Standardization challenges in cell-free biomanufacturing.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Biomanufacturing Background and Objectives
Cell-free biomanufacturing represents a paradigm shift in biotechnology, emerging from decades of research in cell-free protein synthesis (CFPS) that began with Nirenberg and Matthaei's groundbreaking work in the 1960s. This technology has evolved from a research tool for understanding fundamental biological processes to a promising manufacturing platform with diverse applications across pharmaceuticals, diagnostics, and sustainable chemical production.
The field has experienced accelerated development over the past decade, driven by advances in synthetic biology, metabolic engineering, and analytical technologies. The transition from traditional cell-based manufacturing to cell-free systems offers significant advantages, including rapid production cycles, simplified purification processes, and the ability to produce compounds toxic to living cells.
Despite these advantages, cell-free biomanufacturing faces critical standardization challenges that impede its widespread industrial adoption. The lack of standardized protocols for extract preparation, reaction conditions, and quality control creates significant variability in production outcomes. This inconsistency hampers reproducibility across laboratories and scales, presenting a major obstacle to commercial viability.
The primary technical objective in this domain is to establish robust standardization frameworks that enable consistent, scalable, and cost-effective cell-free biomanufacturing processes. This includes developing standardized methods for cell extract preparation, defining universal metrics for extract quality assessment, and creating standardized reaction formats and conditions that ensure reproducible performance.
Additionally, there is a pressing need to standardize analytical methods for monitoring and characterizing cell-free reactions, as well as establishing common reporting formats to facilitate data sharing and comparison across the scientific community. These standardization efforts must balance the need for consistency with the flexibility required to accommodate diverse applications and production targets.
The evolution of cell-free systems has been marked by several technological breakthroughs, including the development of continuous-exchange cell-free (CECF) systems, the integration of non-canonical amino acids, and the creation of lyophilized cell-free reaction components that enhance stability and portability. These innovations have expanded the potential applications while simultaneously increasing the complexity of standardization requirements.
Looking forward, the field aims to transition from laboratory-scale demonstrations to industrial implementation, requiring standardized scaling methodologies and regulatory frameworks. The ultimate goal is to establish cell-free biomanufacturing as a mainstream production platform that complements traditional bioprocessing approaches, offering unique advantages for rapid prototyping, distributed manufacturing, and on-demand production of critical biologics and chemicals.
The field has experienced accelerated development over the past decade, driven by advances in synthetic biology, metabolic engineering, and analytical technologies. The transition from traditional cell-based manufacturing to cell-free systems offers significant advantages, including rapid production cycles, simplified purification processes, and the ability to produce compounds toxic to living cells.
Despite these advantages, cell-free biomanufacturing faces critical standardization challenges that impede its widespread industrial adoption. The lack of standardized protocols for extract preparation, reaction conditions, and quality control creates significant variability in production outcomes. This inconsistency hampers reproducibility across laboratories and scales, presenting a major obstacle to commercial viability.
The primary technical objective in this domain is to establish robust standardization frameworks that enable consistent, scalable, and cost-effective cell-free biomanufacturing processes. This includes developing standardized methods for cell extract preparation, defining universal metrics for extract quality assessment, and creating standardized reaction formats and conditions that ensure reproducible performance.
Additionally, there is a pressing need to standardize analytical methods for monitoring and characterizing cell-free reactions, as well as establishing common reporting formats to facilitate data sharing and comparison across the scientific community. These standardization efforts must balance the need for consistency with the flexibility required to accommodate diverse applications and production targets.
The evolution of cell-free systems has been marked by several technological breakthroughs, including the development of continuous-exchange cell-free (CECF) systems, the integration of non-canonical amino acids, and the creation of lyophilized cell-free reaction components that enhance stability and portability. These innovations have expanded the potential applications while simultaneously increasing the complexity of standardization requirements.
Looking forward, the field aims to transition from laboratory-scale demonstrations to industrial implementation, requiring standardized scaling methodologies and regulatory frameworks. The ultimate goal is to establish cell-free biomanufacturing as a mainstream production platform that complements traditional bioprocessing approaches, offering unique advantages for rapid prototyping, distributed manufacturing, and on-demand production of critical biologics and chemicals.
Market Analysis for Cell-free Production Systems
The global market for cell-free production systems has experienced significant growth in recent years, driven by increasing demand for sustainable biomanufacturing solutions. As of 2023, the market was valued at approximately $1.2 billion, with projections indicating a compound annual growth rate (CAGR) of 9.8% through 2030. This growth trajectory reflects the expanding applications of cell-free systems across pharmaceutical, industrial enzyme, and specialty chemical sectors.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 45% of the total market share. This segment's prominence stems from the advantages cell-free systems offer in producing complex therapeutic proteins, vaccines, and personalized medicines with reduced contamination risks and faster production cycles compared to traditional cell-based methods.
Industrial biotechnology represents the fastest-growing segment, with a projected CAGR of 12.3% through 2030. This acceleration is primarily attributed to increasing adoption of sustainable manufacturing practices and the versatility of cell-free systems in producing industrial enzymes, biofuels, and biomaterials.
Regionally, North America leads the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea, alongside expanding biomanufacturing capabilities in Singapore and India.
Key market drivers include technological advancements in extract preparation and reaction optimization, growing demand for rapid protein production platforms, and increasing focus on sustainable manufacturing processes. The COVID-19 pandemic has further accelerated market growth by highlighting the need for agile biomanufacturing platforms capable of rapid response to emerging biological threats.
Despite promising growth prospects, several factors constrain market expansion. High production costs, limited scalability of current systems, and lack of standardized protocols represent significant barriers. Additionally, regulatory uncertainties surrounding products manufactured using cell-free systems pose challenges for commercial adoption across different regions.
The competitive landscape features a mix of established biotechnology companies, specialized cell-free technology providers, and academic spin-offs. Strategic collaborations between technology developers and end-users are becoming increasingly common, aimed at overcoming technical barriers and expanding application areas.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 45% of the total market share. This segment's prominence stems from the advantages cell-free systems offer in producing complex therapeutic proteins, vaccines, and personalized medicines with reduced contamination risks and faster production cycles compared to traditional cell-based methods.
Industrial biotechnology represents the fastest-growing segment, with a projected CAGR of 12.3% through 2030. This acceleration is primarily attributed to increasing adoption of sustainable manufacturing practices and the versatility of cell-free systems in producing industrial enzymes, biofuels, and biomaterials.
Regionally, North America leads the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea, alongside expanding biomanufacturing capabilities in Singapore and India.
Key market drivers include technological advancements in extract preparation and reaction optimization, growing demand for rapid protein production platforms, and increasing focus on sustainable manufacturing processes. The COVID-19 pandemic has further accelerated market growth by highlighting the need for agile biomanufacturing platforms capable of rapid response to emerging biological threats.
Despite promising growth prospects, several factors constrain market expansion. High production costs, limited scalability of current systems, and lack of standardized protocols represent significant barriers. Additionally, regulatory uncertainties surrounding products manufactured using cell-free systems pose challenges for commercial adoption across different regions.
The competitive landscape features a mix of established biotechnology companies, specialized cell-free technology providers, and academic spin-offs. Strategic collaborations between technology developers and end-users are becoming increasingly common, aimed at overcoming technical barriers and expanding application areas.
Technical Barriers in Standardization
Despite significant advancements in cell-free biomanufacturing, standardization remains a critical challenge that impedes widespread adoption and commercialization. The absence of universally accepted protocols creates substantial barriers to reproducibility across different laboratories and manufacturing facilities. Current cell-free systems exhibit significant batch-to-batch variability, with extract preparation methods differing widely between research groups, resulting in inconsistent protein yields and functionality.
Technical standardization is further complicated by the diverse range of cell-free expression systems available, including those derived from E. coli, wheat germ, rabbit reticulocytes, and insect cells. Each system possesses unique characteristics regarding protein folding capabilities, post-translational modifications, and expression efficiency, making direct comparisons and standardization exceptionally difficult.
The analytical methods used to characterize cell-free reactions present another significant barrier. Different laboratories employ varying techniques for measuring protein yield, activity, and quality, creating challenges in comparing results across studies. The lack of standardized metrics for assessing reaction efficiency and product quality hampers objective evaluation of different cell-free platforms and optimization strategies.
Component sourcing represents a major standardization hurdle, as reagents obtained from different suppliers often exhibit varying levels of purity and activity. Critical components such as nucleotides, amino acids, and energy regeneration systems can significantly impact reaction performance, yet standardized specifications for these inputs remain underdeveloped.
Scale-up processes introduce additional standardization challenges, as parameters optimized at small laboratory scales frequently fail to translate effectively to industrial production volumes. The physical and biochemical dynamics of cell-free reactions change substantially with increasing volume, requiring recalibration of numerous parameters including mixing strategies, temperature control, and feeding regimes.
Regulatory frameworks for cell-free biomanufacturing remain underdeveloped, with limited guidance on quality control standards and validation requirements. This regulatory uncertainty creates significant barriers for companies seeking to commercialize cell-free products, particularly in highly regulated sectors such as pharmaceuticals and food production.
The integration of cell-free systems with automated platforms presents standardization challenges related to hardware-software compatibility and process control. Different automation systems utilize proprietary interfaces and control algorithms, complicating the development of standardized workflows that can be readily transferred between different manufacturing facilities or research institutions.
Technical standardization is further complicated by the diverse range of cell-free expression systems available, including those derived from E. coli, wheat germ, rabbit reticulocytes, and insect cells. Each system possesses unique characteristics regarding protein folding capabilities, post-translational modifications, and expression efficiency, making direct comparisons and standardization exceptionally difficult.
The analytical methods used to characterize cell-free reactions present another significant barrier. Different laboratories employ varying techniques for measuring protein yield, activity, and quality, creating challenges in comparing results across studies. The lack of standardized metrics for assessing reaction efficiency and product quality hampers objective evaluation of different cell-free platforms and optimization strategies.
Component sourcing represents a major standardization hurdle, as reagents obtained from different suppliers often exhibit varying levels of purity and activity. Critical components such as nucleotides, amino acids, and energy regeneration systems can significantly impact reaction performance, yet standardized specifications for these inputs remain underdeveloped.
Scale-up processes introduce additional standardization challenges, as parameters optimized at small laboratory scales frequently fail to translate effectively to industrial production volumes. The physical and biochemical dynamics of cell-free reactions change substantially with increasing volume, requiring recalibration of numerous parameters including mixing strategies, temperature control, and feeding regimes.
Regulatory frameworks for cell-free biomanufacturing remain underdeveloped, with limited guidance on quality control standards and validation requirements. This regulatory uncertainty creates significant barriers for companies seeking to commercialize cell-free products, particularly in highly regulated sectors such as pharmaceuticals and food production.
The integration of cell-free systems with automated platforms presents standardization challenges related to hardware-software compatibility and process control. Different automation systems utilize proprietary interfaces and control algorithms, complicating the development of standardized workflows that can be readily transferred between different manufacturing facilities or research institutions.
Current Standardization Approaches
01 Standardized cell-free protein synthesis systems
Cell-free protein synthesis systems have been developed with standardized components and protocols to ensure reproducibility and scalability in biomanufacturing. These systems typically include purified enzymes, energy regeneration components, and translation machinery that can be precisely controlled. Standardization of these systems enables consistent protein production across different batches and laboratories, which is crucial for industrial applications and regulatory compliance.- Standardized cell-free protein synthesis systems: Cell-free protein synthesis systems have been developed with standardized components and protocols to ensure reproducibility in biomanufacturing. These systems typically include purified enzymes, energy regeneration components, and translation machinery that can be assembled in controlled ratios. Standardization of these systems allows for consistent protein production across different batches and laboratories, which is crucial for industrial applications and regulatory compliance.
- Quality control and measurement standards for cell-free systems: Establishing quality control measures and measurement standards is essential for cell-free biomanufacturing. This includes standardized methods for assessing the activity of cell extracts, quantifying protein yield, and evaluating product purity. Advanced analytical techniques are employed to monitor reaction kinetics and ensure batch-to-batch consistency. These standardized measurements enable reliable comparison of results across different research groups and manufacturing facilities.
- Standardized genetic parts and expression templates: Standardization of genetic components used in cell-free systems is critical for reproducible biomanufacturing. This includes the development of standardized DNA templates, promoters, ribosome binding sites, and terminators that can be reliably assembled and function predictably in cell-free environments. These standardized genetic parts enable modular design approaches and facilitate the rapid prototyping and optimization of biosynthetic pathways for various applications.
- Automation and high-throughput standardization: Automation technologies have been developed to standardize cell-free biomanufacturing processes at scale. These include robotic platforms for reaction setup, monitoring, and analysis that minimize human error and increase throughput. Standardized microfluidic devices and bioreactor designs enable consistent reaction conditions across multiple scales. These automated systems incorporate standardized protocols and data collection methods to ensure reproducibility and facilitate process optimization.
- Regulatory frameworks and industry standards: Regulatory frameworks and industry standards are being established to govern cell-free biomanufacturing processes. These include guidelines for documentation, validation protocols, and quality assurance measures specific to cell-free systems. International standardization organizations are working to develop consensus standards for terminology, methods, and performance criteria. These regulatory standards are essential for commercial adoption of cell-free biomanufacturing technologies and ensuring product safety and efficacy.
02 Quality control and measurement standards for cell-free systems
Standardized methods for quality control and measurement in cell-free biomanufacturing have been established to ensure product consistency and safety. These include analytical techniques for monitoring reaction kinetics, protein yield, and purity. Standardized assays and reference materials allow for reliable comparison of results across different laboratories and manufacturing facilities, supporting regulatory compliance and process validation in cell-free production platforms.Expand Specific Solutions03 Standardized genetic parts and expression vectors
The development of standardized genetic parts and expression vectors has significantly advanced cell-free biomanufacturing. These standardized components include promoters, ribosome binding sites, and terminators that have been characterized for predictable performance in cell-free systems. Libraries of these standardized genetic elements enable rapid prototyping and optimization of expression constructs, facilitating the efficient production of proteins and other biomolecules in cell-free environments.Expand Specific Solutions04 Automated platforms for cell-free biomanufacturing
Automated platforms have been developed to standardize cell-free biomanufacturing processes. These systems integrate liquid handling, reaction monitoring, and data analysis to ensure consistent production conditions. Automation reduces human error and increases throughput, enabling scalable and reproducible manufacturing of biologics. Standardized interfaces and protocols for these automated platforms facilitate technology transfer between research and production environments.Expand Specific Solutions05 Regulatory frameworks and industry standards
Regulatory frameworks and industry standards have been established to guide the implementation of cell-free biomanufacturing processes. These standards address critical aspects such as raw material qualification, process validation, and product characterization. Harmonized guidelines ensure that cell-free produced biologics meet safety and efficacy requirements across different regulatory jurisdictions, facilitating global market access and adoption of cell-free manufacturing technologies.Expand Specific Solutions
Leading Organizations in Cell-free Biomanufacturing
Cell-free biomanufacturing standardization faces significant challenges in a rapidly evolving market currently transitioning from early development to commercial application. The industry, valued at approximately $100-150 million, is projected to grow substantially as technologies mature. Companies like Multiply Labs, Cellino Biotech, and GreenLight Biosciences are pioneering commercial applications, while established players such as Genentech and Lonza Walkersville provide institutional expertise. Academic-industry partnerships, exemplified by collaborations between Tsinghua University and Just-Evotec Biologics, are accelerating technological maturation. However, the field still struggles with reproducibility issues, quality control standardization, and regulatory framework development, requiring coordinated efforts across stakeholders to establish industry-wide protocols that balance innovation with reliability.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary WEPRO® cell-free protein synthesis system based on wheat germ extract, addressing standardization challenges through their ENDEXT® Technology. This platform provides consistent protein expression by utilizing standardized wheat germ extract components and optimized reaction conditions. Their approach includes comprehensive quality control measures for raw materials, standardized extract preparation protocols, and automated production systems that ensure batch-to-batch consistency. The company has implemented real-time monitoring systems for critical parameters during cell-free reactions, allowing for precise control of temperature, pH, and nutrient availability. Additionally, they've developed specialized reaction vessels designed to maintain optimal conditions throughout the manufacturing process, significantly reducing variability in protein yield and quality.
Strengths: Highly reproducible wheat germ-based system with lower endogenous nuclease activity compared to E. coli systems; excellent for producing complex eukaryotic proteins. Weaknesses: Limited scalability compared to some bacterial systems; higher production costs than bacterial-based cell-free systems; potentially lower protein yields for certain applications.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has pioneered a cell-free biomanufacturing platform specifically designed for RNA production, addressing standardization challenges through their proprietary cell-free transcription technology. Their system utilizes highly purified enzymes and nucleotides in a controlled environment to produce RNA molecules with exceptional consistency. The company has developed standardized protocols for component preparation, quality assessment, and reaction optimization that ensure reproducibility across manufacturing scales. Their platform incorporates automated sampling and real-time analytics to monitor critical quality attributes throughout the production process. GreenLight has also implemented a comprehensive digital infrastructure that tracks every aspect of production, from raw material sourcing to final product testing, creating a complete audit trail that supports standardization efforts and regulatory compliance.
Strengths: Specialized in RNA production with high purity and consistency; scalable manufacturing process suitable for commercial applications; reduced contamination risk compared to cell-based systems. Weaknesses: Limited to RNA products rather than proteins; requires highly purified and potentially expensive input materials; technology may be less versatile for diverse biomolecule production.
Key Patents and Innovations
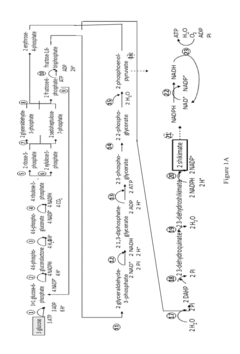
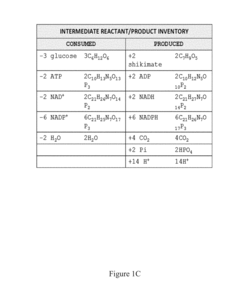
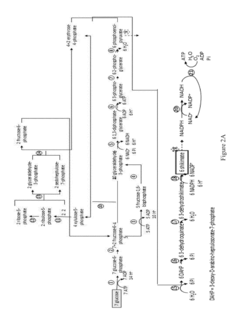
Methods for control of flux in metabolic pathways
PatentInactiveUS20170247724A1
Innovation
- Genetically modifying microbial cells to make mRNA encoding enzymes resistant to ribonuclease activity, allowing for controlled manipulation of key pathway enzymes during growth and production phases, and using interferase-resistant enzymes to maintain or alter enzyme concentrations, thereby avoiding stress responses and optimizing metabolic flux.
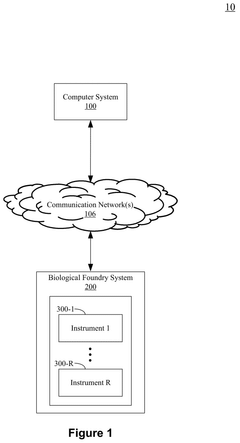
System, method, and apparatus facilitating automated modular manufacture of cell therapy
PatentPendingUS20250257302A1
Innovation
- A modular biological foundry system with a docking device for portable devices, enabling automated, parallelized manufacturing through robotic material transfer and a parallel architecture, allowing for high-throughput, flexible, and scalable production of cellular engineering targets.
Regulatory Framework for Biomanufacturing
The regulatory landscape for cell-free biomanufacturing remains in a nascent stage, presenting significant challenges for industry stakeholders. Current regulatory frameworks were primarily designed for traditional cell-based manufacturing processes, creating a misalignment when applied to cell-free systems. This regulatory gap necessitates urgent attention as the field advances toward commercial applications.
The FDA and EMA have begun addressing these challenges through guidance documents that acknowledge the unique aspects of cell-free systems. However, these guidelines often lack specificity, leaving manufacturers to navigate ambiguous requirements. The absence of standardized protocols for quality control, safety assessment, and product characterization creates regulatory uncertainty that impedes investment and commercialization efforts.
Regulatory considerations for cell-free biomanufacturing span multiple domains, including raw material sourcing, process validation, and product classification. Cell lysates and cell-free reaction components require stringent quality standards, yet current regulations provide limited direction on acceptable sourcing practices or purity requirements. This regulatory ambiguity increases compliance risks and development costs.
Process validation presents another regulatory challenge, as traditional validation approaches may not adequately address the unique aspects of cell-free systems. Regulatory bodies increasingly request comprehensive data on process reproducibility and scalability, but without established standards, manufacturers struggle to determine sufficient validation parameters.
Product classification further complicates the regulatory landscape. Cell-free produced biologics may fall under different regulatory categories depending on their production method rather than their molecular identity, creating inconsistent approval pathways across different jurisdictions. This regulatory fragmentation impedes global market access and increases compliance complexity.
International harmonization efforts have emerged through organizations like the International Council for Harmonisation (ICH) and the World Health Organization (WHO). These initiatives aim to develop globally recognized standards for cell-free biomanufacturing, though progress remains slow due to the technical complexity and diverse stakeholder interests involved.
Industry consortia and public-private partnerships have begun developing voluntary standards to fill regulatory gaps. These collaborative efforts focus on establishing common terminology, analytical methods, and quality benchmarks that could eventually inform formal regulatory frameworks. Such pre-competitive collaboration represents a promising approach to accelerating regulatory development while ensuring scientific rigor and practical applicability.
The FDA and EMA have begun addressing these challenges through guidance documents that acknowledge the unique aspects of cell-free systems. However, these guidelines often lack specificity, leaving manufacturers to navigate ambiguous requirements. The absence of standardized protocols for quality control, safety assessment, and product characterization creates regulatory uncertainty that impedes investment and commercialization efforts.
Regulatory considerations for cell-free biomanufacturing span multiple domains, including raw material sourcing, process validation, and product classification. Cell lysates and cell-free reaction components require stringent quality standards, yet current regulations provide limited direction on acceptable sourcing practices or purity requirements. This regulatory ambiguity increases compliance risks and development costs.
Process validation presents another regulatory challenge, as traditional validation approaches may not adequately address the unique aspects of cell-free systems. Regulatory bodies increasingly request comprehensive data on process reproducibility and scalability, but without established standards, manufacturers struggle to determine sufficient validation parameters.
Product classification further complicates the regulatory landscape. Cell-free produced biologics may fall under different regulatory categories depending on their production method rather than their molecular identity, creating inconsistent approval pathways across different jurisdictions. This regulatory fragmentation impedes global market access and increases compliance complexity.
International harmonization efforts have emerged through organizations like the International Council for Harmonisation (ICH) and the World Health Organization (WHO). These initiatives aim to develop globally recognized standards for cell-free biomanufacturing, though progress remains slow due to the technical complexity and diverse stakeholder interests involved.
Industry consortia and public-private partnerships have begun developing voluntary standards to fill regulatory gaps. These collaborative efforts focus on establishing common terminology, analytical methods, and quality benchmarks that could eventually inform formal regulatory frameworks. Such pre-competitive collaboration represents a promising approach to accelerating regulatory development while ensuring scientific rigor and practical applicability.
Scalability and Cost Considerations
Scaling up cell-free biomanufacturing systems presents significant economic and technical challenges that must be addressed for widespread industrial adoption. Current laboratory-scale cell-free systems typically operate at volumes between 10μL to 100mL, while industrial applications require scaling to tens or hundreds of liters. This substantial volume increase introduces numerous complexities in maintaining reaction efficiency and consistency.
The economic considerations of cell-free systems remain a critical barrier to commercialization. Extract preparation costs, particularly for crude cell extracts, can range from $50 to $1000 per liter depending on the source organism and preparation method. These costs are primarily driven by expensive reagents such as nucleotides, energy regeneration components, and specialized enzymes. Additionally, the limited shelf-life of cell-free components necessitates frequent production cycles, further increasing operational expenses.
Reaction yield optimization represents another significant challenge in scaling cell-free systems. As reaction volumes increase, issues such as oxygen transfer limitations, heat dissipation, and mixing inefficiencies become more pronounced. These factors can lead to decreased productivity and increased variability in larger-scale operations. Current data indicates that productivity often decreases by 30-50% when scaling from milliliter to liter volumes without appropriate engineering interventions.
Infrastructure requirements for large-scale cell-free manufacturing also present substantial capital expenditure considerations. Specialized bioreactors with precise temperature control, continuous monitoring capabilities, and appropriate mixing technologies are necessary but represent significant investments. The absence of standardized equipment specifically designed for cell-free processes further complicates scaling efforts and increases costs.
Recent economic analyses suggest that cell-free biomanufacturing becomes cost-competitive with traditional cell-based methods only at certain production scales and for specific high-value products. For instance, pharmaceutical proteins and specialized enzymes with market values exceeding $1000 per gram may justify the higher production costs. However, for lower-value products, significant cost reductions in extract preparation and reaction components are still required.
Emerging approaches to address these challenges include continuous processing methods, which can reduce reagent requirements by up to 70% compared to batch processes. Additionally, the development of lyophilized cell-free systems has extended shelf-life from days to months, potentially reducing production frequency and associated costs. These innovations, combined with ongoing efforts to standardize production methods, offer promising pathways toward economically viable large-scale cell-free biomanufacturing.
The economic considerations of cell-free systems remain a critical barrier to commercialization. Extract preparation costs, particularly for crude cell extracts, can range from $50 to $1000 per liter depending on the source organism and preparation method. These costs are primarily driven by expensive reagents such as nucleotides, energy regeneration components, and specialized enzymes. Additionally, the limited shelf-life of cell-free components necessitates frequent production cycles, further increasing operational expenses.
Reaction yield optimization represents another significant challenge in scaling cell-free systems. As reaction volumes increase, issues such as oxygen transfer limitations, heat dissipation, and mixing inefficiencies become more pronounced. These factors can lead to decreased productivity and increased variability in larger-scale operations. Current data indicates that productivity often decreases by 30-50% when scaling from milliliter to liter volumes without appropriate engineering interventions.
Infrastructure requirements for large-scale cell-free manufacturing also present substantial capital expenditure considerations. Specialized bioreactors with precise temperature control, continuous monitoring capabilities, and appropriate mixing technologies are necessary but represent significant investments. The absence of standardized equipment specifically designed for cell-free processes further complicates scaling efforts and increases costs.
Recent economic analyses suggest that cell-free biomanufacturing becomes cost-competitive with traditional cell-based methods only at certain production scales and for specific high-value products. For instance, pharmaceutical proteins and specialized enzymes with market values exceeding $1000 per gram may justify the higher production costs. However, for lower-value products, significant cost reductions in extract preparation and reaction components are still required.
Emerging approaches to address these challenges include continuous processing methods, which can reduce reagent requirements by up to 70% compared to batch processes. Additionally, the development of lyophilized cell-free systems has extended shelf-life from days to months, potentially reducing production frequency and associated costs. These innovations, combined with ongoing efforts to standardize production methods, offer promising pathways toward economically viable large-scale cell-free biomanufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!