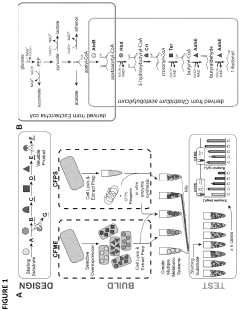
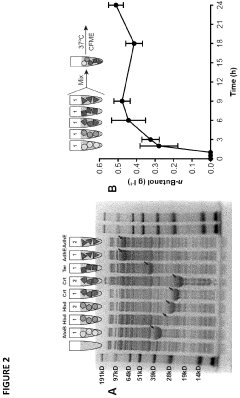
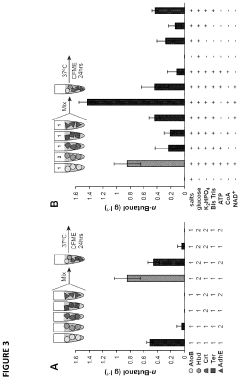
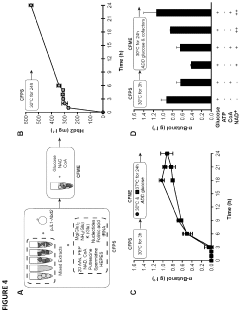
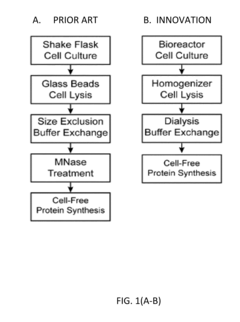
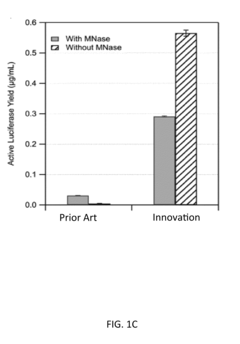
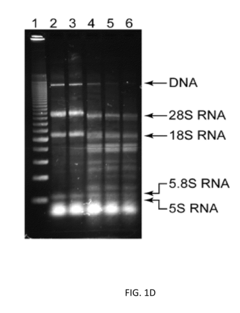
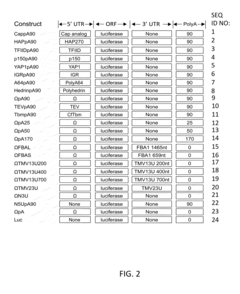
Protein engineering advancements via cell-free systems.
SEP 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Free Protein Engineering Background and Objectives
Protein engineering has evolved significantly over the past several decades, transitioning from traditional in vivo systems to more versatile cell-free platforms. The historical trajectory began with rudimentary site-directed mutagenesis techniques in the 1980s, progressing through directed evolution methodologies in the 1990s, and now embracing sophisticated cell-free systems that offer unprecedented control over protein synthesis and modification processes.
Cell-free protein engineering represents a paradigm shift in biotechnology, eliminating cellular constraints while providing direct access to the protein synthesis machinery. This approach circumvents issues related to cellular toxicity, membrane permeability barriers, and complex cellular regulatory networks that often impede conventional protein engineering efforts. The technology has roots in early cell extract preparations developed for studying fundamental biochemical processes but has since evolved into sophisticated platforms for protein production and engineering.
The current technological landscape features various cell-free systems derived from diverse organisms including Escherichia coli, wheat germ, rabbit reticulocytes, and insect cells. Each system offers distinct advantages in terms of protein folding capabilities, post-translational modifications, and scalability. Recent advancements in cell-free protein synthesis (CFPS) have dramatically improved protein yields, reduced costs, and expanded the repertoire of producible proteins.
The primary objectives of cell-free protein engineering encompass several ambitious goals. First, researchers aim to develop high-throughput screening platforms that can rapidly evaluate thousands of protein variants in parallel, significantly accelerating the protein optimization process. Second, there is a focused effort to enhance the incorporation of non-canonical amino acids, enabling the creation of proteins with novel functionalities beyond those available through the standard genetic code.
Additionally, the field seeks to improve the integration of computational design tools with experimental validation in cell-free environments, creating more predictive and efficient engineering workflows. Researchers are also working toward developing continuous-flow cell-free systems that can sustain protein synthesis for extended periods, overcoming current limitations in reaction longevity.
The ultimate technological goal is to establish cell-free protein engineering as a mainstream platform for the rapid development of therapeutic proteins, industrial enzymes, biosensors, and novel biomaterials. This includes creating standardized, modular cell-free systems that can be readily adapted to diverse protein engineering challenges across multiple industries, from pharmaceuticals to sustainable manufacturing.
Cell-free protein engineering represents a paradigm shift in biotechnology, eliminating cellular constraints while providing direct access to the protein synthesis machinery. This approach circumvents issues related to cellular toxicity, membrane permeability barriers, and complex cellular regulatory networks that often impede conventional protein engineering efforts. The technology has roots in early cell extract preparations developed for studying fundamental biochemical processes but has since evolved into sophisticated platforms for protein production and engineering.
The current technological landscape features various cell-free systems derived from diverse organisms including Escherichia coli, wheat germ, rabbit reticulocytes, and insect cells. Each system offers distinct advantages in terms of protein folding capabilities, post-translational modifications, and scalability. Recent advancements in cell-free protein synthesis (CFPS) have dramatically improved protein yields, reduced costs, and expanded the repertoire of producible proteins.
The primary objectives of cell-free protein engineering encompass several ambitious goals. First, researchers aim to develop high-throughput screening platforms that can rapidly evaluate thousands of protein variants in parallel, significantly accelerating the protein optimization process. Second, there is a focused effort to enhance the incorporation of non-canonical amino acids, enabling the creation of proteins with novel functionalities beyond those available through the standard genetic code.
Additionally, the field seeks to improve the integration of computational design tools with experimental validation in cell-free environments, creating more predictive and efficient engineering workflows. Researchers are also working toward developing continuous-flow cell-free systems that can sustain protein synthesis for extended periods, overcoming current limitations in reaction longevity.
The ultimate technological goal is to establish cell-free protein engineering as a mainstream platform for the rapid development of therapeutic proteins, industrial enzymes, biosensors, and novel biomaterials. This includes creating standardized, modular cell-free systems that can be readily adapted to diverse protein engineering challenges across multiple industries, from pharmaceuticals to sustainable manufacturing.
Market Analysis for Cell-Free Protein Engineering Applications
The global market for cell-free protein engineering applications is experiencing robust growth, driven by increasing demand for novel therapeutics, industrial enzymes, and diagnostic tools. Current market valuations indicate that the cell-free protein synthesis market reached approximately $270 million in 2022 and is projected to grow at a compound annual growth rate of 8.5% through 2030, potentially reaching $500 million by the end of the decade.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 60% of the total market share. This dominance stems from the critical role cell-free systems play in rapid prototyping of therapeutic proteins, antibodies, and vaccines. The COVID-19 pandemic significantly accelerated market growth as cell-free systems demonstrated their value in rapid vaccine development and diagnostic tool creation.
Academic research institutions constitute the second-largest market segment, utilizing cell-free protein engineering for fundamental research and educational purposes. Industrial biotechnology applications, particularly in enzyme engineering for biocatalysis and sustainable manufacturing, represent the fastest-growing segment with annual growth rates exceeding 10%.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate due to increasing biotechnology investments in China, Japan, and South Korea, alongside expanding pharmaceutical manufacturing capabilities.
Key market drivers include technological advancements in cell-free expression systems, increasing R&D investments in synthetic biology, growing demand for personalized medicine, and the shift toward sustainable manufacturing processes. The reduced development time and costs associated with cell-free systems compared to traditional cell-based methods represent significant economic advantages that are fueling market expansion.
Market challenges include high initial setup costs, technical complexities in scaling production, regulatory uncertainties for novel protein products, and competition from established cell-based protein engineering methods. Additionally, limitations in post-translational modifications in some cell-free systems remain a technical barrier for certain applications.
Emerging market opportunities include point-of-care diagnostic applications, on-demand pharmaceutical manufacturing, agricultural biotechnology applications, and educational tools. The integration of cell-free protein engineering with other emerging technologies such as artificial intelligence for protein design and microfluidics for high-throughput screening is creating new market niches with substantial growth potential.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 60% of the total market share. This dominance stems from the critical role cell-free systems play in rapid prototyping of therapeutic proteins, antibodies, and vaccines. The COVID-19 pandemic significantly accelerated market growth as cell-free systems demonstrated their value in rapid vaccine development and diagnostic tool creation.
Academic research institutions constitute the second-largest market segment, utilizing cell-free protein engineering for fundamental research and educational purposes. Industrial biotechnology applications, particularly in enzyme engineering for biocatalysis and sustainable manufacturing, represent the fastest-growing segment with annual growth rates exceeding 10%.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate due to increasing biotechnology investments in China, Japan, and South Korea, alongside expanding pharmaceutical manufacturing capabilities.
Key market drivers include technological advancements in cell-free expression systems, increasing R&D investments in synthetic biology, growing demand for personalized medicine, and the shift toward sustainable manufacturing processes. The reduced development time and costs associated with cell-free systems compared to traditional cell-based methods represent significant economic advantages that are fueling market expansion.
Market challenges include high initial setup costs, technical complexities in scaling production, regulatory uncertainties for novel protein products, and competition from established cell-based protein engineering methods. Additionally, limitations in post-translational modifications in some cell-free systems remain a technical barrier for certain applications.
Emerging market opportunities include point-of-care diagnostic applications, on-demand pharmaceutical manufacturing, agricultural biotechnology applications, and educational tools. The integration of cell-free protein engineering with other emerging technologies such as artificial intelligence for protein design and microfluidics for high-throughput screening is creating new market niches with substantial growth potential.
Current Landscape and Technical Barriers in Cell-Free Systems
Cell-free protein synthesis (CFPS) systems have emerged as powerful platforms for protein engineering, offering distinct advantages over traditional in vivo methods. Currently, the landscape of cell-free systems encompasses several established platforms derived from various organisms, including Escherichia coli, wheat germ, rabbit reticulocyte lysate, and insect cells. Each system presents unique characteristics suitable for different protein engineering applications, with E. coli-based systems dominating due to their cost-effectiveness and scalability.
The technical maturity of cell-free systems has advanced significantly in recent years, with improvements in extract preparation protocols, energy regeneration systems, and reaction optimization. Commercial kits now enable protein expression yields exceeding 2 mg/mL in batch format, representing a substantial improvement over earlier systems. Open-source protocols have democratized access to these technologies, fostering broader adoption across academic and industrial settings.
Despite these advancements, several technical barriers persist in cell-free protein engineering. Extract quality inconsistency remains a significant challenge, with batch-to-batch variations affecting reproducibility and scalability. The limited duration of cell-free reactions—typically 4-8 hours before productivity declines—constrains the synthesis of complex proteins requiring extended folding times or post-translational modifications.
Post-translational modification capabilities represent another major limitation, particularly for eukaryotic proteins. While some progress has been made in incorporating glycosylation, phosphorylation, and disulfide bond formation machinery, these systems remain less efficient than their in vivo counterparts. The absence of membrane structures in most cell-free systems also hinders the production of membrane proteins, which constitute approximately 30% of the proteome and represent important pharmaceutical targets.
Cost considerations continue to impede widespread industrial adoption, with reagent expenses—particularly for energy sources and nucleotides—remaining prohibitively high for large-scale applications. Although recent developments in continuous-exchange cell-free systems have improved economics, further cost reductions are necessary to compete with conventional fermentation-based methods.
The integration of cell-free systems with high-throughput screening technologies presents both opportunities and challenges. While cell-free systems theoretically enable rapid prototyping and testing of protein variants, practical implementation of truly high-throughput workflows remains technically demanding. Current microfluidic and automation solutions have demonstrated proof-of-concept but lack the robustness required for routine industrial applications.
Regulatory considerations also pose barriers to commercial implementation, as the regulatory framework for products developed using cell-free protein engineering remains less defined compared to traditional biotechnology platforms. This regulatory uncertainty can delay translation of promising protein candidates from research to commercial applications.
The technical maturity of cell-free systems has advanced significantly in recent years, with improvements in extract preparation protocols, energy regeneration systems, and reaction optimization. Commercial kits now enable protein expression yields exceeding 2 mg/mL in batch format, representing a substantial improvement over earlier systems. Open-source protocols have democratized access to these technologies, fostering broader adoption across academic and industrial settings.
Despite these advancements, several technical barriers persist in cell-free protein engineering. Extract quality inconsistency remains a significant challenge, with batch-to-batch variations affecting reproducibility and scalability. The limited duration of cell-free reactions—typically 4-8 hours before productivity declines—constrains the synthesis of complex proteins requiring extended folding times or post-translational modifications.
Post-translational modification capabilities represent another major limitation, particularly for eukaryotic proteins. While some progress has been made in incorporating glycosylation, phosphorylation, and disulfide bond formation machinery, these systems remain less efficient than their in vivo counterparts. The absence of membrane structures in most cell-free systems also hinders the production of membrane proteins, which constitute approximately 30% of the proteome and represent important pharmaceutical targets.
Cost considerations continue to impede widespread industrial adoption, with reagent expenses—particularly for energy sources and nucleotides—remaining prohibitively high for large-scale applications. Although recent developments in continuous-exchange cell-free systems have improved economics, further cost reductions are necessary to compete with conventional fermentation-based methods.
The integration of cell-free systems with high-throughput screening technologies presents both opportunities and challenges. While cell-free systems theoretically enable rapid prototyping and testing of protein variants, practical implementation of truly high-throughput workflows remains technically demanding. Current microfluidic and automation solutions have demonstrated proof-of-concept but lack the robustness required for routine industrial applications.
Regulatory considerations also pose barriers to commercial implementation, as the regulatory framework for products developed using cell-free protein engineering remains less defined compared to traditional biotechnology platforms. This regulatory uncertainty can delay translation of promising protein candidates from research to commercial applications.
Contemporary Cell-Free Protein Engineering Methodologies
01 Cell-free protein synthesis systems for rapid protein production
Cell-free protein synthesis systems enable rapid production of proteins without the constraints of living cells. These systems utilize extracted cellular components to perform transcription and translation in vitro, allowing for direct manipulation of reaction conditions. This approach offers advantages in speed, scalability, and the ability to produce proteins that might be toxic to living cells. Recent advancements have improved the efficiency and yield of these systems, making them valuable tools for protein engineering applications.- Cell-free protein synthesis systems for rapid protein engineering: Cell-free protein synthesis systems enable rapid protein engineering by eliminating the constraints of cell viability. These systems allow for direct manipulation of the translation machinery, incorporation of non-natural amino acids, and high-throughput screening of protein variants. The cell-free approach significantly accelerates the protein engineering cycle by bypassing time-consuming cell cultivation and extraction steps, making it ideal for directed evolution experiments and functional protein studies.
- Integration of microfluidic technologies with cell-free systems: Microfluidic platforms integrated with cell-free protein synthesis systems represent a significant advancement in protein engineering. These technologies enable miniaturization, parallelization, and automation of protein expression and screening processes. Droplet-based microfluidics allow for compartmentalization of individual reactions, facilitating high-throughput screening of protein variants with minimal reagent consumption. This integration enhances the efficiency and precision of protein engineering workflows.
- Enhanced cell-free systems with optimized components: Advanced cell-free protein synthesis systems feature optimized components to improve protein yield, folding, and functionality. These enhancements include engineered ribosomes, modified tRNA synthetases, chaperone proteins, and energy regeneration systems. By carefully controlling the biochemical environment and supplementing with specific cofactors, these systems can produce complex proteins with post-translational modifications that closely resemble those produced in living cells, expanding the range of proteins that can be engineered.
- Cell-free directed evolution techniques: Cell-free directed evolution represents a powerful approach for protein engineering that combines in vitro selection methods with cell-free protein synthesis. These techniques enable the rapid generation and screening of large protein variant libraries without cellular transformation steps. Methods such as ribosome display, mRNA display, and in vitro compartmentalization allow for the selection of proteins with desired properties from libraries of unprecedented size and diversity, accelerating the discovery of novel protein functions and improved protein variants.
- Applications of cell-free protein engineering in therapeutics and diagnostics: Cell-free protein engineering has enabled significant advancements in the development of therapeutic proteins, antibodies, vaccines, and diagnostic tools. The ability to rapidly prototype and optimize protein-based pharmaceuticals in cell-free systems accelerates the drug discovery pipeline. Additionally, cell-free systems allow for the production of proteins that would be toxic to living cells, expanding the range of potential therapeutic targets. These systems also facilitate the development of point-of-care diagnostic devices through the integration of stabilized cell-free reactions with biosensing elements.
02 High-throughput screening and directed evolution in cell-free systems
Cell-free systems provide platforms for high-throughput screening and directed evolution of proteins. By eliminating cellular growth requirements, these systems allow for rapid iterative cycles of mutation, selection, and amplification. This approach accelerates the evolution of proteins with desired properties such as improved stability, activity, or specificity. Advanced techniques combine cell-free expression with automated screening methods to efficiently explore large protein variant libraries, significantly speeding up the protein engineering process.Expand Specific Solutions03 Integration of artificial and non-canonical amino acids in engineered proteins
Cell-free systems enable the incorporation of artificial and non-canonical amino acids into proteins, expanding the chemical diversity beyond the standard 20 amino acids. This capability allows for the creation of proteins with novel functions, improved properties, or specific reactive groups for further modification. The open nature of cell-free systems permits direct addition of modified tRNAs, synthetases, and non-standard amino acids without cellular barriers, facilitating the production of proteins with precisely positioned chemical modifications.Expand Specific Solutions04 Membrane protein production and engineering
Cell-free systems offer unique advantages for the production and engineering of membrane proteins, which are often challenging to express in conventional cellular systems. These systems can be supplemented with lipids, detergents, or nanodiscs to create environments that support proper folding and function of membrane proteins. This approach enables structural studies, functional characterization, and engineering of membrane proteins for applications in drug discovery, biosensing, and synthetic biology.Expand Specific Solutions05 Miniaturized and microfluidic cell-free protein engineering platforms
Advancements in miniaturization and microfluidic technologies have enabled the development of sophisticated cell-free protein engineering platforms. These systems allow for precise control of reaction conditions, reduced reagent consumption, and parallel processing of multiple samples. Microfluidic devices integrated with cell-free expression systems facilitate rapid prototyping, high-throughput screening, and automation of protein engineering workflows. These platforms are particularly valuable for applications requiring extensive optimization or screening of large protein libraries.Expand Specific Solutions
Leading Organizations and Research Groups in the Field
Protein engineering via cell-free systems is currently in a growth phase, with increasing market adoption driven by its advantages in rapid prototyping and scalability. The global market is expanding steadily, estimated to reach significant value as applications in therapeutics, diagnostics, and industrial enzymes grow. Technologically, the field shows moderate maturity with established players like Shimadzu Corp. and Life Technologies providing instrumentation, while specialized companies such as Cellfree Sciences and Aldevron focus on cell-free protein expression platforms. Academic institutions including Northwestern University, Cornell University, and Tsinghua University are advancing fundamental research, while commercial entities like Kangma Biological Technology are developing application-specific implementations. The ecosystem demonstrates a healthy balance between established infrastructure providers and innovative startups exploring novel applications.
Northwestern University
Technical Solution: Northwestern University has developed the PURE (Protein synthesis Using Recombinant Elements) cell-free system, representing a significant advancement in protein engineering. Unlike extract-based systems, their PURE technology utilizes individually purified components of the E. coli translation machinery, creating a highly defined environment for protein synthesis. This approach enables unprecedented control over reaction conditions and eliminates interference from unwanted enzymatic activities present in crude extracts. Northwestern researchers have engineered specialized ribosomes with modified rRNA sequences that enhance the incorporation of non-canonical amino acids, expanding the chemical diversity available for protein engineering. Their platform incorporates custom-designed tRNA synthetases with altered specificity, enabling site-specific incorporation of over 200 different non-standard amino acids into proteins. The university has also pioneered computational tools that optimize genetic sequences specifically for cell-free expression, significantly improving yields for difficult targets. Additionally, they've developed microfluidic devices that enable continuous-flow cell-free protein synthesis, dramatically extending reaction lifetimes and increasing yields by over 10-fold compared to batch reactions.
Strengths: Unparalleled control over reaction components enabling precise manipulation of the translation process; exceptional purity of synthesized proteins; superior capability for incorporating non-canonical amino acids. Weaknesses: Higher cost and technical complexity compared to extract-based systems; lower protein yields for some targets; requires specialized expertise to implement effectively.
Life Technologies Corp.
Technical Solution: Life Technologies has developed the ExpiCHO™ and Expi293™ cell-free protein synthesis platforms that leverage optimized mammalian cell extracts to produce complex proteins with authentic post-translational modifications. Their technology incorporates proprietary reaction components including specialized translation factors and chaperones that enhance folding efficiency of complex multi-domain proteins. The company has engineered custom redox environments within their cell-free reactions to facilitate proper disulfide bond formation, critical for many therapeutic proteins. Their platform features pre-optimized reaction conditions for different protein classes, reducing development time for new targets. Life Technologies has also pioneered high-throughput screening applications of their cell-free systems, enabling rapid testing of thousands of protein variants in parallel using miniaturized reaction formats. The technology supports both batch and continuous-exchange cell-free protein synthesis modes, with the latter enabling significantly higher protein yields through continuous supply of substrates and removal of inhibitory byproducts.
Strengths: Superior capability for producing proteins requiring complex post-translational modifications; excellent reproducibility across different protein targets; comprehensive technical support infrastructure. Weaknesses: Higher cost compared to bacterial cell-free systems; more complex setup requirements; limited shelf-life of reaction components requiring cold-chain logistics.
Critical Patents and Publications in Cell-Free Systems
Cell-free protein synthesis driven metabolic engineering
PatentActiveUS11913052B2
Innovation
- The development of cell-free systems and methods for metabolic engineering that allow for the expression of enzymes outside cells, enabling the optimization of biosynthetic pathways through cell-free protein synthesis and combinatorial approaches to reduce the costs and time associated with traditional metabolic engineering methods.
Methods for Cell-Free Protein Synthesis
PatentActiveUS20140295492A1
Innovation
- A novel cell-free protein synthesis platform using Saccharomyces cerevisiae cellular extract, optimized with mid-exponential to late-exponential phase cultures, combined transcription/translation reactions, and specific reaction conditions to enhance protein synthesis yield and reduce costs, leveraging cap-independent translation elements and linear DNA templates for high-throughput protein expression.
Biosafety and Regulatory Considerations for Cell-Free Technologies
Cell-free protein engineering systems present unique biosafety and regulatory challenges that differ significantly from traditional cell-based approaches. These systems operate outside the constraints of living cells, eliminating concerns about organism escape or environmental contamination through reproduction. However, this advantage does not exempt cell-free technologies from rigorous safety assessment and regulatory oversight.
The regulatory landscape for cell-free protein engineering remains in development, with frameworks primarily adapted from existing biotechnology regulations. Current approaches typically evaluate these technologies based on their end products rather than the production process itself. This creates a regulatory gap where the unique aspects of cell-free systems may not be adequately addressed by conventional frameworks designed for whole-cell systems.
Biosafety considerations for cell-free protein engineering include potential bioactive molecule release, unintended interactions with biological systems, and the possibility of reconstitution into viable systems. The absence of cellular containment means engineered proteins or nucleic acids could potentially interact with environmental components in unpredicted ways. Regulatory bodies increasingly recognize these distinct risk profiles and are developing specialized guidelines.
International harmonization of regulatory approaches presents another significant challenge. Different jurisdictions apply varying standards to cell-free technologies, creating compliance complexities for global research and commercialization efforts. Organizations such as the International Organization for Standardization (ISO) and the World Health Organization (WHO) are working to establish standardized frameworks specifically addressing cell-free biotechnologies.
Risk assessment methodologies for cell-free systems require adaptation from traditional approaches. Current best practices include comprehensive characterization of starting materials, process validation with defined containment strategies, and thorough product purification protocols. The development of standardized safety testing protocols specifically designed for cell-free produced proteins represents an ongoing area of regulatory evolution.
Intellectual property considerations intersect with regulatory compliance in this domain. Patent landscapes around cell-free technologies often include safety mechanisms and containment strategies as key components of protected innovations. This creates a complex interplay between proprietary technologies and regulatory requirements that researchers and companies must navigate carefully.
Looking forward, the regulatory framework for cell-free protein engineering will likely evolve toward a more tailored approach that recognizes the unique characteristics of these systems while maintaining appropriate safety standards. Proactive engagement between researchers, industry stakeholders, and regulatory authorities will be essential to develop proportionate, science-based regulations that enable innovation while protecting public health and environmental safety.
The regulatory landscape for cell-free protein engineering remains in development, with frameworks primarily adapted from existing biotechnology regulations. Current approaches typically evaluate these technologies based on their end products rather than the production process itself. This creates a regulatory gap where the unique aspects of cell-free systems may not be adequately addressed by conventional frameworks designed for whole-cell systems.
Biosafety considerations for cell-free protein engineering include potential bioactive molecule release, unintended interactions with biological systems, and the possibility of reconstitution into viable systems. The absence of cellular containment means engineered proteins or nucleic acids could potentially interact with environmental components in unpredicted ways. Regulatory bodies increasingly recognize these distinct risk profiles and are developing specialized guidelines.
International harmonization of regulatory approaches presents another significant challenge. Different jurisdictions apply varying standards to cell-free technologies, creating compliance complexities for global research and commercialization efforts. Organizations such as the International Organization for Standardization (ISO) and the World Health Organization (WHO) are working to establish standardized frameworks specifically addressing cell-free biotechnologies.
Risk assessment methodologies for cell-free systems require adaptation from traditional approaches. Current best practices include comprehensive characterization of starting materials, process validation with defined containment strategies, and thorough product purification protocols. The development of standardized safety testing protocols specifically designed for cell-free produced proteins represents an ongoing area of regulatory evolution.
Intellectual property considerations intersect with regulatory compliance in this domain. Patent landscapes around cell-free technologies often include safety mechanisms and containment strategies as key components of protected innovations. This creates a complex interplay between proprietary technologies and regulatory requirements that researchers and companies must navigate carefully.
Looking forward, the regulatory framework for cell-free protein engineering will likely evolve toward a more tailored approach that recognizes the unique characteristics of these systems while maintaining appropriate safety standards. Proactive engagement between researchers, industry stakeholders, and regulatory authorities will be essential to develop proportionate, science-based regulations that enable innovation while protecting public health and environmental safety.
Scalability and Industrial Implementation Challenges
Despite the promising advancements in cell-free protein engineering, scaling these systems from laboratory to industrial implementation presents significant challenges. Current cell-free protein synthesis (CFPS) platforms typically operate at microliter to milliliter scales, which are adequate for research but insufficient for commercial production. The transition to industrial volumes requires overcoming several technical hurdles related to reaction efficiency, component stability, and economic viability.
One primary challenge is maintaining consistent protein yield and quality during scale-up. As reaction volumes increase, issues such as oxygen transfer limitations, heat dissipation, and mixing inefficiencies become more pronounced. These factors can lead to heterogeneous reaction environments that compromise protein folding and functionality. Companies like Sutro Biopharma and GreenLight Biosciences have made progress in addressing these issues through specialized bioreactor designs, but standardized solutions remain elusive.
The economic aspects of scaling cell-free systems present another significant barrier. The cost of extract preparation and nucleotide triphosphates (NTPs) remains prohibitively high for many applications. Current estimates suggest that cell-free protein production costs range from $50 to $1000 per gram of protein, significantly higher than conventional cell-based systems. Reducing these costs requires innovations in extract preparation methods, energy regeneration systems, and feedstock utilization.
Supply chain considerations also impact industrial implementation. Reliable sources of high-quality components, including engineered enzymes, tRNAs, and ribosomes, are essential for consistent production. The development of robust supply networks and quality control standards represents a critical step toward industrial viability. Companies like Arbor Biosciences and New England Biolabs have begun addressing this need by offering standardized reagents for cell-free applications.
Regulatory frameworks present additional complexity for industrial adoption. Current regulations for biopharmaceuticals and other protein products are primarily designed for cell-based production systems. Establishing appropriate quality control metrics, validation protocols, and regulatory pathways for cell-free produced proteins requires collaborative efforts between industry stakeholders and regulatory agencies. The FDA and EMA have begun preliminary discussions on this topic, but comprehensive guidelines remain under development.
Despite these challenges, several companies have made significant progress in scaling cell-free systems. Sutro Biopharma has developed a 100-liter scale platform for therapeutic protein production, while Synvitrobio (now Tierra Biosciences) has created automated platforms for parallel protein synthesis at intermediate scales. These pioneering efforts demonstrate the potential for overcoming current limitations through continued technological innovation and strategic investment.
One primary challenge is maintaining consistent protein yield and quality during scale-up. As reaction volumes increase, issues such as oxygen transfer limitations, heat dissipation, and mixing inefficiencies become more pronounced. These factors can lead to heterogeneous reaction environments that compromise protein folding and functionality. Companies like Sutro Biopharma and GreenLight Biosciences have made progress in addressing these issues through specialized bioreactor designs, but standardized solutions remain elusive.
The economic aspects of scaling cell-free systems present another significant barrier. The cost of extract preparation and nucleotide triphosphates (NTPs) remains prohibitively high for many applications. Current estimates suggest that cell-free protein production costs range from $50 to $1000 per gram of protein, significantly higher than conventional cell-based systems. Reducing these costs requires innovations in extract preparation methods, energy regeneration systems, and feedstock utilization.
Supply chain considerations also impact industrial implementation. Reliable sources of high-quality components, including engineered enzymes, tRNAs, and ribosomes, are essential for consistent production. The development of robust supply networks and quality control standards represents a critical step toward industrial viability. Companies like Arbor Biosciences and New England Biolabs have begun addressing this need by offering standardized reagents for cell-free applications.
Regulatory frameworks present additional complexity for industrial adoption. Current regulations for biopharmaceuticals and other protein products are primarily designed for cell-based production systems. Establishing appropriate quality control metrics, validation protocols, and regulatory pathways for cell-free produced proteins requires collaborative efforts between industry stakeholders and regulatory agencies. The FDA and EMA have begun preliminary discussions on this topic, but comprehensive guidelines remain under development.
Despite these challenges, several companies have made significant progress in scaling cell-free systems. Sutro Biopharma has developed a 100-liter scale platform for therapeutic protein production, while Synvitrobio (now Tierra Biosciences) has created automated platforms for parallel protein synthesis at intermediate scales. These pioneering efforts demonstrate the potential for overcoming current limitations through continued technological innovation and strategic investment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!