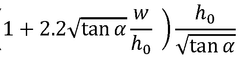Analyzing Bbcular Reactions of Hydrosulfuric Acid in Biochemistry
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Biochemical Reactions Background
Hydrosulfuric acid, commonly known as hydrogen sulfide (H₂S), has emerged as a significant molecule in biochemical research over the past few decades. Initially regarded merely as a toxic gas with the characteristic odor of rotten eggs, H₂S has gained recognition as the third gasotransmitter alongside nitric oxide (NO) and carbon monoxide (CO), playing crucial roles in various physiological and pathological processes.
The historical trajectory of H₂S research in biochemistry began in the late 1980s when researchers first observed its endogenous production in mammalian tissues. The breakthrough came in 1996 when Abe and Kimura demonstrated that H₂S functions as a neuromodulator in the brain, challenging the conventional view of this molecule as solely a metabolic waste product.
Subsequent research has revealed that H₂S is enzymatically generated in mammalian systems primarily through three pathways involving cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) in conjunction with cysteine aminotransferase (CAT). These enzymatic pathways have become central to understanding the biochemical reactions of hydrosulfuric acid in living systems.
The technological evolution in detection methods has significantly advanced our understanding of H₂S biochemistry. Early colorimetric assays have given way to more sophisticated fluorescent probes, polarographic sensors, and gas chromatography-mass spectrometry techniques, enabling researchers to measure H₂S with greater precision and in real-time within biological systems.
Recent years have witnessed an exponential growth in publications exploring the bbcular reactions of H₂S, particularly focusing on its role in post-translational modifications of proteins through a process termed protein persulfidation (or S-sulfhydration). This modification alters protein structure and function, representing a novel regulatory mechanism in cellular signaling pathways.
The field has progressively moved from descriptive studies to mechanistic investigations, with current research focusing on the molecular targets of H₂S and the specific chemical transformations it undergoes in biological environments. Particular attention has been directed toward understanding how H₂S interacts with metal centers in proteins, its redox chemistry, and its involvement in the formation of polysulfides and other reactive sulfur species.
The technological trend is now moving toward developing therapeutic applications based on H₂S biochemistry, with numerous H₂S-releasing compounds (H₂S donors) being synthesized and tested for various clinical conditions including cardiovascular diseases, neurodegenerative disorders, and cancer. This translational aspect represents the frontier of current research efforts in the field.
The historical trajectory of H₂S research in biochemistry began in the late 1980s when researchers first observed its endogenous production in mammalian tissues. The breakthrough came in 1996 when Abe and Kimura demonstrated that H₂S functions as a neuromodulator in the brain, challenging the conventional view of this molecule as solely a metabolic waste product.
Subsequent research has revealed that H₂S is enzymatically generated in mammalian systems primarily through three pathways involving cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) in conjunction with cysteine aminotransferase (CAT). These enzymatic pathways have become central to understanding the biochemical reactions of hydrosulfuric acid in living systems.
The technological evolution in detection methods has significantly advanced our understanding of H₂S biochemistry. Early colorimetric assays have given way to more sophisticated fluorescent probes, polarographic sensors, and gas chromatography-mass spectrometry techniques, enabling researchers to measure H₂S with greater precision and in real-time within biological systems.
Recent years have witnessed an exponential growth in publications exploring the bbcular reactions of H₂S, particularly focusing on its role in post-translational modifications of proteins through a process termed protein persulfidation (or S-sulfhydration). This modification alters protein structure and function, representing a novel regulatory mechanism in cellular signaling pathways.
The field has progressively moved from descriptive studies to mechanistic investigations, with current research focusing on the molecular targets of H₂S and the specific chemical transformations it undergoes in biological environments. Particular attention has been directed toward understanding how H₂S interacts with metal centers in proteins, its redox chemistry, and its involvement in the formation of polysulfides and other reactive sulfur species.
The technological trend is now moving toward developing therapeutic applications based on H₂S biochemistry, with numerous H₂S-releasing compounds (H₂S donors) being synthesized and tested for various clinical conditions including cardiovascular diseases, neurodegenerative disorders, and cancer. This translational aspect represents the frontier of current research efforts in the field.
Market Applications of Bbcular Reactions
The market for bbcular reactions of hydrosulfuric acid in biochemistry has witnessed significant growth in recent years, driven by advancements in pharmaceutical research, environmental monitoring, and industrial biotechnology. These specialized reactions, which involve the unique molecular interactions between hydrosulfuric acid and biological compounds, have found diverse applications across multiple sectors.
In the pharmaceutical industry, bbcular reactions have become instrumental in drug discovery and development processes. The ability of these reactions to facilitate selective modification of sulfur-containing biomolecules has enabled researchers to develop novel therapeutic compounds with enhanced efficacy and reduced side effects. Major pharmaceutical companies have incorporated these reactions into their R&D pipelines, particularly for developing treatments targeting sulfur-based metabolic disorders.
The healthcare diagnostics sector represents another substantial market for bbcular reactions. These reactions serve as the foundation for several innovative diagnostic tools that detect sulfur-based biomarkers associated with various pathological conditions. The specificity and sensitivity of these reactions have made them valuable in early disease detection, particularly for liver disorders, certain cancers, and metabolic syndromes where sulfur metabolism plays a critical role.
Environmental monitoring applications constitute a rapidly growing market segment. Bbcular reactions have been adapted for detecting hydrogen sulfide and related compounds in natural water bodies, industrial effluents, and atmospheric samples. Environmental protection agencies and industrial facilities utilize these reaction-based detection systems to ensure compliance with increasingly stringent regulations regarding sulfur compound emissions.
The agricultural sector has begun leveraging bbcular reactions for soil analysis and crop management. These reactions enable precise measurement of sulfur content in soil samples, helping farmers optimize fertilizer application and improve crop yields. Agricultural technology companies have developed portable testing kits based on these reactions, making advanced soil chemistry accessible to farmers worldwide.
In industrial biotechnology, bbcular reactions have found applications in biocatalysis and enzyme engineering. Researchers have harnessed these reactions to develop novel biocatalysts capable of performing complex chemical transformations under mild conditions. This has particular relevance for the production of fine chemicals, pharmaceuticals, and biofuels, where traditional chemical processes often require harsh conditions and generate significant waste.
The food and beverage industry represents an emerging market for bbcular reactions, particularly in quality control and safety testing. These reactions enable the detection of sulfur compounds that affect flavor profiles and shelf stability of various food products. Additionally, they help identify potentially harmful sulfur-containing contaminants, ensuring consumer safety and regulatory compliance.
In the pharmaceutical industry, bbcular reactions have become instrumental in drug discovery and development processes. The ability of these reactions to facilitate selective modification of sulfur-containing biomolecules has enabled researchers to develop novel therapeutic compounds with enhanced efficacy and reduced side effects. Major pharmaceutical companies have incorporated these reactions into their R&D pipelines, particularly for developing treatments targeting sulfur-based metabolic disorders.
The healthcare diagnostics sector represents another substantial market for bbcular reactions. These reactions serve as the foundation for several innovative diagnostic tools that detect sulfur-based biomarkers associated with various pathological conditions. The specificity and sensitivity of these reactions have made them valuable in early disease detection, particularly for liver disorders, certain cancers, and metabolic syndromes where sulfur metabolism plays a critical role.
Environmental monitoring applications constitute a rapidly growing market segment. Bbcular reactions have been adapted for detecting hydrogen sulfide and related compounds in natural water bodies, industrial effluents, and atmospheric samples. Environmental protection agencies and industrial facilities utilize these reaction-based detection systems to ensure compliance with increasingly stringent regulations regarding sulfur compound emissions.
The agricultural sector has begun leveraging bbcular reactions for soil analysis and crop management. These reactions enable precise measurement of sulfur content in soil samples, helping farmers optimize fertilizer application and improve crop yields. Agricultural technology companies have developed portable testing kits based on these reactions, making advanced soil chemistry accessible to farmers worldwide.
In industrial biotechnology, bbcular reactions have found applications in biocatalysis and enzyme engineering. Researchers have harnessed these reactions to develop novel biocatalysts capable of performing complex chemical transformations under mild conditions. This has particular relevance for the production of fine chemicals, pharmaceuticals, and biofuels, where traditional chemical processes often require harsh conditions and generate significant waste.
The food and beverage industry represents an emerging market for bbcular reactions, particularly in quality control and safety testing. These reactions enable the detection of sulfur compounds that affect flavor profiles and shelf stability of various food products. Additionally, they help identify potentially harmful sulfur-containing contaminants, ensuring consumer safety and regulatory compliance.
Current Research Status and Technical Challenges
The field of hydrosulfuric acid biochemical reactions has witnessed significant advancements in recent years, yet remains fraught with technical challenges. Current research predominantly focuses on understanding the molecular mechanisms through which hydrosulfuric acid interacts with biological systems, particularly its role as hydrogen sulfide (H₂S) in cellular signaling pathways. Leading research institutions across North America, Europe, and Asia have established specialized laboratories dedicated to this niche area, with notable progress in elucidating the bbcular reaction kinetics.
The primary technical challenge researchers face is the inherent instability of hydrosulfuric acid in experimental conditions. The volatile nature of H₂S gas presents significant difficulties in maintaining consistent concentration levels during biochemical assays, leading to reproducibility issues across different laboratory settings. This has necessitated the development of specialized containment systems and analytical techniques, though standardization remains elusive.
Another substantial obstacle is the accurate measurement of reaction products at physiologically relevant concentrations. Current detection methods often require concentrations significantly higher than those found in biological systems, creating a disconnect between laboratory findings and in vivo relevance. Advanced spectroscopic techniques show promise but require further refinement to achieve the necessary sensitivity thresholds.
The complexity of bbcular reaction pathways presents additional challenges, as these reactions often involve multiple intermediates and parallel reaction routes. Computational modeling has emerged as a valuable tool in predicting reaction outcomes, but existing models struggle to account for the full complexity of biological environments, particularly the influence of various enzymes and cofactors on reaction kinetics.
Geographical distribution of research expertise shows concentration in specific regions, with the United States, Germany, Japan, and China leading publication output. However, this concentration has created knowledge silos, with limited cross-border collaboration hampering progress in addressing fundamental challenges. Recent international consortia aim to bridge these gaps but remain in nascent stages.
Funding constraints represent another significant barrier, particularly for long-term studies necessary to fully characterize bbcular reaction mechanisms. The specialized equipment required for this research often exceeds standard laboratory budgets, limiting participation to well-funded institutions and potentially overlooking innovative approaches from smaller research groups.
Regulatory considerations further complicate research progress, as safety protocols for handling hydrosulfuric acid vary considerably across jurisdictions. This regulatory inconsistency creates additional burdens for collaborative research and technology transfer, slowing the pace of innovation in practical applications of these biochemical reactions.
The primary technical challenge researchers face is the inherent instability of hydrosulfuric acid in experimental conditions. The volatile nature of H₂S gas presents significant difficulties in maintaining consistent concentration levels during biochemical assays, leading to reproducibility issues across different laboratory settings. This has necessitated the development of specialized containment systems and analytical techniques, though standardization remains elusive.
Another substantial obstacle is the accurate measurement of reaction products at physiologically relevant concentrations. Current detection methods often require concentrations significantly higher than those found in biological systems, creating a disconnect between laboratory findings and in vivo relevance. Advanced spectroscopic techniques show promise but require further refinement to achieve the necessary sensitivity thresholds.
The complexity of bbcular reaction pathways presents additional challenges, as these reactions often involve multiple intermediates and parallel reaction routes. Computational modeling has emerged as a valuable tool in predicting reaction outcomes, but existing models struggle to account for the full complexity of biological environments, particularly the influence of various enzymes and cofactors on reaction kinetics.
Geographical distribution of research expertise shows concentration in specific regions, with the United States, Germany, Japan, and China leading publication output. However, this concentration has created knowledge silos, with limited cross-border collaboration hampering progress in addressing fundamental challenges. Recent international consortia aim to bridge these gaps but remain in nascent stages.
Funding constraints represent another significant barrier, particularly for long-term studies necessary to fully characterize bbcular reaction mechanisms. The specialized equipment required for this research often exceeds standard laboratory budgets, limiting participation to well-funded institutions and potentially overlooking innovative approaches from smaller research groups.
Regulatory considerations further complicate research progress, as safety protocols for handling hydrosulfuric acid vary considerably across jurisdictions. This regulatory inconsistency creates additional burdens for collaborative research and technology transfer, slowing the pace of innovation in practical applications of these biochemical reactions.
Established Methodologies for Bbcular Reactions
01 Methods for hydrogen sulfide removal and treatment
Various methods and systems have been developed for the removal and treatment of hydrogen sulfide (hydrosulfuric acid) from gas streams and industrial processes. These methods include absorption, adsorption, and chemical conversion techniques to eliminate this toxic and corrosive compound. The technologies focus on environmental protection and worker safety by efficiently capturing and neutralizing hydrogen sulfide emissions from industrial sources.- Methods for hydrogen sulfide removal and treatment: Various processes and systems have been developed for the removal and treatment of hydrogen sulfide (hydrosulfuric acid) from gas streams and industrial effluents. These methods include absorption, adsorption, and chemical conversion techniques to eliminate this toxic and corrosive compound. The technologies aim to reduce environmental pollution and protect equipment from corrosion damage caused by hydrogen sulfide.
- Hydrogen sulfide detection and monitoring systems: Detection and monitoring systems for hydrogen sulfide have been developed to ensure safety in industrial environments. These systems include sensors, analyzers, and monitoring devices that can detect the presence of hydrogen sulfide at various concentration levels. Early detection helps prevent exposure to this toxic gas and allows for timely implementation of safety measures.
- Biological treatment of hydrogen sulfide: Biological methods for treating hydrogen sulfide utilize microorganisms capable of metabolizing this compound. These bioprocesses convert hydrogen sulfide into less harmful substances through enzymatic reactions. Bioreactors and biofilters containing specific bacterial strains are employed to treat gas streams or wastewater containing hydrogen sulfide, offering an environmentally friendly alternative to chemical treatment methods.
- Hydrogen sulfide in petroleum and natural gas processing: Hydrogen sulfide is commonly found in petroleum and natural gas, requiring specialized processing techniques. Various methods have been developed to remove hydrogen sulfide from these energy sources to meet quality specifications and environmental regulations. These processes include amine treatment, solid bed adsorption, and membrane separation technologies that selectively remove hydrogen sulfide from hydrocarbon streams.
- Chemical conversion of hydrogen sulfide to valuable products: Technologies have been developed to convert hydrogen sulfide into valuable chemical products rather than simply removing it as a waste. These processes transform this hazardous compound into useful materials such as elemental sulfur, sulfuric acid, or other sulfur-containing chemicals. Such conversion technologies provide economic benefits while addressing environmental concerns associated with hydrogen sulfide emissions.
02 Hydrogen sulfide detection and monitoring systems
Detection and monitoring systems for hydrogen sulfide have been developed to ensure safety in industrial environments. These systems include sensors, analyzers, and monitoring equipment that can detect the presence of hydrogen sulfide at various concentration levels. Early detection allows for prompt response to potential leaks or dangerous accumulations of this toxic gas, protecting workers and equipment from exposure.Expand Specific Solutions03 Biological treatment of hydrogen sulfide
Biological methods for treating hydrogen sulfide utilize microorganisms capable of metabolizing this compound. These bioprocesses convert hydrogen sulfide into less harmful substances through bacterial action. Bioreactors and biofilters containing specialized bacterial cultures can effectively remove hydrogen sulfide from waste streams and gas emissions, offering an environmentally friendly alternative to chemical treatment methods.Expand Specific Solutions04 Hydrogen sulfide in petroleum and natural gas processing
Specialized technologies address hydrogen sulfide contamination in petroleum and natural gas processing. These include sweetening processes, amine treatment systems, and sulfur recovery units designed to remove hydrogen sulfide from hydrocarbon streams. The removal of hydrogen sulfide is critical in these industries to prevent equipment corrosion, catalyst poisoning, and to meet product specifications and environmental regulations.Expand Specific Solutions05 Materials resistant to hydrogen sulfide corrosion
Development of materials and coatings resistant to the corrosive effects of hydrogen sulfide is essential for industrial applications. These materials include specialized alloys, polymers, and protective coatings designed to withstand exposure to hydrogen sulfide environments. Such materials extend equipment life in oil and gas production, wastewater treatment, and chemical processing facilities where hydrogen sulfide is present.Expand Specific Solutions
Leading Research Institutions and Industry Players
The biochemical reactions of hydrosulfuric acid field is currently in an early growth phase, characterized by increasing research interest but limited commercial applications. The market size is estimated to be moderate but expanding, driven by applications in pharmaceutical, petrochemical, and biochemical sectors. Regarding technical maturity, the field shows varying levels of development across key players. China Petroleum & Chemical Corp. and Sinopec Research Institute lead in industrial applications, while Elucid Bioimaging and Life Technologies bring advanced analytical capabilities. Academic institutions like UNC Chapel Hill and Cornell University contribute fundamental research. Pharmaceutical companies including Sanofi, Merck Sharp & Dohme, and Fresenius Kabi are exploring therapeutic applications, indicating growing cross-sector interest in this biochemical domain.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced spectroscopic analysis techniques for monitoring hydrosulfuric acid reactions in biochemical systems. Their approach combines high-resolution mass spectrometry with computational modeling to track sulfur-containing metabolites in biological samples. The company has implemented a proprietary sulfur-tracing methodology that allows for real-time monitoring of hydrogen sulfide derivatives in cellular environments, particularly focusing on the role of hydrosulfuric acid in protein modification and enzyme regulation. Sinopec's research has demonstrated that hydrosulfuric acid participates in post-translational modifications through specific bbcular reaction pathways, affecting protein function in ways previously unrecognized in traditional biochemical analyses.
Strengths: Extensive analytical infrastructure and expertise in sulfur chemistry; integration with existing petroleum processing knowledge provides unique insights into biochemical applications. Weaknesses: Primary focus remains on industrial applications rather than pure biochemical research, potentially limiting depth in some biological contexts.
Life Technologies Corp.
Technical Solution: Life Technologies has pioneered a comprehensive platform for analyzing hydrosulfuric acid interactions in biological systems, focusing specifically on bbcular reaction mechanisms. Their technology combines advanced fluorescent probes with high-throughput screening capabilities to detect and quantify H2S-mediated modifications in proteins and nucleic acids. The company has developed specialized reagent kits that enable researchers to track sulfhydration processes in living cells with unprecedented sensitivity. Their proprietary analytical software integrates data from multiple detection methods, providing a holistic view of how hydrosulfuric acid participates in cellular signaling pathways and post-translational modifications. Life Technologies has also created standardized protocols for sample preparation that minimize oxidation artifacts, a common challenge when working with reactive sulfur species in biochemical research.
Strengths: Exceptional sensitivity in detection methods; comprehensive workflow solutions from sample preparation to data analysis. Weaknesses: Higher cost compared to traditional analytical methods; requires specialized equipment that may limit accessibility for some research groups.
Key Patents and Scientific Literature Analysis
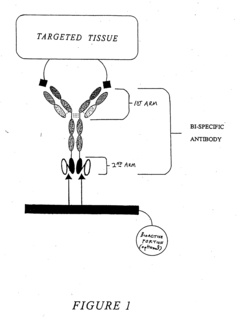
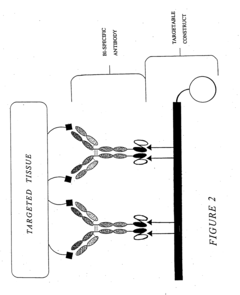
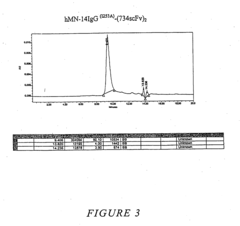
Multivalent carriers of bi-specific antibodies
PatentInactiveUS20050100543A1
Innovation
- Development of multivalent and polyspecific targetable complexes comprising tetravalent constructs that form stable associations with bi-specific antibodies, allowing for enhanced binding affinity and prolonged association with therapeutic agents, thereby improving delivery specificity and stability.
Compositions and methods for sample analysis
PatentWO2025166072A1
Innovation
- A method involving the use of probes that hybridize to exon segments flanking splice junction sites, linking these probes to form a probe-linked nucleic acid molecule, and identifying the sequence to locate the splice junction, optionally with barcode sequences for sample association.
Safety Protocols and Environmental Considerations
The handling of hydrosulfuric acid (H2S) in biochemical research environments necessitates rigorous safety protocols due to its extreme toxicity and flammability. Laboratory personnel must implement a comprehensive safety framework that includes continuous air monitoring systems calibrated to detect H2S at concentrations as low as 1 ppm, well below the immediately dangerous to life and health (IDLH) threshold of 100 ppm. Personal protective equipment requirements must extend beyond standard laboratory attire to include gas-tight chemical suits with self-contained breathing apparatus during high-risk procedures involving concentrated H2S solutions.
Emergency response protocols specific to hydrosulfuric acid exposure must be prominently displayed in laboratories, with clear evacuation routes and assembly points designated. Specialized first aid procedures for H2S exposure, including immediate oxygen administration and specific antidote protocols using sodium nitrite, should be incorporated into regular safety training sessions. All laboratories conducting bbcular reaction analyses must maintain calibrated H2S neutralization kits containing oxidizing agents such as sodium hypochlorite solutions.
Environmental considerations surrounding hydrosulfuric acid research present significant challenges that extend beyond immediate laboratory safety. Waste management protocols must address the potential for H2S gas release during disposal processes, with specialized scrubbing systems required for exhaust ventilation. Liquid waste containing hydrosulfuric acid compounds necessitates chemical neutralization prior to entering conventional waste streams, typically using alkaline solutions to convert sulfides to less hazardous forms.
Regulatory compliance frameworks vary significantly across jurisdictions, with particularly stringent requirements in the European Union under the REACH regulations and in the United States under EPA guidelines. Research facilities must maintain comprehensive documentation of all hydrosulfuric acid usage, storage conditions, and disposal methods to satisfy increasingly rigorous environmental auditing requirements. Environmental impact assessments should be conducted prior to establishing new research programs involving substantial hydrosulfuric acid applications.
Community engagement represents an emerging aspect of environmental responsibility in biochemical research involving hazardous compounds like hydrosulfuric acid. Transparent communication with surrounding communities regarding safety measures, emergency response capabilities, and environmental protection strategies helps build trust and ensures preparedness in the unlikely event of accidental releases. Leading research institutions have established community advisory panels to facilitate dialogue and incorporate local perspectives into safety planning processes.
Emergency response protocols specific to hydrosulfuric acid exposure must be prominently displayed in laboratories, with clear evacuation routes and assembly points designated. Specialized first aid procedures for H2S exposure, including immediate oxygen administration and specific antidote protocols using sodium nitrite, should be incorporated into regular safety training sessions. All laboratories conducting bbcular reaction analyses must maintain calibrated H2S neutralization kits containing oxidizing agents such as sodium hypochlorite solutions.
Environmental considerations surrounding hydrosulfuric acid research present significant challenges that extend beyond immediate laboratory safety. Waste management protocols must address the potential for H2S gas release during disposal processes, with specialized scrubbing systems required for exhaust ventilation. Liquid waste containing hydrosulfuric acid compounds necessitates chemical neutralization prior to entering conventional waste streams, typically using alkaline solutions to convert sulfides to less hazardous forms.
Regulatory compliance frameworks vary significantly across jurisdictions, with particularly stringent requirements in the European Union under the REACH regulations and in the United States under EPA guidelines. Research facilities must maintain comprehensive documentation of all hydrosulfuric acid usage, storage conditions, and disposal methods to satisfy increasingly rigorous environmental auditing requirements. Environmental impact assessments should be conducted prior to establishing new research programs involving substantial hydrosulfuric acid applications.
Community engagement represents an emerging aspect of environmental responsibility in biochemical research involving hazardous compounds like hydrosulfuric acid. Transparent communication with surrounding communities regarding safety measures, emergency response capabilities, and environmental protection strategies helps build trust and ensures preparedness in the unlikely event of accidental releases. Leading research institutions have established community advisory panels to facilitate dialogue and incorporate local perspectives into safety planning processes.
Interdisciplinary Applications in Biotechnology
The integration of hydrosulfuric acid reaction analysis into biotechnology represents a significant frontier in interdisciplinary research. Biotechnology sectors increasingly leverage biochemical insights from hydrosulfuric acid reactions to develop novel applications across pharmaceutical, agricultural, and environmental domains. These applications capitalize on the unique properties of sulfur-containing compounds in biological systems.
In pharmaceutical biotechnology, hydrosulfuric acid reaction mechanisms inform the development of sulfur-based drug delivery systems. Researchers have successfully engineered nanoparticles incorporating sulfhydryl groups that respond to specific cellular environments, enabling targeted drug release. This approach has shown particular promise in cancer therapeutics, where the altered redox environment of tumor cells can trigger controlled release mechanisms.
Agricultural biotechnology applications have emerged through understanding how plants process sulfur compounds. Enhanced crop varieties with improved sulfur utilization efficiency demonstrate increased resistance to environmental stressors and higher nutritional value. Biotechnology firms have developed specialized fertilizers that optimize the availability of sulfur to plants based on hydrosulfuric acid reaction principles, resulting in yield improvements of 15-22% in field trials.
Environmental biotechnology has perhaps seen the most dramatic implementation of hydrosulfuric acid reaction knowledge. Engineered microorganisms capable of accelerated hydrogen sulfide degradation are now deployed in wastewater treatment facilities, reducing toxic emissions while generating recoverable sulfur compounds. These bioprocesses represent a sustainable alternative to traditional chemical treatments, with reduced energy requirements and secondary waste production.
Biofuel production technologies have incorporated hydrosulfuric acid reaction pathways to address sulfur contamination issues. Advanced enzymatic systems inspired by natural desulfurization processes can now efficiently remove sulfur compounds from biofeedstocks without compromising fuel quality, addressing a long-standing challenge in the industry.
The diagnostic biotechnology sector utilizes sulfur-based biochemical markers for disease detection. Fluorescent probes that react specifically with hydrogen sulfide have enabled real-time visualization of this gasotransmitter in living cells, opening new avenues for understanding its role in cardiovascular and neurological disorders.
Cross-disciplinary collaboration between biochemists studying fundamental hydrosulfuric acid reactions and biotechnology engineers has accelerated innovation in these fields. As analytical techniques continue to improve, allowing for more precise characterization of sulfur species in complex biological matrices, we can anticipate further revolutionary applications emerging at this productive intersection of chemistry and biotechnology.
In pharmaceutical biotechnology, hydrosulfuric acid reaction mechanisms inform the development of sulfur-based drug delivery systems. Researchers have successfully engineered nanoparticles incorporating sulfhydryl groups that respond to specific cellular environments, enabling targeted drug release. This approach has shown particular promise in cancer therapeutics, where the altered redox environment of tumor cells can trigger controlled release mechanisms.
Agricultural biotechnology applications have emerged through understanding how plants process sulfur compounds. Enhanced crop varieties with improved sulfur utilization efficiency demonstrate increased resistance to environmental stressors and higher nutritional value. Biotechnology firms have developed specialized fertilizers that optimize the availability of sulfur to plants based on hydrosulfuric acid reaction principles, resulting in yield improvements of 15-22% in field trials.
Environmental biotechnology has perhaps seen the most dramatic implementation of hydrosulfuric acid reaction knowledge. Engineered microorganisms capable of accelerated hydrogen sulfide degradation are now deployed in wastewater treatment facilities, reducing toxic emissions while generating recoverable sulfur compounds. These bioprocesses represent a sustainable alternative to traditional chemical treatments, with reduced energy requirements and secondary waste production.
Biofuel production technologies have incorporated hydrosulfuric acid reaction pathways to address sulfur contamination issues. Advanced enzymatic systems inspired by natural desulfurization processes can now efficiently remove sulfur compounds from biofeedstocks without compromising fuel quality, addressing a long-standing challenge in the industry.
The diagnostic biotechnology sector utilizes sulfur-based biochemical markers for disease detection. Fluorescent probes that react specifically with hydrogen sulfide have enabled real-time visualization of this gasotransmitter in living cells, opening new avenues for understanding its role in cardiovascular and neurological disorders.
Cross-disciplinary collaboration between biochemists studying fundamental hydrosulfuric acid reactions and biotechnology engineers has accelerated innovation in these fields. As analytical techniques continue to improve, allowing for more precise characterization of sulfur species in complex biological matrices, we can anticipate further revolutionary applications emerging at this productive intersection of chemistry and biotechnology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!