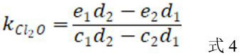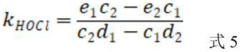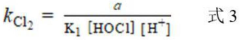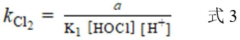Determine the Reaction Rate Constants for Hydrosulfuric Acid
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Kinetics Background and Objectives
Hydrosulfuric acid (H2S) kinetics has evolved significantly over the past century, with early studies dating back to the 1920s focusing primarily on basic dissociation properties in aqueous solutions. The understanding of this compound's reaction mechanisms has progressed from rudimentary equilibrium studies to sophisticated computational modeling of reaction pathways and transition states. Recent advancements in analytical techniques, particularly in spectroscopy and chromatography, have enabled more precise measurements of reaction intermediates and rate constants, revolutionizing our comprehension of H2S reaction dynamics.
The technological importance of hydrosulfuric acid kinetics has grown exponentially with the expansion of the petroleum, natural gas, and wastewater treatment industries. As a corrosive and toxic compound commonly encountered in these sectors, precise knowledge of H2S reaction rates is crucial for designing effective removal processes, corrosion prevention strategies, and safety protocols. Additionally, the emerging recognition of H2S as a gasotransmitter in biological systems has sparked interest in its physiological reaction kinetics, opening new research frontiers in biochemistry and medicine.
Current research trends indicate a shift toward understanding H2S kinetics under extreme conditions, including high-pressure environments relevant to deep-well petroleum extraction and high-temperature scenarios in industrial processing. Computational chemistry approaches have become increasingly sophisticated, allowing for more accurate predictions of reaction mechanisms and rate constants without extensive experimental work.
The primary objective of this technical research is to determine precise reaction rate constants for hydrosulfuric acid across various environmental conditions and reaction pathways. Specifically, we aim to establish comprehensive kinetic models for H2S oxidation, dissociation, and interaction with metal surfaces and catalysts. These models should account for temperature dependencies (10-200°C), pressure variations (1-100 bar), and the influence of common industrial contaminants.
Secondary objectives include developing standardized methodologies for measuring H2S reaction kinetics in complex matrices, creating predictive models that can be integrated into process simulation software, and identifying potential catalysts or inhibitors that could manipulate reaction rates for industrial applications. The research also seeks to bridge the gap between theoretical calculations and experimental measurements, providing validation protocols for computational kinetics models.
The ultimate goal is to compile a comprehensive database of hydrosulfuric acid reaction rate constants that can serve as a reference for industrial process design, environmental remediation strategies, and safety protocol development. This knowledge will enable more efficient H2S management across multiple industries while minimizing environmental impact and occupational hazards associated with this challenging compound.
The technological importance of hydrosulfuric acid kinetics has grown exponentially with the expansion of the petroleum, natural gas, and wastewater treatment industries. As a corrosive and toxic compound commonly encountered in these sectors, precise knowledge of H2S reaction rates is crucial for designing effective removal processes, corrosion prevention strategies, and safety protocols. Additionally, the emerging recognition of H2S as a gasotransmitter in biological systems has sparked interest in its physiological reaction kinetics, opening new research frontiers in biochemistry and medicine.
Current research trends indicate a shift toward understanding H2S kinetics under extreme conditions, including high-pressure environments relevant to deep-well petroleum extraction and high-temperature scenarios in industrial processing. Computational chemistry approaches have become increasingly sophisticated, allowing for more accurate predictions of reaction mechanisms and rate constants without extensive experimental work.
The primary objective of this technical research is to determine precise reaction rate constants for hydrosulfuric acid across various environmental conditions and reaction pathways. Specifically, we aim to establish comprehensive kinetic models for H2S oxidation, dissociation, and interaction with metal surfaces and catalysts. These models should account for temperature dependencies (10-200°C), pressure variations (1-100 bar), and the influence of common industrial contaminants.
Secondary objectives include developing standardized methodologies for measuring H2S reaction kinetics in complex matrices, creating predictive models that can be integrated into process simulation software, and identifying potential catalysts or inhibitors that could manipulate reaction rates for industrial applications. The research also seeks to bridge the gap between theoretical calculations and experimental measurements, providing validation protocols for computational kinetics models.
The ultimate goal is to compile a comprehensive database of hydrosulfuric acid reaction rate constants that can serve as a reference for industrial process design, environmental remediation strategies, and safety protocol development. This knowledge will enable more efficient H2S management across multiple industries while minimizing environmental impact and occupational hazards associated with this challenging compound.
Industrial Applications and Market Demand Analysis
The accurate determination of reaction rate constants for hydrosulfuric acid (H2S) has become increasingly critical across multiple industrial sectors, driving significant market demand for improved analytical methods and technologies. The global hydrogen sulfide removal market, which heavily relies on precise H2S reaction kinetics data, was valued at approximately 1.5 billion USD in 2022 and is projected to grow at a compound annual growth rate of 5.8% through 2030, underscoring the economic importance of this technical domain.
Oil and gas industries represent the largest market segment requiring precise H2S reaction rate constants, as these parameters are essential for designing efficient desulfurization processes and equipment. With environmental regulations becoming increasingly stringent worldwide, petroleum refineries are investing substantially in advanced sulfur removal technologies, creating a robust demand for accurate kinetic data that can optimize process efficiency and reduce operational costs.
The natural gas processing sector similarly demonstrates strong market pull for improved H2S reaction rate determination methods. As global natural gas consumption continues to rise, particularly in emerging economies, the need to efficiently remove H2S contaminants has intensified. Industry reports indicate that approximately 40% of global natural gas reserves contain significant H2S concentrations requiring treatment, representing a substantial addressable market.
Water treatment applications constitute another rapidly expanding market segment. Municipal wastewater facilities and industrial water treatment systems increasingly require precise understanding of H2S reaction kinetics to design effective removal processes and prevent infrastructure corrosion. The industrial wastewater treatment market specifically related to sulfur compound removal is growing at 6.2% annually, driven by both regulatory requirements and sustainability initiatives.
Mining operations, particularly in copper, zinc, and gold extraction, represent an additional significant market for H2S reaction rate constant determination technologies. These industries utilize hydrometallurgical processes where precise control of sulfide chemistry directly impacts production efficiency and environmental compliance.
The biogas industry has emerged as a high-growth market segment requiring H2S kinetic data. With the global push toward renewable energy sources, biogas production from agricultural waste, landfills, and wastewater treatment plants has accelerated, creating demand for cost-effective H2S removal solutions based on accurate reaction kinetics.
Market analysis reveals a notable shift toward real-time monitoring and control systems that incorporate reaction kinetics data, allowing for dynamic process optimization. This trend is particularly evident in smart manufacturing initiatives within the chemical processing industry, where improved H2S reaction rate constant determination can deliver substantial operational and environmental benefits.
Oil and gas industries represent the largest market segment requiring precise H2S reaction rate constants, as these parameters are essential for designing efficient desulfurization processes and equipment. With environmental regulations becoming increasingly stringent worldwide, petroleum refineries are investing substantially in advanced sulfur removal technologies, creating a robust demand for accurate kinetic data that can optimize process efficiency and reduce operational costs.
The natural gas processing sector similarly demonstrates strong market pull for improved H2S reaction rate determination methods. As global natural gas consumption continues to rise, particularly in emerging economies, the need to efficiently remove H2S contaminants has intensified. Industry reports indicate that approximately 40% of global natural gas reserves contain significant H2S concentrations requiring treatment, representing a substantial addressable market.
Water treatment applications constitute another rapidly expanding market segment. Municipal wastewater facilities and industrial water treatment systems increasingly require precise understanding of H2S reaction kinetics to design effective removal processes and prevent infrastructure corrosion. The industrial wastewater treatment market specifically related to sulfur compound removal is growing at 6.2% annually, driven by both regulatory requirements and sustainability initiatives.
Mining operations, particularly in copper, zinc, and gold extraction, represent an additional significant market for H2S reaction rate constant determination technologies. These industries utilize hydrometallurgical processes where precise control of sulfide chemistry directly impacts production efficiency and environmental compliance.
The biogas industry has emerged as a high-growth market segment requiring H2S kinetic data. With the global push toward renewable energy sources, biogas production from agricultural waste, landfills, and wastewater treatment plants has accelerated, creating demand for cost-effective H2S removal solutions based on accurate reaction kinetics.
Market analysis reveals a notable shift toward real-time monitoring and control systems that incorporate reaction kinetics data, allowing for dynamic process optimization. This trend is particularly evident in smart manufacturing initiatives within the chemical processing industry, where improved H2S reaction rate constant determination can deliver substantial operational and environmental benefits.
Current Methodologies and Technical Challenges
The determination of reaction rate constants for hydrosulfuric acid (H2S) involves several established methodologies, each with specific advantages and limitations. Traditional approaches include spectroscopic methods such as UV-visible spectroscopy, infrared spectroscopy, and Raman spectroscopy, which monitor concentration changes of reactants or products over time. These techniques provide real-time data but often struggle with the volatile and toxic nature of H2S, requiring specialized containment systems and safety protocols.
Electrochemical methods represent another significant approach, utilizing potentiometric, amperometric, and voltammetric techniques to measure electron transfer processes during H2S reactions. While these methods offer high sensitivity, they frequently encounter electrode fouling issues due to sulfur deposition, leading to signal drift and reduced accuracy in long-term measurements.
Chromatographic techniques, particularly gas chromatography coupled with mass spectrometry (GC-MS), have emerged as powerful tools for analyzing H2S reaction kinetics. These methods excel at separating complex reaction mixtures but require careful sample handling to prevent H2S degradation before analysis. The time delay between sampling and measurement can introduce significant errors in fast reaction rate determinations.
Computational chemistry approaches have gained prominence in recent years, employing quantum mechanical calculations and molecular dynamics simulations to predict reaction pathways and rate constants. However, these methods face challenges in accurately modeling the complex solvation effects and multiple reaction pathways characteristic of H2S chemistry, often necessitating experimental validation.
The technical challenges in determining H2S reaction rate constants are multifaceted. The compound's high volatility (boiling point of -60°C) and toxicity (immediately dangerous to life at concentrations above 100 ppm) create significant experimental difficulties, requiring specialized equipment and strict safety protocols that limit widespread research.
Analytical interference presents another major challenge, as H2S readily reacts with many common laboratory materials, including certain metals and rubber components, potentially skewing measurements. Additionally, the compound's sensitivity to oxygen and light exposure can lead to unintended side reactions, complicating kinetic analyses.
Temperature and pressure dependencies further complicate measurements, as H2S reaction rates exhibit strong non-linear responses to these variables. This necessitates precise environmental control during experiments, which becomes increasingly difficult at the extreme conditions often encountered in industrial applications such as petroleum refining and geothermal energy production.
Standardization remains problematic across the field, with different research groups employing varied methodologies that yield inconsistent rate constants, sometimes differing by orders of magnitude. This lack of standardization hinders comparative analyses and the development of comprehensive kinetic models for H2S reactions.
Electrochemical methods represent another significant approach, utilizing potentiometric, amperometric, and voltammetric techniques to measure electron transfer processes during H2S reactions. While these methods offer high sensitivity, they frequently encounter electrode fouling issues due to sulfur deposition, leading to signal drift and reduced accuracy in long-term measurements.
Chromatographic techniques, particularly gas chromatography coupled with mass spectrometry (GC-MS), have emerged as powerful tools for analyzing H2S reaction kinetics. These methods excel at separating complex reaction mixtures but require careful sample handling to prevent H2S degradation before analysis. The time delay between sampling and measurement can introduce significant errors in fast reaction rate determinations.
Computational chemistry approaches have gained prominence in recent years, employing quantum mechanical calculations and molecular dynamics simulations to predict reaction pathways and rate constants. However, these methods face challenges in accurately modeling the complex solvation effects and multiple reaction pathways characteristic of H2S chemistry, often necessitating experimental validation.
The technical challenges in determining H2S reaction rate constants are multifaceted. The compound's high volatility (boiling point of -60°C) and toxicity (immediately dangerous to life at concentrations above 100 ppm) create significant experimental difficulties, requiring specialized equipment and strict safety protocols that limit widespread research.
Analytical interference presents another major challenge, as H2S readily reacts with many common laboratory materials, including certain metals and rubber components, potentially skewing measurements. Additionally, the compound's sensitivity to oxygen and light exposure can lead to unintended side reactions, complicating kinetic analyses.
Temperature and pressure dependencies further complicate measurements, as H2S reaction rates exhibit strong non-linear responses to these variables. This necessitates precise environmental control during experiments, which becomes increasingly difficult at the extreme conditions often encountered in industrial applications such as petroleum refining and geothermal energy production.
Standardization remains problematic across the field, with different research groups employing varied methodologies that yield inconsistent rate constants, sometimes differing by orders of magnitude. This lack of standardization hinders comparative analyses and the development of comprehensive kinetic models for H2S reactions.
Established Experimental Approaches for Rate Constant Determination
01 Measurement and determination of hydrosulfuric acid reaction rate constants
Various methods and apparatus are used to measure and determine the reaction rate constants of hydrosulfuric acid in different chemical processes. These measurements are crucial for understanding the kinetics of reactions involving hydrosulfuric acid and optimizing industrial processes. The techniques include specialized reactors, analytical instruments, and computational models that can accurately track reaction progress and calculate rate constants under various conditions.- Measurement of hydrosulfuric acid reaction rate constants: Various methods and apparatus for measuring the reaction rate constants of hydrosulfuric acid in different chemical processes. These measurements are crucial for understanding the kinetics of reactions involving H2S and optimizing industrial processes where hydrosulfuric acid plays a role. The techniques include specialized equipment for monitoring reaction progress and determining rate constants under different conditions of temperature, pressure, and catalyst presence.
- Catalytic processes affecting hydrosulfuric acid reaction rates: The use of catalysts to influence the reaction rates of hydrosulfuric acid in various chemical transformations. Different catalytic materials can significantly alter the kinetics of H2S reactions, either accelerating desirable reactions or inhibiting corrosive processes. These catalysts are designed to operate under specific conditions to achieve optimal reaction rate constants for industrial applications.
- Equipment for controlling hydrosulfuric acid reactions: Specialized equipment and apparatus designed for controlling reactions involving hydrosulfuric acid, with particular focus on maintaining optimal reaction rates. These systems include monitoring devices, reaction vessels with precise temperature and pressure controls, and safety mechanisms to handle the toxic and corrosive nature of H2S. The equipment is engineered to ensure consistent reaction kinetics across various industrial processes.
- Environmental and industrial applications of hydrosulfuric acid reaction kinetics: Applications of hydrosulfuric acid reaction rate constants in environmental protection and industrial processes. Understanding the kinetics of H2S reactions is essential for developing effective treatments for wastewater, gas streams, and industrial emissions containing sulfur compounds. These applications leverage known reaction rate constants to design efficient removal or conversion processes for hydrosulfuric acid in various environmental contexts.
- Computational methods for predicting hydrosulfuric acid reaction rates: Computational approaches and models used to predict the reaction rate constants of hydrosulfuric acid under various conditions. These methods include quantum chemical calculations, molecular dynamics simulations, and machine learning algorithms that can estimate reaction kinetics without extensive experimental work. Such computational techniques are valuable for understanding reaction mechanisms and screening conditions before conducting laboratory experiments.
02 Catalytic processes affecting hydrosulfuric acid reaction rates
Catalysts play a significant role in modifying the reaction rate constants of hydrosulfuric acid. Different catalytic materials and systems can either accelerate or inhibit reactions involving hydrosulfuric acid, allowing for better control of industrial processes. The selection and optimization of catalysts can significantly impact reaction efficiency, selectivity, and overall process economics when dealing with hydrosulfuric acid reactions.Expand Specific Solutions03 Environmental and safety systems for hydrosulfuric acid reactions
Specialized equipment and systems are designed to safely handle hydrosulfuric acid reactions and monitor their rates under controlled conditions. These systems incorporate safety features to prevent hazardous situations arising from the toxic and corrosive nature of hydrosulfuric acid. Monitoring devices track reaction progress and can adjust parameters to maintain optimal reaction rates while ensuring environmental protection and worker safety.Expand Specific Solutions04 Temperature and pressure effects on hydrosulfuric acid reaction kinetics
The reaction rate constants of hydrosulfuric acid are significantly influenced by temperature and pressure conditions. Research has focused on quantifying these relationships to optimize process conditions. Studies demonstrate how varying temperature and pressure can be used to control reaction rates, with mathematical models developed to predict kinetic behavior under different operating conditions for industrial applications involving hydrosulfuric acid.Expand Specific Solutions05 Novel applications utilizing controlled hydrosulfuric acid reaction rates
Innovative applications have been developed that leverage precise control of hydrosulfuric acid reaction rate constants. These include specialized treatment processes, synthesis methods for valuable chemicals, and purification techniques. By understanding and manipulating the reaction kinetics of hydrosulfuric acid, these applications achieve improved efficiency, selectivity, and product quality across various industrial sectors.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
The hydrosulfuric acid reaction rate constants research field is currently in a growth phase, with increasing market demand driven by environmental regulations and industrial applications. The global market for sulfur chemistry is estimated at $10-12 billion annually, with steady growth projected. Technologically, the field shows moderate maturity with established methodologies but ongoing innovation. Leading players include major chemical corporations like Sumitomo Chemical and Sinopec, who leverage extensive R&D infrastructure, alongside specialized entities like Efirm New Material focusing on sulfur-containing compounds. Academic institutions such as Zhejiang University and Nanyang Technological University contribute fundamental research, while environmental technology companies like Zhejiang Tiandi develop practical applications. The collaboration between industry and academia is accelerating technological advancement in this domain.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive approach for determining hydrosulfuric acid reaction rate constants using advanced microreactor technology coupled with in-situ spectroscopic analysis. Their methodology employs continuous flow microreactors with precise temperature and pressure control systems that allow for real-time monitoring of H2S reactions under various industrial conditions. The company utilizes Raman spectroscopy and mass spectrometry for direct measurement of reaction intermediates and products, enabling accurate determination of reaction kinetics. Sinopec has specifically focused on understanding H2S reaction mechanisms in petroleum refining processes, developing mathematical models that incorporate multiple reaction pathways and catalytic effects. Their research has established a database of reaction rate constants across different temperature ranges (25-200°C) and pressure conditions (1-100 bar), which is crucial for optimizing desulfurization processes and preventing equipment corrosion in refineries.
Strengths: Extensive industrial application experience allows for practical validation of theoretical models; proprietary microreactor technology enables precise control of reaction conditions. Weaknesses: Their approach requires sophisticated analytical equipment with high maintenance costs; models may be optimized primarily for petroleum industry applications rather than broader scientific applications.
Korea Institute of Geoscience & Mineral Resources
Technical Solution: The Korea Institute of Geoscience & Mineral Resources (KIGAM) has developed a specialized methodology for determining hydrosulfuric acid reaction rate constants in geological and mineral processing contexts. Their approach combines electrochemical impedance spectroscopy (EIS) with potentiodynamic polarization techniques to measure reaction kinetics at mineral-solution interfaces. KIGAM researchers have created custom-designed flow-through reactors that simulate geochemical conditions in various mineral deposits, allowing for precise measurement of H2S reaction rates with different mineral surfaces. Their methodology incorporates isotope tracing techniques using 35S-labeled compounds to track reaction pathways and determine elementary reaction steps. The institute has established a comprehensive database of hydrosulfuric acid reaction rate constants with various minerals and metals under conditions ranging from ambient to hydrothermal (up to 350°C), which has significant applications in mineral processing optimization, acid mine drainage prediction, and geothermal resource development. Their research has particularly focused on the catalytic effects of various mineral surfaces on H2S reaction kinetics, providing insights into natural geochemical processes.
Strengths: Specialized expertise in geochemical applications; excellent capabilities for studying mineral-acid interactions under various environmental conditions. Weaknesses: Narrower focus on geological applications may limit applicability to some industrial processes; methodology may require adaptation for non-mineral reaction systems.
Critical Analysis of Recent Kinetic Studies and Patents
A method for determining the secondary reaction rate constants of different free chlorine and reactants
PatentActiveCN114636782B
Innovation
- By changing the Cl-concentration and FAC dosage at pH less than 6 and 8, the pseudo-first-order reaction rate constant of the reactants was obtained by fitting. Combined with the reaction rate equation, the second-order reaction rates of HOCl, Cl2, and Cl2O with the reactants were calculated. constant.
Reaction rate constant measuring device
PatentInactiveJP4701190B2
Innovation
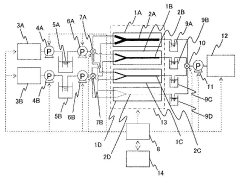
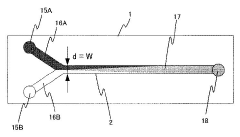
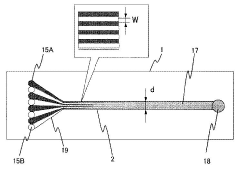
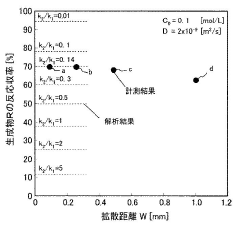
- A microreactor system with multiple microchannels of varying diffusion distances is used to quantify reaction products, combined with concentration adjustment, temperature control, and analytical means to calculate reaction rate constants.
Environmental Impact and Safety Considerations
The determination of reaction rate constants for hydrosulfuric acid (H2S) carries significant environmental and safety implications that must be thoroughly considered in both research and industrial applications. Hydrosulfuric acid, commonly encountered as hydrogen sulfide gas in aqueous solution, presents substantial environmental hazards due to its toxicity and corrosive properties. When released into the environment, H2S can contaminate water bodies, affecting aquatic ecosystems through oxygen depletion and direct toxicity to organisms. Understanding reaction kinetics allows for accurate modeling of its environmental fate and transport, which is crucial for predicting potential ecological impacts.
From an air quality perspective, H2S emissions contribute to atmospheric pollution, creating not only the characteristic "rotten egg" odor detectable at extremely low concentrations (0.5 ppb), but also posing serious health risks at higher concentrations. Precise reaction rate constants enable better dispersion modeling and help establish appropriate buffer zones around industrial facilities where H2S is produced or utilized.
Safety considerations in laboratories and industrial settings are paramount when working with hydrosulfuric acid. The gas is highly flammable and can form explosive mixtures with air, with concentrations between 4.3% and 46% by volume being particularly dangerous. Accurate reaction kinetics data facilitates the design of safer processes by enabling engineers to predict heat generation rates, pressure buildup potential, and reaction completion times. This information directly informs emergency response protocols and the design of control systems to prevent runaway reactions.
Occupational exposure limits for H2S are strictly regulated (typically 10-20 ppm for short-term exposure), reflecting its acute toxicity profile. At concentrations above 100 ppm, H2S can cause respiratory paralysis and death within minutes. By understanding reaction kinetics, industrial hygienists can better assess potential exposure scenarios and implement appropriate engineering controls and personal protective equipment requirements.
The neutralization and treatment of hydrosulfuric acid waste streams also benefits from precise kinetic data. Treatment processes such as oxidation, precipitation, and biological remediation all depend on reaction rates to determine detention times, reagent dosages, and equipment sizing. This ensures that discharged effluents meet environmental regulations and minimize ecological harm.
Climate considerations must also be addressed, as H2S can be oxidized in the atmosphere to form sulfur dioxide and ultimately sulfate aerosols, which contribute to acid rain and climate forcing effects. Quantifying these transformation rates through accurate kinetic constants helps in assessing the broader environmental footprint of processes involving hydrosulfuric acid.
From an air quality perspective, H2S emissions contribute to atmospheric pollution, creating not only the characteristic "rotten egg" odor detectable at extremely low concentrations (0.5 ppb), but also posing serious health risks at higher concentrations. Precise reaction rate constants enable better dispersion modeling and help establish appropriate buffer zones around industrial facilities where H2S is produced or utilized.
Safety considerations in laboratories and industrial settings are paramount when working with hydrosulfuric acid. The gas is highly flammable and can form explosive mixtures with air, with concentrations between 4.3% and 46% by volume being particularly dangerous. Accurate reaction kinetics data facilitates the design of safer processes by enabling engineers to predict heat generation rates, pressure buildup potential, and reaction completion times. This information directly informs emergency response protocols and the design of control systems to prevent runaway reactions.
Occupational exposure limits for H2S are strictly regulated (typically 10-20 ppm for short-term exposure), reflecting its acute toxicity profile. At concentrations above 100 ppm, H2S can cause respiratory paralysis and death within minutes. By understanding reaction kinetics, industrial hygienists can better assess potential exposure scenarios and implement appropriate engineering controls and personal protective equipment requirements.
The neutralization and treatment of hydrosulfuric acid waste streams also benefits from precise kinetic data. Treatment processes such as oxidation, precipitation, and biological remediation all depend on reaction rates to determine detention times, reagent dosages, and equipment sizing. This ensures that discharged effluents meet environmental regulations and minimize ecological harm.
Climate considerations must also be addressed, as H2S can be oxidized in the atmosphere to form sulfur dioxide and ultimately sulfate aerosols, which contribute to acid rain and climate forcing effects. Quantifying these transformation rates through accurate kinetic constants helps in assessing the broader environmental footprint of processes involving hydrosulfuric acid.
Analytical Instrumentation Requirements and Advancements
Accurate determination of reaction rate constants for hydrosulfuric acid requires sophisticated analytical instrumentation capable of precise measurement under challenging conditions. Modern spectroscopic techniques form the foundation of these analytical approaches, with UV-visible spectrophotometry enabling real-time monitoring of concentration changes during reactions. This method requires specialized quartz cells resistant to the corrosive nature of H2S and its derivatives, along with high-resolution detectors capable of capturing rapid kinetic processes.
Mass spectrometry represents another critical analytical tool, with recent advancements in time-of-flight and quadrupole technologies significantly improving the ability to track reaction intermediates. These instruments must incorporate specialized sample introduction systems designed to handle volatile and potentially hazardous sulfur compounds while maintaining analytical integrity. Corrosion-resistant components, including specialized alloys and coatings for sample pathways, have emerged as essential design features.
Electrochemical methods, particularly potentiometric and amperometric techniques, offer complementary approaches for monitoring hydrosulfuric acid reactions. Recent innovations in electrode materials, including boron-doped diamond and modified carbon surfaces, have enhanced sensitivity while resisting sulfide poisoning that plagued earlier sensor designs. Microfluidic platforms integrated with these electrochemical sensors now enable reaction monitoring with minimal sample volumes and reduced exposure risks.
Data acquisition systems have evolved in parallel with these instrumental advances, with modern systems capable of microsecond time resolution necessary for capturing fast reaction kinetics. Machine learning algorithms increasingly supplement traditional kinetic analysis software, improving the extraction of rate constants from complex, multi-step reaction profiles typical of sulfur chemistry.
Environmental and safety considerations have driven significant instrumentation modifications, including closed-loop sampling systems, automated dilution capabilities, and integrated scrubbers to neutralize exhaust gases. These engineering solutions address the toxic and corrosive nature of hydrosulfuric acid while maintaining analytical performance.
Looking forward, emerging technologies such as operando spectroscopy and advanced neutron scattering techniques promise to reveal reaction mechanisms at unprecedented molecular detail. Quantum sensing approaches, though still primarily in research settings, demonstrate potential for detecting subtle electronic changes during bond formation and breaking, potentially revolutionizing our understanding of hydrosulfuric acid reaction pathways and enabling more precise determination of rate constants.
Mass spectrometry represents another critical analytical tool, with recent advancements in time-of-flight and quadrupole technologies significantly improving the ability to track reaction intermediates. These instruments must incorporate specialized sample introduction systems designed to handle volatile and potentially hazardous sulfur compounds while maintaining analytical integrity. Corrosion-resistant components, including specialized alloys and coatings for sample pathways, have emerged as essential design features.
Electrochemical methods, particularly potentiometric and amperometric techniques, offer complementary approaches for monitoring hydrosulfuric acid reactions. Recent innovations in electrode materials, including boron-doped diamond and modified carbon surfaces, have enhanced sensitivity while resisting sulfide poisoning that plagued earlier sensor designs. Microfluidic platforms integrated with these electrochemical sensors now enable reaction monitoring with minimal sample volumes and reduced exposure risks.
Data acquisition systems have evolved in parallel with these instrumental advances, with modern systems capable of microsecond time resolution necessary for capturing fast reaction kinetics. Machine learning algorithms increasingly supplement traditional kinetic analysis software, improving the extraction of rate constants from complex, multi-step reaction profiles typical of sulfur chemistry.
Environmental and safety considerations have driven significant instrumentation modifications, including closed-loop sampling systems, automated dilution capabilities, and integrated scrubbers to neutralize exhaust gases. These engineering solutions address the toxic and corrosive nature of hydrosulfuric acid while maintaining analytical performance.
Looking forward, emerging technologies such as operando spectroscopy and advanced neutron scattering techniques promise to reveal reaction mechanisms at unprecedented molecular detail. Quantum sensing approaches, though still primarily in research settings, demonstrate potential for detecting subtle electronic changes during bond formation and breaking, potentially revolutionizing our understanding of hydrosulfuric acid reaction pathways and enabling more precise determination of rate constants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!