The role of cell-free systems in synthetic biology advancements.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Systems Background and Objectives
Cell-free systems represent a transformative approach in synthetic biology, emerging from the pioneering work of Nirenberg and Matthaei in the 1960s who utilized cell extracts to decipher the genetic code. These systems have evolved from simple protein synthesis platforms to sophisticated tools enabling complex biological processes outside living cells. The fundamental concept involves extracting cellular machinery while eliminating cellular barriers, creating controlled environments for studying and engineering biological processes.
The evolution of cell-free systems has accelerated dramatically over the past two decades, transitioning from academic curiosity to practical biotechnology applications. Early systems focused primarily on protein production, while contemporary platforms support diverse functions including genetic circuit testing, metabolic engineering, and biosensor development. This progression reflects significant technological advancements in extract preparation, energy regeneration systems, and reaction optimization.
Current cell-free systems derive from various organisms including Escherichia coli, Saccharomyces cerevisiae, insect cells, and mammalian cell lines, each offering distinct advantages for specific applications. The diversity of source organisms has expanded the toolkit available to synthetic biologists, enabling more nuanced approaches to biological engineering challenges.
The primary objectives of cell-free synthetic biology research encompass several dimensions. First, researchers aim to develop more efficient and cost-effective cell-free platforms with enhanced protein yield, extended reaction durations, and simplified preparation protocols. Second, there is significant focus on expanding the functional capabilities of these systems to support increasingly complex biological processes and pathways.
Third, scientists seek to leverage cell-free systems as prototyping platforms for rapid design-build-test cycles in synthetic biology, dramatically accelerating the development timeline for engineered biological systems. Fourth, researchers are working to scale cell-free reactions from laboratory demonstrations to industrial applications, addressing challenges in reaction volume, stability, and reproducibility.
Finally, the field aims to integrate cell-free systems with complementary technologies including microfluidics, biosensors, and artificial cells, creating hybrid platforms with enhanced capabilities. These objectives collectively drive toward establishing cell-free systems as a foundational technology for next-generation biotechnology applications including on-demand biomanufacturing, point-of-care diagnostics, and environmental sensing.
The trajectory of cell-free systems research suggests potential breakthroughs in portable biomanufacturing, personalized medicine, and sustainable chemical production, positioning this technology at the forefront of synthetic biology innovation for the coming decade.
The evolution of cell-free systems has accelerated dramatically over the past two decades, transitioning from academic curiosity to practical biotechnology applications. Early systems focused primarily on protein production, while contemporary platforms support diverse functions including genetic circuit testing, metabolic engineering, and biosensor development. This progression reflects significant technological advancements in extract preparation, energy regeneration systems, and reaction optimization.
Current cell-free systems derive from various organisms including Escherichia coli, Saccharomyces cerevisiae, insect cells, and mammalian cell lines, each offering distinct advantages for specific applications. The diversity of source organisms has expanded the toolkit available to synthetic biologists, enabling more nuanced approaches to biological engineering challenges.
The primary objectives of cell-free synthetic biology research encompass several dimensions. First, researchers aim to develop more efficient and cost-effective cell-free platforms with enhanced protein yield, extended reaction durations, and simplified preparation protocols. Second, there is significant focus on expanding the functional capabilities of these systems to support increasingly complex biological processes and pathways.
Third, scientists seek to leverage cell-free systems as prototyping platforms for rapid design-build-test cycles in synthetic biology, dramatically accelerating the development timeline for engineered biological systems. Fourth, researchers are working to scale cell-free reactions from laboratory demonstrations to industrial applications, addressing challenges in reaction volume, stability, and reproducibility.
Finally, the field aims to integrate cell-free systems with complementary technologies including microfluidics, biosensors, and artificial cells, creating hybrid platforms with enhanced capabilities. These objectives collectively drive toward establishing cell-free systems as a foundational technology for next-generation biotechnology applications including on-demand biomanufacturing, point-of-care diagnostics, and environmental sensing.
The trajectory of cell-free systems research suggests potential breakthroughs in portable biomanufacturing, personalized medicine, and sustainable chemical production, positioning this technology at the forefront of synthetic biology innovation for the coming decade.
Market Applications of Cell-Free Synthetic Biology
Cell-free synthetic biology has rapidly evolved from a research tool to a commercially viable technology with diverse market applications. The global market for cell-free protein synthesis was valued at approximately $250 million in 2021 and is projected to reach $500 million by 2027, growing at a CAGR of 10-12%. This growth is driven by increasing demand across multiple sectors including pharmaceuticals, diagnostics, and industrial biotechnology.
In the pharmaceutical industry, cell-free systems are revolutionizing drug discovery and development processes. Companies like Sutro Biopharma and Greenlight Biosciences have successfully employed cell-free platforms for rapid production of therapeutic proteins and antibodies. This approach significantly reduces development timelines from months to weeks, enabling faster response to emerging health threats. The COVID-19 pandemic highlighted this advantage when cell-free systems were utilized to rapidly produce diagnostic reagents and vaccine components.
The diagnostics sector represents another high-growth application area. Paper-based cell-free diagnostic tools developed by companies such as Mammoth Biosciences and Sherlock Biosciences offer point-of-care testing capabilities with minimal infrastructure requirements. These systems can detect pathogens, genetic markers, and environmental contaminants with high sensitivity and specificity. The market for such portable diagnostic tools is expanding particularly in resource-limited settings and field applications.
Industrial biotechnology has embraced cell-free systems for the production of enzymes, fine chemicals, and biofuels. The technology enables the synthesis of products that would be toxic to living cells or require non-natural reaction conditions. Companies like Genomatica and Arzeda are leveraging cell-free platforms to design novel enzymatic pathways for sustainable chemical production, addressing growing consumer demand for environmentally friendly products.
Agricultural applications are emerging as farmers seek sustainable alternatives to conventional pesticides and fertilizers. Cell-free systems can produce biological crop protection agents and growth promoters with precise specifications and reduced environmental impact. This segment is expected to grow significantly as regulatory frameworks increasingly favor biological solutions over chemical interventions.
The food and beverage industry has begun adopting cell-free technology for ingredient production, flavor development, and quality control. Alternative protein manufacturers are exploring cell-free systems to produce animal proteins without animal involvement, addressing both ethical concerns and sustainability challenges in food production.
As manufacturing scales improve and production costs decrease, cell-free synthetic biology is poised to penetrate additional markets including cosmetics, materials science, and environmental remediation. The technology's flexibility, precision, and reduced regulatory complexity compared to GMO-based approaches position it as a transformative platform across diverse industrial applications.
In the pharmaceutical industry, cell-free systems are revolutionizing drug discovery and development processes. Companies like Sutro Biopharma and Greenlight Biosciences have successfully employed cell-free platforms for rapid production of therapeutic proteins and antibodies. This approach significantly reduces development timelines from months to weeks, enabling faster response to emerging health threats. The COVID-19 pandemic highlighted this advantage when cell-free systems were utilized to rapidly produce diagnostic reagents and vaccine components.
The diagnostics sector represents another high-growth application area. Paper-based cell-free diagnostic tools developed by companies such as Mammoth Biosciences and Sherlock Biosciences offer point-of-care testing capabilities with minimal infrastructure requirements. These systems can detect pathogens, genetic markers, and environmental contaminants with high sensitivity and specificity. The market for such portable diagnostic tools is expanding particularly in resource-limited settings and field applications.
Industrial biotechnology has embraced cell-free systems for the production of enzymes, fine chemicals, and biofuels. The technology enables the synthesis of products that would be toxic to living cells or require non-natural reaction conditions. Companies like Genomatica and Arzeda are leveraging cell-free platforms to design novel enzymatic pathways for sustainable chemical production, addressing growing consumer demand for environmentally friendly products.
Agricultural applications are emerging as farmers seek sustainable alternatives to conventional pesticides and fertilizers. Cell-free systems can produce biological crop protection agents and growth promoters with precise specifications and reduced environmental impact. This segment is expected to grow significantly as regulatory frameworks increasingly favor biological solutions over chemical interventions.
The food and beverage industry has begun adopting cell-free technology for ingredient production, flavor development, and quality control. Alternative protein manufacturers are exploring cell-free systems to produce animal proteins without animal involvement, addressing both ethical concerns and sustainability challenges in food production.
As manufacturing scales improve and production costs decrease, cell-free synthetic biology is poised to penetrate additional markets including cosmetics, materials science, and environmental remediation. The technology's flexibility, precision, and reduced regulatory complexity compared to GMO-based approaches position it as a transformative platform across diverse industrial applications.
Technical Landscape and Barriers in Cell-Free Systems
Cell-free systems represent a significant technological advancement in synthetic biology, offering a platform that bypasses the complexities of living cells while retaining their biochemical capabilities. Currently, these systems exist in various forms, including crude cell extracts, purified enzyme systems, and hybrid approaches that combine elements of both. Each variant presents unique advantages and challenges for different applications in biotechnology, pharmaceuticals, and materials science.
The global landscape of cell-free technology shows uneven development patterns. North America, particularly the United States, leads in research output and commercial applications, with major academic institutions like Stanford, MIT, and Northwestern University driving innovation. Europe follows closely, with strong contributions from the UK, Germany, and Switzerland. The Asia-Pacific region, especially China, Japan, and Singapore, is rapidly expanding its research capacity in this field, though still lagging in commercial translation compared to Western counterparts.
Despite promising advancements, cell-free systems face several critical technical barriers. Extract preparation remains inconsistent, with batch-to-batch variability hampering reproducibility and scalability. The limited operational lifetime of these systems—typically hours to days—restricts their application in continuous production processes. Energy regeneration systems, essential for sustained biochemical reactions, often become depleted quickly, further constraining reaction duration and yield.
Post-translational modifications, crucial for producing functional proteins, remain challenging to implement effectively in cell-free environments. This limitation particularly affects the production of complex therapeutic proteins and enzymes. Additionally, the cost of components for cell-free systems, especially for purified enzyme approaches, remains prohibitively high for many large-scale applications, creating economic barriers to widespread adoption.
Regulatory frameworks for cell-free products are still evolving, creating uncertainty for commercial development. The lack of standardized protocols and components further complicates technology transfer and industrial implementation. These challenges are compounded by intellectual property landscapes that can restrict access to key technologies and methodologies.
Environmental considerations also present challenges, as the disposal of spent reaction mixtures containing biological materials requires careful management. The field must address these sustainability concerns to align with growing demands for environmentally responsible biotechnologies.
The global landscape of cell-free technology shows uneven development patterns. North America, particularly the United States, leads in research output and commercial applications, with major academic institutions like Stanford, MIT, and Northwestern University driving innovation. Europe follows closely, with strong contributions from the UK, Germany, and Switzerland. The Asia-Pacific region, especially China, Japan, and Singapore, is rapidly expanding its research capacity in this field, though still lagging in commercial translation compared to Western counterparts.
Despite promising advancements, cell-free systems face several critical technical barriers. Extract preparation remains inconsistent, with batch-to-batch variability hampering reproducibility and scalability. The limited operational lifetime of these systems—typically hours to days—restricts their application in continuous production processes. Energy regeneration systems, essential for sustained biochemical reactions, often become depleted quickly, further constraining reaction duration and yield.
Post-translational modifications, crucial for producing functional proteins, remain challenging to implement effectively in cell-free environments. This limitation particularly affects the production of complex therapeutic proteins and enzymes. Additionally, the cost of components for cell-free systems, especially for purified enzyme approaches, remains prohibitively high for many large-scale applications, creating economic barriers to widespread adoption.
Regulatory frameworks for cell-free products are still evolving, creating uncertainty for commercial development. The lack of standardized protocols and components further complicates technology transfer and industrial implementation. These challenges are compounded by intellectual property landscapes that can restrict access to key technologies and methodologies.
Environmental considerations also present challenges, as the disposal of spent reaction mixtures containing biological materials requires careful management. The field must address these sustainability concerns to align with growing demands for environmentally responsible biotechnologies.
Current Cell-Free Expression Platforms
01 Cell-free protein synthesis systems
Cell-free protein synthesis systems allow for the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, nucleotides, and amino acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells.- Cell-free protein synthesis systems: Cell-free protein synthesis systems allow for the production of proteins without the use of living cells. These systems typically contain all the necessary components for transcription and translation, including ribosomes, enzymes, and nucleic acids. They offer advantages such as rapid protein production, the ability to produce toxic proteins, and simplified purification processes. These systems can be derived from various organisms including bacteria, yeast, and mammalian cells, and can be optimized for specific applications in biotechnology and pharmaceutical research.
- Cell-free nucleic acid amplification and detection: Cell-free systems for nucleic acid amplification and detection enable the identification of specific DNA or RNA sequences without the need for cell culture. These methods include PCR-based techniques, isothermal amplification, and various detection strategies that can be performed in vitro. Such systems are particularly valuable for diagnostic applications, allowing for rapid detection of pathogens, genetic mutations, or biomarkers. The cell-free format increases speed, sensitivity, and adaptability for point-of-care testing and high-throughput screening applications.
- Cell-free metabolic engineering and biosynthesis: Cell-free metabolic engineering involves reconstructing biochemical pathways outside of living cells to produce valuable compounds. By eliminating cellular constraints such as growth requirements and toxicity issues, these systems can achieve higher yields and conversion rates. They allow for direct manipulation of reaction conditions, enzyme concentrations, and cofactor regeneration. Applications include the production of biofuels, pharmaceuticals, fine chemicals, and natural products. These systems also serve as platforms for studying metabolic pathways and optimizing enzymatic reactions.
- Cell-free gene expression and regulation systems: Cell-free gene expression systems provide platforms for studying transcription, translation, and regulatory mechanisms outside of cellular environments. These systems allow for precise control over reaction components and conditions, enabling the investigation of gene regulation, promoter activity, and RNA processing. They can be used to express reporter genes, test genetic circuits, and evaluate the effects of various factors on gene expression. Such systems are valuable tools for synthetic biology, genetic engineering, and fundamental research on gene function and regulation.
- Cell-free diagnostic and therapeutic applications: Cell-free systems have been developed for various diagnostic and therapeutic applications, including the detection of disease biomarkers, drug screening, and the production of therapeutic proteins. These systems can analyze cell-free DNA, RNA, or proteins present in bodily fluids to diagnose conditions such as cancer, infectious diseases, or genetic disorders. They also enable rapid screening of drug candidates and the production of personalized therapeutics. The cell-free format offers advantages in terms of speed, scalability, and the ability to work with samples that would be challenging in cell-based systems.
02 Cell-free diagnostic applications
Cell-free systems are utilized in diagnostic applications for detecting various diseases and conditions. These diagnostic platforms often use cell-free nucleic acids (DNA or RNA) present in bodily fluids such as blood, urine, or saliva. The technology enables non-invasive testing for genetic disorders, infectious diseases, and cancer biomarkers. Cell-free diagnostic systems offer advantages including rapid results, high sensitivity, and the ability to detect multiple targets simultaneously.Expand Specific Solutions03 Cell-free gene expression and regulation
Cell-free systems provide platforms for studying gene expression and regulation mechanisms without cellular complexity. These systems allow researchers to investigate transcription, translation, and post-translational modifications in controlled environments. They enable the study of regulatory elements such as promoters, enhancers, and repressors, as well as the effects of various factors on gene expression. Cell-free gene expression systems are valuable tools for synthetic biology and metabolic engineering applications.Expand Specific Solutions04 Cell-free biosensors and environmental monitoring
Cell-free biosensors utilize biological components outside of living cells to detect specific analytes or environmental conditions. These systems often incorporate enzymes, antibodies, or nucleic acids as recognition elements coupled with signal transduction mechanisms. Cell-free biosensors offer advantages including stability, portability, and customizability for detecting various targets such as pollutants, toxins, pathogens, and biomarkers. They can be designed for use in resource-limited settings and for rapid environmental monitoring applications.Expand Specific Solutions05 Cell-free therapeutic production systems
Cell-free systems are employed for the production of therapeutic compounds including proteins, peptides, and small molecules. These systems allow for the synthesis of pharmaceuticals without the constraints of cellular metabolism or viability. They can be optimized for high-yield production of specific therapeutics and enable the incorporation of non-natural amino acids or modifications. Cell-free therapeutic production systems offer advantages in terms of scalability, product purity, and reduced contamination risks compared to traditional cell-based manufacturing methods.Expand Specific Solutions
Leading Organizations in Cell-Free Synthetic Biology
Cell-free synthetic biology is currently in a growth phase, with market size expanding due to increasing applications in pharmaceuticals, diagnostics, and biomanufacturing. The technology is approaching early commercial maturity, with key players demonstrating diverse capabilities. Academic institutions like MIT, Harvard, and Tsinghua University are driving fundamental research, while companies are commercializing applications. GreenLight Biosciences and Debut Biotechnology have developed scalable cell-free platforms for RNA and enzyme production. Cellfree Sciences and Tierra Biosciences (formerly Synvitrobio) offer specialized protein expression systems. Kangma Biological Technology is advancing diagnostic applications, demonstrating the technology's transition from research to commercial applications across multiple sectors.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free RNA production platform that enables the rapid, scalable synthesis of RNA molecules for various applications including vaccines, therapeutics, and agricultural products. Their proprietary cell-free system utilizes optimized enzymatic reactions to produce RNA without the limitations of traditional fermentation-based methods. The company's platform incorporates a continuous-flow bioreactor system that allows for consistent production quality and scalability from research quantities to commercial volumes. Their technology employs specially engineered enzymes and reaction conditions that minimize RNA degradation and maximize yield. GreenLight's cell-free approach enables the production of RNA molecules that would be difficult to produce in living cells due to toxicity or structural complexity. The platform has demonstrated particular success in producing double-stranded RNA for agricultural applications and mRNA for therapeutic and vaccine applications, with production costs significantly lower than competing technologies.
Strengths: Exceptional scalability from micrograms to kilograms; high purity of RNA products; rapid production timelines compared to cell-based methods; flexibility to produce diverse RNA structures. Weaknesses: Higher energy requirements compared to some cell-based methods; challenges with very long RNA sequences; dependency on specialized enzymatic components; potential regulatory complexities for therapeutic applications.
The Regents of the University of California
Technical Solution: The University of California system has been at the forefront of cell-free synthetic biology research across multiple campuses. Researchers have developed PURE (Protein synthesis Using Recombinant Elements) system variants that utilize entirely defined components rather than crude cell extracts, enabling unprecedented control over reaction conditions. UC Berkeley teams have pioneered cell-free metabolic engineering approaches that combine enzyme cascades for the production of valuable chemicals and biofuels. UC San Diego researchers have developed cell-free systems specifically optimized for glycoprotein synthesis, incorporating eukaryotic translational machinery and glycosylation enzymes to enable the production of properly folded and modified proteins. The UC system has also been instrumental in developing cell-free biosensors for environmental monitoring and diagnostics, utilizing transcription-translation reactions coupled with reporter systems to detect specific analytes. Additionally, UC researchers have created cell-free platforms for directed evolution of enzymes, allowing for rapid screening of mutant libraries without the constraints of cell viability.
Strengths: Cutting-edge research across diverse cell-free applications; strong integration between computational design and experimental implementation; extensive intellectual property portfolio; access to multidisciplinary expertise. Weaknesses: Focus on fundamental research rather than commercial applications; fragmentation of technologies across different research groups; potential challenges in technology transfer and commercialization; varying levels of optimization across different platforms.
Key Patents and Breakthroughs in Cell-Free Systems
Methods for control of FLUX in metabolic pathways through enzyme relocation
PatentWO2011140516A2
Innovation
- The method involves genetically modifying cells to relocate key enzymes in a metabolic pathway to the periplasmic space during the cell growth phase, and then combining the enzyme with cytoplasmic enzymes in a cell-free system during the production phase to control metabolic flux and increase product yield.
Cell-free system for synthesizing membrane proteins cell free method for synthesizing membrane proteins
PatentActiveUS20110244524A1
Innovation
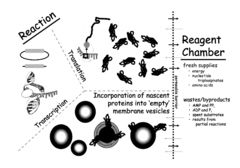
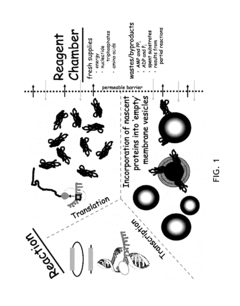
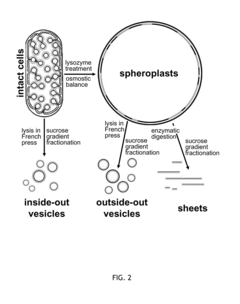
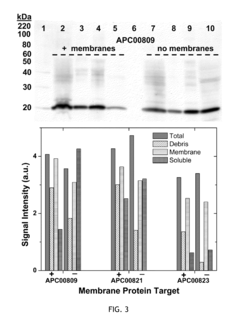
- A cell-free system utilizing Rhodobacter extracts with a coupled transcription/translation system, where intracytoplasmic membranes (ICM) from genetically modified organisms are used to produce and encapsulate heterologous membrane proteins, reducing native protein content and optimizing membrane space for maximal protein encapsulation, allowing for simultaneous production and sequestration.
Scalability and Manufacturing Challenges
Cell-free systems (CFS) in synthetic biology face significant scalability and manufacturing challenges that currently limit their widespread industrial application. Despite their promising advantages in bypassing cellular constraints, the transition from laboratory-scale experiments to industrial production remains problematic. The primary challenge lies in the economic viability of large-scale cell-free protein synthesis (CFPS), with current production costs estimated at $0.26-0.66 per milligram of protein, substantially higher than traditional cell-based systems.
The manufacturing process for cell-free systems involves complex multi-step procedures including cell cultivation, cell lysis, extract preparation, and reaction optimization. Each step introduces variability that affects reproducibility and scalability. Cell lysis methods, particularly, present a critical bottleneck - laboratory techniques like sonication or high-pressure homogenization are difficult to scale efficiently without compromising extract quality.
Extract preparation standardization represents another major hurdle. Batch-to-batch variations in extract performance significantly impact product consistency and process reliability. Recent research has focused on developing continuous-flow systems for extract preparation to address these inconsistencies, but these technologies remain in early development stages.
Energy regeneration systems within CFS constitute a substantial cost factor. ATP and other high-energy molecules required to drive protein synthesis are expensive and degrade rapidly. Current approaches using phosphoenolpyruvate (PEP) or creatine phosphate are prohibitively expensive for industrial-scale applications, necessitating the development of more cost-effective alternatives.
Reaction environment control presents additional challenges. Cell-free reactions are highly sensitive to parameters such as pH, temperature, and ion concentrations. Maintaining optimal conditions becomes increasingly difficult as reaction volumes increase, often resulting in decreased productivity at larger scales.
Storage stability remains problematic for widespread commercialization. Current cell-free extracts typically require ultra-low temperature storage (-80°C), creating logistical complications and increasing costs for distribution and long-term storage. Research into lyophilization and other preservation methods shows promise but has yet to yield commercially viable solutions.
Recent innovations addressing these challenges include the development of continuous-flow cell-free systems, which allow for substrate replenishment and product removal during synthesis. Additionally, companies like Sutro Biopharma and Greenlight Biosciences have made significant progress in scaling CFPS for pharmaceutical applications, demonstrating the potential for overcoming these manufacturing barriers through continued technological innovation and process engineering.
The manufacturing process for cell-free systems involves complex multi-step procedures including cell cultivation, cell lysis, extract preparation, and reaction optimization. Each step introduces variability that affects reproducibility and scalability. Cell lysis methods, particularly, present a critical bottleneck - laboratory techniques like sonication or high-pressure homogenization are difficult to scale efficiently without compromising extract quality.
Extract preparation standardization represents another major hurdle. Batch-to-batch variations in extract performance significantly impact product consistency and process reliability. Recent research has focused on developing continuous-flow systems for extract preparation to address these inconsistencies, but these technologies remain in early development stages.
Energy regeneration systems within CFS constitute a substantial cost factor. ATP and other high-energy molecules required to drive protein synthesis are expensive and degrade rapidly. Current approaches using phosphoenolpyruvate (PEP) or creatine phosphate are prohibitively expensive for industrial-scale applications, necessitating the development of more cost-effective alternatives.
Reaction environment control presents additional challenges. Cell-free reactions are highly sensitive to parameters such as pH, temperature, and ion concentrations. Maintaining optimal conditions becomes increasingly difficult as reaction volumes increase, often resulting in decreased productivity at larger scales.
Storage stability remains problematic for widespread commercialization. Current cell-free extracts typically require ultra-low temperature storage (-80°C), creating logistical complications and increasing costs for distribution and long-term storage. Research into lyophilization and other preservation methods shows promise but has yet to yield commercially viable solutions.
Recent innovations addressing these challenges include the development of continuous-flow cell-free systems, which allow for substrate replenishment and product removal during synthesis. Additionally, companies like Sutro Biopharma and Greenlight Biosciences have made significant progress in scaling CFPS for pharmaceutical applications, demonstrating the potential for overcoming these manufacturing barriers through continued technological innovation and process engineering.
Regulatory Framework for Cell-Free Biologics
The regulatory landscape for cell-free biologics represents a complex and evolving framework that significantly impacts the development and commercialization of synthetic biology applications. Currently, cell-free systems occupy an ambiguous position within existing regulatory structures, as they do not neatly fit into traditional categories established for living organisms or conventional pharmaceutical products. This regulatory uncertainty poses substantial challenges for researchers and companies seeking to bring cell-free technologies to market.
In the United States, the Food and Drug Administration (FDA) has begun addressing cell-free biologics through its framework for biological products, though specific guidelines remain limited. The Center for Biologics Evaluation and Research (CBER) typically oversees these products, applying risk-based approaches that consider both the manufacturing process and final product characteristics. Meanwhile, the Environmental Protection Agency (EPA) may assert jurisdiction over certain cell-free applications under the Toxic Substances Control Act, particularly when environmental release is possible.
The European regulatory framework, administered through the European Medicines Agency (EMA), has established more explicit pathways for advanced therapy medicinal products (ATMPs), which may encompass some cell-free biologics. However, classification challenges persist, with significant variations in how member states interpret and implement these regulations. The Advanced Therapies Classification Procedure offers a mechanism for developers to obtain regulatory clarity, though the process remains time-consuming.
Internationally, regulatory harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun incorporating considerations for novel biologics, including cell-free systems. However, significant disparities exist between developed and developing nations, creating a fragmented global landscape that complicates multinational development programs.
Key regulatory considerations specific to cell-free biologics include characterization requirements for cell-free extracts, quality control standards for non-living biological components, and safety assessment protocols that address potential immunogenicity concerns. Additionally, intellectual property protection presents unique challenges, as patent offices worldwide struggle to establish consistent examination standards for these innovative technologies.
Looking forward, regulatory agencies are increasingly adopting adaptive licensing approaches that allow for staged approval processes, potentially accelerating market access for cell-free applications while maintaining appropriate oversight. Industry stakeholders and academic researchers are actively engaging with regulators through public-private partnerships and formal consultation processes to shape emerging frameworks that balance innovation with safety considerations.
In the United States, the Food and Drug Administration (FDA) has begun addressing cell-free biologics through its framework for biological products, though specific guidelines remain limited. The Center for Biologics Evaluation and Research (CBER) typically oversees these products, applying risk-based approaches that consider both the manufacturing process and final product characteristics. Meanwhile, the Environmental Protection Agency (EPA) may assert jurisdiction over certain cell-free applications under the Toxic Substances Control Act, particularly when environmental release is possible.
The European regulatory framework, administered through the European Medicines Agency (EMA), has established more explicit pathways for advanced therapy medicinal products (ATMPs), which may encompass some cell-free biologics. However, classification challenges persist, with significant variations in how member states interpret and implement these regulations. The Advanced Therapies Classification Procedure offers a mechanism for developers to obtain regulatory clarity, though the process remains time-consuming.
Internationally, regulatory harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun incorporating considerations for novel biologics, including cell-free systems. However, significant disparities exist between developed and developing nations, creating a fragmented global landscape that complicates multinational development programs.
Key regulatory considerations specific to cell-free biologics include characterization requirements for cell-free extracts, quality control standards for non-living biological components, and safety assessment protocols that address potential immunogenicity concerns. Additionally, intellectual property protection presents unique challenges, as patent offices worldwide struggle to establish consistent examination standards for these innovative technologies.
Looking forward, regulatory agencies are increasingly adopting adaptive licensing approaches that allow for staged approval processes, potentially accelerating market access for cell-free applications while maintaining appropriate oversight. Industry stakeholders and academic researchers are actively engaging with regulators through public-private partnerships and formal consultation processes to shape emerging frameworks that balance innovation with safety considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!