Analyze Thermodynamic Properties of Hydrosulfuric Acid Reactions
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Reaction Thermodynamics Background and Objectives
Hydrogen sulfide (H2S) has been a subject of scientific inquiry since its discovery in the late 18th century. This pungent, toxic gas occurs naturally in volcanic gases, natural gas deposits, and as a byproduct of various industrial processes. The thermodynamic properties of hydrosulfuric acid reactions have gained increasing attention due to their significance in environmental science, industrial applications, and energy production sectors.
The evolution of research on H2S reaction thermodynamics has progressed from basic characterization studies to sophisticated computational modeling approaches. Early investigations focused primarily on qualitative descriptions of reaction behaviors, while modern research employs advanced spectroscopic techniques and quantum mechanical calculations to elucidate reaction mechanisms at the molecular level.
Current technological trends in this field include the development of more accurate computational methods for predicting thermodynamic parameters, in-situ monitoring technologies for real-time reaction analysis, and novel catalytic systems that can modulate H2S reaction pathways. These advancements are driven by both environmental concerns regarding sulfur emissions and industrial needs for more efficient processes.
The primary technical objectives of analyzing H2S reaction thermodynamics include establishing comprehensive thermodynamic databases for various H2S reactions under different conditions, developing predictive models for reaction outcomes in complex systems, and identifying optimal conditions for desired reaction pathways. These objectives serve both fundamental scientific understanding and practical applications in multiple industries.
From an environmental perspective, understanding the thermodynamics of H2S reactions is crucial for developing effective abatement technologies and predicting the fate of sulfur compounds in natural systems. The increasing stringency of environmental regulations worldwide has accelerated research in this area, particularly focusing on low-energy pathways for H2S conversion to less harmful compounds.
In the energy sector, H2S thermodynamics plays a vital role in natural gas processing, petroleum refining, and emerging technologies such as sulfur-based energy storage systems. The push toward cleaner energy sources has heightened interest in efficient methods for handling sulfur compounds in fossil fuels and exploring the potential of H2S as a hydrogen source.
Industrial applications extend to metallurgy, where H2S reactions are utilized in metal extraction and purification processes, and to chemical manufacturing, where H2S serves as a reagent for various syntheses. Accurate thermodynamic data is essential for optimizing these processes and ensuring safety in operations involving this hazardous compound.
The evolution of research on H2S reaction thermodynamics has progressed from basic characterization studies to sophisticated computational modeling approaches. Early investigations focused primarily on qualitative descriptions of reaction behaviors, while modern research employs advanced spectroscopic techniques and quantum mechanical calculations to elucidate reaction mechanisms at the molecular level.
Current technological trends in this field include the development of more accurate computational methods for predicting thermodynamic parameters, in-situ monitoring technologies for real-time reaction analysis, and novel catalytic systems that can modulate H2S reaction pathways. These advancements are driven by both environmental concerns regarding sulfur emissions and industrial needs for more efficient processes.
The primary technical objectives of analyzing H2S reaction thermodynamics include establishing comprehensive thermodynamic databases for various H2S reactions under different conditions, developing predictive models for reaction outcomes in complex systems, and identifying optimal conditions for desired reaction pathways. These objectives serve both fundamental scientific understanding and practical applications in multiple industries.
From an environmental perspective, understanding the thermodynamics of H2S reactions is crucial for developing effective abatement technologies and predicting the fate of sulfur compounds in natural systems. The increasing stringency of environmental regulations worldwide has accelerated research in this area, particularly focusing on low-energy pathways for H2S conversion to less harmful compounds.
In the energy sector, H2S thermodynamics plays a vital role in natural gas processing, petroleum refining, and emerging technologies such as sulfur-based energy storage systems. The push toward cleaner energy sources has heightened interest in efficient methods for handling sulfur compounds in fossil fuels and exploring the potential of H2S as a hydrogen source.
Industrial applications extend to metallurgy, where H2S reactions are utilized in metal extraction and purification processes, and to chemical manufacturing, where H2S serves as a reagent for various syntheses. Accurate thermodynamic data is essential for optimizing these processes and ensuring safety in operations involving this hazardous compound.
Market Applications and Demand Analysis for H2S Processing
The global market for hydrogen sulfide (H2S) processing technologies has been experiencing significant growth, driven primarily by stringent environmental regulations and increasing industrial activities. The oil and gas sector remains the largest consumer of H2S processing technologies, accounting for approximately 60% of the total market share. This dominance is attributed to the high concentration of H2S in natural gas and crude oil streams, necessitating efficient removal processes to meet product specifications and environmental standards.
The petrochemical industry represents another substantial market segment, where H2S processing is critical for preventing catalyst poisoning and equipment corrosion. With the global petrochemical market expanding at a compound annual growth rate of 5.1%, the demand for advanced H2S treatment solutions continues to rise proportionally.
Environmental regulations worldwide have become increasingly stringent regarding sulfur emissions, particularly in developed regions like North America and Europe. The implementation of regulations such as the EPA's National Emission Standards for Hazardous Air Pollutants (NESHAP) in the United States and the Industrial Emissions Directive (IED) in Europe has significantly boosted the demand for efficient H2S processing technologies.
Emerging economies, particularly in Asia-Pacific and the Middle East, are witnessing rapid industrialization and urbanization, leading to increased energy consumption and consequently higher demand for H2S processing solutions. China and India, with their expanding industrial bases and growing environmental consciousness, represent particularly promising markets with projected growth rates exceeding the global average.
The mining industry presents another significant application area for H2S processing technologies. The treatment of acid mine drainage and metallurgical processes often involves dealing with hydrogen sulfide, creating substantial demand for specialized processing solutions. This sector is expected to grow at a steady rate of 4.3% annually over the next five years.
Recent market trends indicate a growing preference for regenerative H2S removal processes over conventional disposal methods, driven by sustainability concerns and economic considerations. Technologies that can recover sulfur in usable forms, such as elemental sulfur or sulfuric acid, are gaining particular traction due to their potential for value addition and reduced waste generation.
The water treatment sector represents an emerging application area with significant growth potential. Municipal wastewater treatment facilities increasingly require H2S control to prevent odor issues and infrastructure damage, creating new market opportunities for specialized processing technologies that can operate effectively in aqueous environments.
The petrochemical industry represents another substantial market segment, where H2S processing is critical for preventing catalyst poisoning and equipment corrosion. With the global petrochemical market expanding at a compound annual growth rate of 5.1%, the demand for advanced H2S treatment solutions continues to rise proportionally.
Environmental regulations worldwide have become increasingly stringent regarding sulfur emissions, particularly in developed regions like North America and Europe. The implementation of regulations such as the EPA's National Emission Standards for Hazardous Air Pollutants (NESHAP) in the United States and the Industrial Emissions Directive (IED) in Europe has significantly boosted the demand for efficient H2S processing technologies.
Emerging economies, particularly in Asia-Pacific and the Middle East, are witnessing rapid industrialization and urbanization, leading to increased energy consumption and consequently higher demand for H2S processing solutions. China and India, with their expanding industrial bases and growing environmental consciousness, represent particularly promising markets with projected growth rates exceeding the global average.
The mining industry presents another significant application area for H2S processing technologies. The treatment of acid mine drainage and metallurgical processes often involves dealing with hydrogen sulfide, creating substantial demand for specialized processing solutions. This sector is expected to grow at a steady rate of 4.3% annually over the next five years.
Recent market trends indicate a growing preference for regenerative H2S removal processes over conventional disposal methods, driven by sustainability concerns and economic considerations. Technologies that can recover sulfur in usable forms, such as elemental sulfur or sulfuric acid, are gaining particular traction due to their potential for value addition and reduced waste generation.
The water treatment sector represents an emerging application area with significant growth potential. Municipal wastewater treatment facilities increasingly require H2S control to prevent odor issues and infrastructure damage, creating new market opportunities for specialized processing technologies that can operate effectively in aqueous environments.
Current State and Challenges in H2S Thermodynamic Analysis
The thermodynamic analysis of hydrosulfuric acid (H2S) reactions currently faces significant challenges despite considerable advancements in analytical techniques. Globally, research institutions and energy companies have made substantial progress in understanding the fundamental properties of H2S, yet comprehensive thermodynamic data remains incomplete, particularly for complex reaction environments encountered in industrial settings.
Current analytical methods primarily rely on calorimetry, spectroscopy, and computational chemistry approaches. Differential scanning calorimetry (DSC) and isothermal titration calorimetry (ITC) provide valuable insights into reaction enthalpies and heat capacities, but often struggle with the highly corrosive nature of H2S and its reaction products. This corrosivity presents a persistent challenge for instrument longevity and measurement accuracy.
Computational approaches using density functional theory (DFT) have emerged as powerful alternatives, with recent models achieving improved accuracy in predicting thermodynamic parameters. However, significant discrepancies still exist between theoretical predictions and experimental measurements, particularly in high-pressure and high-temperature conditions relevant to geothermal and petroleum applications.
The geographic distribution of H2S thermodynamic research shows concentration in regions with significant oil and gas industries, including the United States, China, Russia, and Middle Eastern countries. European research tends to focus more on environmental applications and remediation technologies, while Asian research emphasizes industrial process optimization.
A critical limitation in current research is the lack of standardized methodologies for measuring thermodynamic properties across different temperature and pressure ranges. This has resulted in fragmented datasets that are difficult to integrate into comprehensive thermodynamic models. The scientific community has recognized this issue, with recent international collaborations attempting to establish unified experimental protocols.
Safety considerations significantly constrain experimental work with H2S due to its high toxicity, limiting the scope and scale of laboratory investigations. This has driven increased reliance on computational methods, which themselves face validation challenges without robust experimental data.
The most pressing technical challenge remains accurately modeling phase equilibria in multicomponent systems containing H2S, particularly in the presence of electrolytes and under supercritical conditions. Current equations of state struggle to capture the complex molecular interactions in these systems, leading to significant uncertainties in process design calculations for industries dealing with H2S.
Recent technological innovations in in-situ monitoring and microreactor technologies show promise for overcoming some of these limitations, potentially enabling more accurate measurements under industrially relevant conditions while maintaining safety standards.
Current analytical methods primarily rely on calorimetry, spectroscopy, and computational chemistry approaches. Differential scanning calorimetry (DSC) and isothermal titration calorimetry (ITC) provide valuable insights into reaction enthalpies and heat capacities, but often struggle with the highly corrosive nature of H2S and its reaction products. This corrosivity presents a persistent challenge for instrument longevity and measurement accuracy.
Computational approaches using density functional theory (DFT) have emerged as powerful alternatives, with recent models achieving improved accuracy in predicting thermodynamic parameters. However, significant discrepancies still exist between theoretical predictions and experimental measurements, particularly in high-pressure and high-temperature conditions relevant to geothermal and petroleum applications.
The geographic distribution of H2S thermodynamic research shows concentration in regions with significant oil and gas industries, including the United States, China, Russia, and Middle Eastern countries. European research tends to focus more on environmental applications and remediation technologies, while Asian research emphasizes industrial process optimization.
A critical limitation in current research is the lack of standardized methodologies for measuring thermodynamic properties across different temperature and pressure ranges. This has resulted in fragmented datasets that are difficult to integrate into comprehensive thermodynamic models. The scientific community has recognized this issue, with recent international collaborations attempting to establish unified experimental protocols.
Safety considerations significantly constrain experimental work with H2S due to its high toxicity, limiting the scope and scale of laboratory investigations. This has driven increased reliance on computational methods, which themselves face validation challenges without robust experimental data.
The most pressing technical challenge remains accurately modeling phase equilibria in multicomponent systems containing H2S, particularly in the presence of electrolytes and under supercritical conditions. Current equations of state struggle to capture the complex molecular interactions in these systems, leading to significant uncertainties in process design calculations for industries dealing with H2S.
Recent technological innovations in in-situ monitoring and microreactor technologies show promise for overcoming some of these limitations, potentially enabling more accurate measurements under industrially relevant conditions while maintaining safety standards.
Established Methodologies for H2S Thermodynamic Property Calculation
01 Thermodynamic modeling of hydrosulfuric acid reactions
Various computational methods and models are used to predict the thermodynamic properties of hydrosulfuric acid reactions. These models incorporate parameters such as enthalpy, entropy, and Gibbs free energy to determine reaction feasibility and equilibrium constants. Advanced simulation techniques help in understanding the behavior of hydrosulfuric acid under different temperature and pressure conditions, which is crucial for industrial applications and process optimization.- Thermodynamic properties of hydrosulfuric acid reactions: The thermodynamic properties of hydrosulfuric acid reactions are essential for understanding reaction kinetics and equilibrium states. These properties include enthalpy, entropy, Gibbs free energy, and heat capacity, which determine reaction spontaneity and energy requirements. Accurate thermodynamic data enables prediction of reaction behavior under various conditions of temperature and pressure, which is crucial for industrial applications involving hydrogen sulfide chemistry.
- Catalytic reactions involving hydrosulfuric acid: Catalysts play a significant role in hydrosulfuric acid reactions by altering reaction pathways and reducing activation energy. Various catalytic materials, including metal oxides, supported metals, and zeolites, can enhance the conversion efficiency of hydrogen sulfide in oxidation, decomposition, and other transformation processes. The selection of appropriate catalysts can improve reaction rates, selectivity, and yield while operating under milder conditions, which is particularly important for industrial applications.
- Environmental applications of hydrosulfuric acid reactions: Hydrosulfuric acid reactions are utilized in various environmental applications, particularly in waste treatment and pollution control. These reactions are employed in processes for removing hydrogen sulfide from industrial gas streams, wastewater treatment, and soil remediation. Understanding the thermodynamic properties of these reactions is crucial for developing efficient and sustainable environmental technologies that can effectively neutralize or convert toxic hydrogen sulfide into less harmful substances.
- Measurement and modeling of hydrosulfuric acid reaction properties: Advanced techniques and methodologies are employed to measure and model the thermodynamic properties of hydrosulfuric acid reactions. These include calorimetry, spectroscopy, chromatography, and computational chemistry approaches. Accurate measurement and modeling of reaction parameters such as reaction rates, equilibrium constants, and activation energies are essential for process optimization and scale-up. These methods enable researchers to predict reaction behavior under various conditions and develop more efficient processes.
- Industrial processes utilizing hydrosulfuric acid reactions: Hydrosulfuric acid reactions are integral to numerous industrial processes, including natural gas processing, petroleum refining, and chemical manufacturing. The thermodynamic properties of these reactions influence process design, equipment selection, and operating conditions. Understanding these properties enables optimization of reaction conditions to maximize yield and selectivity while minimizing energy consumption and environmental impact. Industrial applications often involve complex reaction networks where hydrosulfuric acid participates in multiple simultaneous reactions.
02 Catalytic reactions involving hydrosulfuric acid
Catalysts play a significant role in hydrosulfuric acid reactions by altering reaction pathways and improving thermodynamic efficiency. Various catalytic materials, including metal oxides and supported metals, can enhance the conversion of hydrosulfuric acid in industrial processes. The thermodynamic properties of these catalytic reactions are important for designing efficient desulfurization processes and hydrogen production systems from hydrogen sulfide.Expand Specific Solutions03 Phase equilibria and solubility properties of hydrosulfuric acid
The phase behavior and solubility characteristics of hydrosulfuric acid in various solvents are critical for understanding its thermodynamic properties. Research focuses on vapor-liquid equilibria, liquid-liquid equilibria, and solid-liquid equilibria involving hydrosulfuric acid systems. These properties are essential for designing separation processes, gas treating systems, and predicting the behavior of hydrosulfuric acid in natural and industrial environments.Expand Specific Solutions04 Reaction kinetics and thermodynamics in hydrosulfuric acid treatment
The kinetics and thermodynamics of hydrosulfuric acid reactions are studied to optimize treatment processes in industrial applications. Understanding the rate constants, activation energies, and thermodynamic parameters helps in designing efficient systems for removing hydrogen sulfide from gas streams and wastewater. These properties are particularly important in petroleum refining, natural gas processing, and environmental remediation technologies.Expand Specific Solutions05 Biological interactions and thermodynamic properties of hydrosulfuric acid
The thermodynamic properties of hydrosulfuric acid play a significant role in biological systems and biochemical reactions. Research examines how hydrosulfuric acid interacts with proteins, enzymes, and cellular components, as well as its role as a signaling molecule. Understanding these thermodynamic properties is important for developing medical applications, studying biological sulfur cycles, and addressing toxicological concerns related to hydrogen sulfide exposure.Expand Specific Solutions
Key Industry Players in Sulfur Chemistry Research
The thermodynamic properties of hydrosulfuric acid reactions market is in a growth phase, with increasing applications across petrochemical, chemical manufacturing, and environmental sectors. Major players include China Petroleum & Chemical Corp. (Sinopec), ExxonMobil, BASF, and Sumitomo Chemical, who are investing in research to improve process efficiency and reduce environmental impact. The technology is relatively mature but evolving, with research institutions like MIT, Johns Hopkins University, and Nanyang Technological University collaborating with industry leaders to develop advanced catalysts and reaction mechanisms. Sinopec's specialized research institutes are particularly focused on safety engineering aspects, while companies like Haldor Topsøe are developing innovative solutions for sulfur management in industrial processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced thermodynamic modeling systems for hydrosulfuric acid reactions in petroleum refining processes. Their technology incorporates modified Peng-Robinson and Soave-Redlich-Kwong equations of state specifically calibrated for H2S-containing systems across varying temperature and pressure conditions. Sinopec's approach includes comprehensive phase equilibrium calculations that account for the non-ideal behavior of hydrosulfuric acid in hydrocarbon mixtures, enabling accurate prediction of reaction pathways and product distributions. Their proprietary simulation software integrates kinetic models with thermodynamic data to optimize sulfur removal processes while minimizing energy consumption. Recent developments include machine learning algorithms that enhance prediction accuracy by incorporating historical plant data with theoretical models, resulting in reported 15-20% improvements in process efficiency and significant reductions in catalyst deactivation rates due to better understanding of H2S reaction mechanisms.
Strengths: Extensive real-world validation from numerous refinery operations provides highly accurate models specific to petroleum processing conditions. Their integrated approach combining thermodynamics with reaction kinetics offers comprehensive process optimization capabilities. Weaknesses: Models are primarily optimized for petroleum refining applications and may require significant adaptation for other chemical processes involving hydrosulfuric acid reactions.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has pioneered advanced computational fluid dynamics (CFD) models coupled with detailed thermochemical analysis for hydrosulfuric acid reactions across their refining and gas processing operations. Their proprietary EMRE (ExxonMobil Research and Engineering) thermodynamic package incorporates specialized parameters for H2S-containing systems, accounting for the unique behavior of sulfur compounds under varying process conditions. The company has developed high-fidelity molecular simulation techniques that predict reaction enthalpies, Gibbs free energies, and equilibrium constants with exceptional accuracy, particularly for acid gas treatment processes. Their approach integrates quantum chemistry calculations with experimental validation to characterize the thermodynamic properties of complex H2S reaction networks, including interactions with various catalysts and solvents. ExxonMobil's technology enables precise prediction of phase behavior in multiphase systems containing hydrosulfuric acid, critical for designing efficient separation processes and preventing equipment corrosion. Recent innovations include machine learning algorithms that optimize thermodynamic model parameters based on plant operating data, resulting in reported improvements of up to 25% in prediction accuracy for complex sulfur chemistry systems.
Strengths: Exceptional integration of molecular-level modeling with process-scale simulations provides comprehensive understanding across multiple scales. Their models excel at predicting corrosion potential and materials compatibility issues in H2S environments. Weaknesses: Proprietary nature of their modeling approaches limits broader scientific validation and application outside their own operations. Models may require substantial computational resources for full implementation.
Critical Research Findings in H2S Reaction Mechanisms
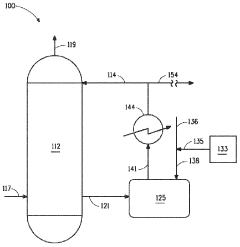
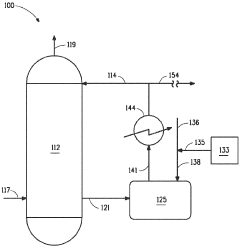
Method for determining thermodynamic and molecular properties in the liquid phase
PatentInactiveUS5739423A
Innovation
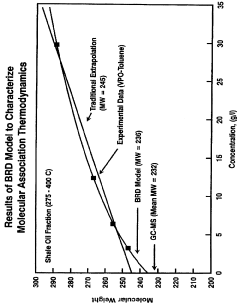
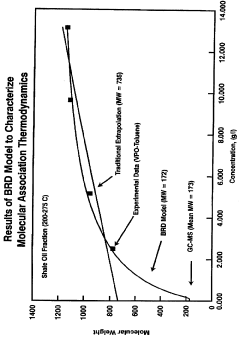
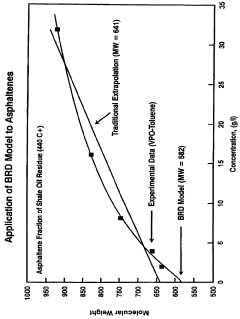
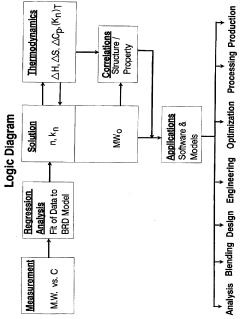
- The Bunget-Russell-Devineni Molecular Association Thermodynamic Method (BRD method) calculates average molecular weight and thermodynamic properties by measuring deviations in vapor pressure and temperature, using equations to account for molecular association and cluster formation, providing a more accurate and chemically derived approach compared to empirical methods.
Process for producing sulfuric acid with low levels of nitrogen oxides
PatentActiveIN1341DELNP2015A
Innovation
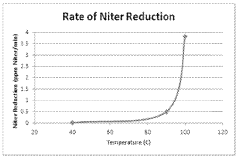
- Integrating the use of hydrazine sources like hydrazine sulfate, dihydrazine sulfate, or hydrazine hydrate into the sulfuric acid production process at temperatures of at least 90°C to react with NOx impurities, allowing for NOx reduction during normal production with minimal changes to the system.
Environmental Impact and Regulatory Considerations for H2S Processing
The processing of hydrogen sulfide (H2S) presents significant environmental challenges that necessitate stringent regulatory oversight. H2S is classified as a hazardous air pollutant under various environmental protection frameworks globally, including the U.S. Clean Air Act and the European Union's Industrial Emissions Directive. These regulations establish strict emission limits, typically in the range of 5-10 ppm for workplace environments and significantly lower thresholds for ambient air quality.
Environmental impacts of H2S processing are multifaceted. When released into the atmosphere, H2S oxidizes to form sulfur dioxide (SO2) and eventually sulfuric acid (H2SO4), contributing to acid rain formation. This process can significantly alter ecosystem pH levels, particularly affecting aquatic environments and soil chemistry. The thermodynamic properties of these reactions indicate high exothermicity, releasing approximately 518 kJ/mol during complete oxidation, which has implications for atmospheric heat balance in areas with substantial H2S emissions.
Water contamination represents another critical environmental concern. H2S dissolves readily in water, forming a weak acid that can mobilize heavy metals in soil and sediments. The thermodynamic equilibrium constants for these dissolution reactions vary significantly with temperature and pH, creating complex environmental fate scenarios that regulatory frameworks must address through comprehensive water quality standards.
Regulatory approaches to H2S management have evolved toward lifecycle assessment methodologies. Modern regulations increasingly focus on Best Available Techniques (BAT) that consider the thermodynamic efficiency of conversion processes. The Claus process, commonly employed for H2S treatment, achieves sulfur recovery rates of 95-98% under optimal conditions, with regulatory requirements in many jurisdictions mandating minimum recovery efficiencies of 99.5% through tail gas treatment units.
Carbon pricing mechanisms and emissions trading schemes are increasingly incorporating sulfur compounds, creating economic incentives for improved H2S management. The thermodynamic analysis of alternative processing routes has become essential for regulatory compliance, with authorities requiring detailed energy balances and reaction pathway analyses to validate proposed treatment technologies.
International agreements, including the Convention on Long-range Transboundary Air Pollution, establish frameworks for cross-border management of sulfur emissions. These agreements recognize the atmospheric transport potential of H2S and its oxidation products, necessitating coordinated regulatory approaches that account for the thermodynamic behavior of these compounds under varying atmospheric conditions.
Environmental impacts of H2S processing are multifaceted. When released into the atmosphere, H2S oxidizes to form sulfur dioxide (SO2) and eventually sulfuric acid (H2SO4), contributing to acid rain formation. This process can significantly alter ecosystem pH levels, particularly affecting aquatic environments and soil chemistry. The thermodynamic properties of these reactions indicate high exothermicity, releasing approximately 518 kJ/mol during complete oxidation, which has implications for atmospheric heat balance in areas with substantial H2S emissions.
Water contamination represents another critical environmental concern. H2S dissolves readily in water, forming a weak acid that can mobilize heavy metals in soil and sediments. The thermodynamic equilibrium constants for these dissolution reactions vary significantly with temperature and pH, creating complex environmental fate scenarios that regulatory frameworks must address through comprehensive water quality standards.
Regulatory approaches to H2S management have evolved toward lifecycle assessment methodologies. Modern regulations increasingly focus on Best Available Techniques (BAT) that consider the thermodynamic efficiency of conversion processes. The Claus process, commonly employed for H2S treatment, achieves sulfur recovery rates of 95-98% under optimal conditions, with regulatory requirements in many jurisdictions mandating minimum recovery efficiencies of 99.5% through tail gas treatment units.
Carbon pricing mechanisms and emissions trading schemes are increasingly incorporating sulfur compounds, creating economic incentives for improved H2S management. The thermodynamic analysis of alternative processing routes has become essential for regulatory compliance, with authorities requiring detailed energy balances and reaction pathway analyses to validate proposed treatment technologies.
International agreements, including the Convention on Long-range Transboundary Air Pollution, establish frameworks for cross-border management of sulfur emissions. These agreements recognize the atmospheric transport potential of H2S and its oxidation products, necessitating coordinated regulatory approaches that account for the thermodynamic behavior of these compounds under varying atmospheric conditions.
Safety Protocols and Risk Assessment in H2S Research
The research on hydrosulfuric acid reactions necessitates stringent safety protocols due to the highly toxic and flammable nature of hydrogen sulfide (H₂S). Laboratory environments conducting thermodynamic analyses must implement comprehensive safety measures beginning with proper ventilation systems equipped with H₂S detectors calibrated to trigger alarms at concentrations as low as 10 ppm. These systems should include emergency shutdown protocols that activate automatically when dangerous levels are detected.
Personal protective equipment requirements for researchers include chemical-resistant gloves, face shields, and respiratory protection appropriate for H₂S exposure. Self-contained breathing apparatus (SCBA) must be readily available in laboratories where higher concentrations may be encountered during experimental procedures. Regular inspection and maintenance of this equipment is essential to ensure functionality during emergencies.
Risk assessment frameworks specific to H₂S research should employ both qualitative and quantitative methodologies. The Hazard and Operability Study (HAZOP) approach has proven effective for identifying potential failure points in experimental setups involving hydrosulfuric acid reactions. Quantitative risk assessment should calculate probability-consequence matrices for various exposure scenarios, with particular attention to the thermodynamic conditions that may lead to rapid H₂S release.
Emergency response protocols must be tailored to the unique challenges of H₂S exposure. These include clearly defined evacuation routes, designated assembly points, and specific medical intervention procedures for H₂S poisoning. Regular drills should be conducted to ensure all laboratory personnel can execute these protocols efficiently under stress conditions.
Storage and handling guidelines for hydrosulfuric acid and its precursors require specialized containment systems with temperature and pressure monitoring. The thermodynamic properties being studied directly impact safety requirements, as exothermic reactions may create hazardous conditions if not properly controlled. Double containment systems and chemical neutralization stations should be standard in all storage areas.
Waste management protocols must address the environmental hazards associated with hydrosulfuric acid reactions. Neutralization procedures using appropriate bases should be clearly documented, with verification testing to confirm complete neutralization before disposal. Regulatory compliance with local hazardous waste regulations must be maintained through proper documentation and disposal tracking.
Training requirements for personnel working with H₂S should include certification in handling toxic gases, specific instruction on the thermodynamic properties being studied, and regular refresher courses. Simulation-based training using virtual reality systems has shown promising results in preparing researchers for emergency scenarios without exposure risk.
Personal protective equipment requirements for researchers include chemical-resistant gloves, face shields, and respiratory protection appropriate for H₂S exposure. Self-contained breathing apparatus (SCBA) must be readily available in laboratories where higher concentrations may be encountered during experimental procedures. Regular inspection and maintenance of this equipment is essential to ensure functionality during emergencies.
Risk assessment frameworks specific to H₂S research should employ both qualitative and quantitative methodologies. The Hazard and Operability Study (HAZOP) approach has proven effective for identifying potential failure points in experimental setups involving hydrosulfuric acid reactions. Quantitative risk assessment should calculate probability-consequence matrices for various exposure scenarios, with particular attention to the thermodynamic conditions that may lead to rapid H₂S release.
Emergency response protocols must be tailored to the unique challenges of H₂S exposure. These include clearly defined evacuation routes, designated assembly points, and specific medical intervention procedures for H₂S poisoning. Regular drills should be conducted to ensure all laboratory personnel can execute these protocols efficiently under stress conditions.
Storage and handling guidelines for hydrosulfuric acid and its precursors require specialized containment systems with temperature and pressure monitoring. The thermodynamic properties being studied directly impact safety requirements, as exothermic reactions may create hazardous conditions if not properly controlled. Double containment systems and chemical neutralization stations should be standard in all storage areas.
Waste management protocols must address the environmental hazards associated with hydrosulfuric acid reactions. Neutralization procedures using appropriate bases should be clearly documented, with verification testing to confirm complete neutralization before disposal. Regulatory compliance with local hazardous waste regulations must be maintained through proper documentation and disposal tracking.
Training requirements for personnel working with H₂S should include certification in handling toxic gases, specific instruction on the thermodynamic properties being studied, and regular refresher courses. Simulation-based training using virtual reality systems has shown promising results in preparing researchers for emergency scenarios without exposure risk.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!