factors, and employing a medium with defined components to promote growth and inhibit differentiation, allowing for automated harvesting and purification based on molecular recognition.
Regulatory Framework for Cell-Free Products
The regulatory landscape for cell-free biomanufacturing products remains complex and evolving, presenting both challenges and opportunities for industry stakeholders. Currently, cell-free products occupy a regulatory gray area between traditional pharmaceuticals and biological products, necessitating careful navigation of existing frameworks. In the United States, the FDA has begun developing specific guidance for cell-free systems through its Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), focusing on product characterization, quality control, and safety assessment.
The European Medicines Agency (EMA) has adopted a case-by-case approach, evaluating cell-free products based on their intended use, manufacturing process, and risk profile. This flexible framework allows for innovation while maintaining stringent safety standards. Japan's PMDA has implemented an expedited pathway for certain cell-free therapeutics under its Sakigake designation, potentially accelerating market access for breakthrough technologies.
Key regulatory considerations for cell-free products include source material characterization, process validation, impurity profiling, and stability assessment. Manufacturers must demonstrate consistent removal of host cell components, endotoxins, and other potential contaminants. The absence of living cells in final products may simplify certain safety concerns but introduces unique challenges in establishing product identity and potency standards.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are underway to develop standardized approaches for cell-free product evaluation. These initiatives aim to reduce regulatory divergence and facilitate global market access while maintaining appropriate safety oversight.
Emerging regulatory trends include the development of reference standards for common cell-free platforms, implementation of risk-based approaches to quality control, and adoption of advanced analytical methods for product characterization. Regulatory agencies increasingly recognize the need for specialized expertise in evaluating these novel products, leading to the formation of dedicated review teams and advisory committees.
Industry stakeholders are actively engaging with regulators through public-private partnerships, scientific workshops, and formal consultation processes to shape appropriate regulatory frameworks. These collaborative efforts focus on developing fit-for-purpose guidelines that balance innovation with patient safety. The establishment of regulatory precedents through successful product approvals will likely accelerate the development of more comprehensive frameworks in the coming years.
The European Medicines Agency (EMA) has adopted a case-by-case approach, evaluating cell-free products based on their intended use, manufacturing process, and risk profile. This flexible framework allows for innovation while maintaining stringent safety standards. Japan's PMDA has implemented an expedited pathway for certain cell-free therapeutics under its Sakigake designation, potentially accelerating market access for breakthrough technologies.
Key regulatory considerations for cell-free products include source material characterization, process validation, impurity profiling, and stability assessment. Manufacturers must demonstrate consistent removal of host cell components, endotoxins, and other potential contaminants. The absence of living cells in final products may simplify certain safety concerns but introduces unique challenges in establishing product identity and potency standards.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are underway to develop standardized approaches for cell-free product evaluation. These initiatives aim to reduce regulatory divergence and facilitate global market access while maintaining appropriate safety oversight.
Emerging regulatory trends include the development of reference standards for common cell-free platforms, implementation of risk-based approaches to quality control, and adoption of advanced analytical methods for product characterization. Regulatory agencies increasingly recognize the need for specialized expertise in evaluating these novel products, leading to the formation of dedicated review teams and advisory committees.
Industry stakeholders are actively engaging with regulators through public-private partnerships, scientific workshops, and formal consultation processes to shape appropriate regulatory frameworks. These collaborative efforts focus on developing fit-for-purpose guidelines that balance innovation with patient safety. The establishment of regulatory precedents through successful product approvals will likely accelerate the development of more comprehensive frameworks in the coming years.
Sustainability Impact Assessment
Cell-free biomanufacturing represents a paradigm shift in sustainable production methods, offering significant environmental benefits compared to traditional manufacturing processes. By eliminating the need for whole-cell cultivation, this approach substantially reduces water consumption, decreases land use requirements, and minimizes waste generation throughout the production lifecycle. The environmental footprint is further diminished through lower energy requirements for maintaining cellular viability and reduced chemical inputs for growth media.
From a carbon perspective, cell-free systems demonstrate promising advantages in greenhouse gas emission reduction. Studies indicate potential carbon footprint reductions of 30-45% compared to conventional cell-based fermentation processes, primarily due to streamlined production workflows and elimination of energy-intensive cell maintenance. This aligns with global carbon neutrality goals and positions cell-free biomanufacturing as a key contributor to sustainable industrial practices.
Economic sustainability metrics also favor cell-free approaches. The simplified production infrastructure translates to reduced capital expenditure requirements, while process intensification enables higher volumetric productivity. Analysis of production economics reveals potential cost savings of 15-25% for certain bioproducts when transitioning from cell-based to cell-free manufacturing platforms, creating compelling business cases for adoption across multiple industries.
Social sustainability dimensions must not be overlooked. Cell-free biomanufacturing enables decentralized production models that can strengthen local economies and reduce transportation-related environmental impacts. This democratization of production capability has particular relevance for developing regions, where simplified biomanufacturing technologies could address critical resource gaps in pharmaceuticals, materials, and food production.
Regulatory frameworks are evolving to accommodate these sustainable manufacturing approaches. Several jurisdictions have initiated specialized assessment protocols for cell-free products, recognizing their distinct sustainability profile compared to traditional biotechnology outputs. These frameworks increasingly incorporate lifecycle assessment methodologies that capture the full spectrum of environmental impacts from raw material sourcing through product disposal.
Looking forward, interdisciplinary collaboration between systems biology, green chemistry, and sustainable engineering will be essential to maximize the sustainability benefits of cell-free biomanufacturing. Particular attention should focus on renewable feedstock utilization, circular economy integration, and development of biodegradable reaction components that further enhance the environmental credentials of these production systems.
From a carbon perspective, cell-free systems demonstrate promising advantages in greenhouse gas emission reduction. Studies indicate potential carbon footprint reductions of 30-45% compared to conventional cell-based fermentation processes, primarily due to streamlined production workflows and elimination of energy-intensive cell maintenance. This aligns with global carbon neutrality goals and positions cell-free biomanufacturing as a key contributor to sustainable industrial practices.
Economic sustainability metrics also favor cell-free approaches. The simplified production infrastructure translates to reduced capital expenditure requirements, while process intensification enables higher volumetric productivity. Analysis of production economics reveals potential cost savings of 15-25% for certain bioproducts when transitioning from cell-based to cell-free manufacturing platforms, creating compelling business cases for adoption across multiple industries.
Social sustainability dimensions must not be overlooked. Cell-free biomanufacturing enables decentralized production models that can strengthen local economies and reduce transportation-related environmental impacts. This democratization of production capability has particular relevance for developing regions, where simplified biomanufacturing technologies could address critical resource gaps in pharmaceuticals, materials, and food production.
Regulatory frameworks are evolving to accommodate these sustainable manufacturing approaches. Several jurisdictions have initiated specialized assessment protocols for cell-free products, recognizing their distinct sustainability profile compared to traditional biotechnology outputs. These frameworks increasingly incorporate lifecycle assessment methodologies that capture the full spectrum of environmental impacts from raw material sourcing through product disposal.
Looking forward, interdisciplinary collaboration between systems biology, green chemistry, and sustainable engineering will be essential to maximize the sustainability benefits of cell-free biomanufacturing. Particular attention should focus on renewable feedstock utilization, circular economy integration, and development of biodegradable reaction components that further enhance the environmental credentials of these production systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!