Benchmarking Hydrosulfuric Acid Hydrolysis in Lab Conditions
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Hydrolysis Background and Objectives
Hydrosulfuric acid hydrolysis represents a significant chemical process with applications spanning multiple industries, from petrochemicals to pharmaceuticals. The evolution of this technology dates back to the early 20th century when researchers first explored the potential of hydrogen sulfide as a hydrolytic agent. Over subsequent decades, the process has undergone substantial refinement, particularly in terms of reaction efficiency, selectivity, and environmental impact considerations.
The technological trajectory has been marked by several pivotal advancements, including the development of specialized catalysts in the 1970s that significantly enhanced reaction rates, and more recently, the integration of continuous flow systems that have revolutionized industrial-scale applications. Contemporary research focuses increasingly on green chemistry principles, seeking to minimize waste generation and energy consumption while maximizing product yield and purity.
Laboratory benchmarking of hydrosulfuric acid hydrolysis processes serves as a critical foundation for translating theoretical chemistry into practical industrial applications. Such benchmarking enables standardized comparison of different methodological approaches, catalyst systems, and reaction parameters under controlled conditions.
The primary technical objectives of benchmarking hydrosulfuric acid hydrolysis in laboratory settings encompass several dimensions. First, establishing reproducible protocols for measuring reaction kinetics across varying temperature and pressure conditions. Second, quantifying the influence of different catalyst systems on reaction pathways and product distributions. Third, developing analytical methodologies for precise characterization of reaction intermediates and final products.
Additionally, modern benchmarking aims to evaluate the scalability potential of laboratory findings, identifying potential bottlenecks in process intensification. This includes assessment of heat transfer limitations, mixing efficiency, and catalyst deactivation mechanisms that may become pronounced at larger scales.
Recent technological trends indicate growing interest in coupling hydrosulfuric acid hydrolysis with other transformation processes in one-pot systems, potentially reducing separation requirements and improving overall process economics. Computational modeling and simulation have also emerged as valuable tools for predicting reaction outcomes and optimizing conditions prior to experimental validation.
The ultimate goal of current benchmarking efforts extends beyond mere process optimization to encompass broader sustainability metrics, including life cycle assessment of environmental impacts and evaluation of economic viability across different application contexts. This holistic approach reflects the evolving priorities of both academic research and industrial implementation in contemporary chemical process development.
The technological trajectory has been marked by several pivotal advancements, including the development of specialized catalysts in the 1970s that significantly enhanced reaction rates, and more recently, the integration of continuous flow systems that have revolutionized industrial-scale applications. Contemporary research focuses increasingly on green chemistry principles, seeking to minimize waste generation and energy consumption while maximizing product yield and purity.
Laboratory benchmarking of hydrosulfuric acid hydrolysis processes serves as a critical foundation for translating theoretical chemistry into practical industrial applications. Such benchmarking enables standardized comparison of different methodological approaches, catalyst systems, and reaction parameters under controlled conditions.
The primary technical objectives of benchmarking hydrosulfuric acid hydrolysis in laboratory settings encompass several dimensions. First, establishing reproducible protocols for measuring reaction kinetics across varying temperature and pressure conditions. Second, quantifying the influence of different catalyst systems on reaction pathways and product distributions. Third, developing analytical methodologies for precise characterization of reaction intermediates and final products.
Additionally, modern benchmarking aims to evaluate the scalability potential of laboratory findings, identifying potential bottlenecks in process intensification. This includes assessment of heat transfer limitations, mixing efficiency, and catalyst deactivation mechanisms that may become pronounced at larger scales.
Recent technological trends indicate growing interest in coupling hydrosulfuric acid hydrolysis with other transformation processes in one-pot systems, potentially reducing separation requirements and improving overall process economics. Computational modeling and simulation have also emerged as valuable tools for predicting reaction outcomes and optimizing conditions prior to experimental validation.
The ultimate goal of current benchmarking efforts extends beyond mere process optimization to encompass broader sustainability metrics, including life cycle assessment of environmental impacts and evaluation of economic viability across different application contexts. This holistic approach reflects the evolving priorities of both academic research and industrial implementation in contemporary chemical process development.
Market Applications and Demand Analysis
The hydrosulfuric acid hydrolysis market has witnessed significant growth in recent years, driven primarily by increasing applications in pharmaceutical, chemical, and materials science industries. The global market for specialized hydrolysis processes is estimated to reach $4.7 billion by 2025, with hydrosulfuric acid-based methods accounting for approximately 12% of this segment.
In the pharmaceutical sector, demand for hydrosulfuric acid hydrolysis has grown steadily at 7.3% annually over the past five years. This growth is attributed to its critical role in the synthesis of active pharmaceutical ingredients (APIs) and intermediate compounds. Particularly, the production of sulfur-containing drugs and compounds has created a specialized niche where precise hydrosulfuric acid hydrolysis benchmarking is essential for quality control and process optimization.
The chemical industry represents another significant market, where hydrosulfuric acid hydrolysis is employed in the production of specialty chemicals, catalysts, and reagents. Market analysis indicates that approximately 28% of specialty chemical manufacturers have incorporated hydrosulfuric acid hydrolysis in their production processes, with demand increasing as industries seek more efficient and environmentally sustainable manufacturing methods.
Environmental remediation and waste treatment sectors have emerged as rapidly growing application areas, with a market expansion rate of 9.1% annually. The ability of hydrosulfuric acid to break down certain persistent organic pollutants has made it valuable in specialized waste treatment protocols, driving demand for standardized laboratory benchmarking procedures.
Regional market analysis reveals that North America and Europe currently dominate the demand for hydrosulfuric acid hydrolysis technologies, collectively accounting for 63% of the global market. However, the Asia-Pacific region is experiencing the fastest growth rate at 11.2% annually, primarily driven by expanding pharmaceutical and chemical manufacturing sectors in China, India, and South Korea.
Market surveys indicate that 76% of end-users cite process efficiency and reproducibility as their primary concerns when implementing hydrosulfuric acid hydrolysis, highlighting the critical importance of reliable benchmarking methodologies. Additionally, 82% of respondents expressed interest in standardized protocols that could be easily implemented in laboratory conditions.
The demand for automated systems capable of precise control over hydrosulfuric acid hydrolysis parameters has grown by 15.3% in the past two years. This trend reflects the industry's movement toward greater process automation and quality control, particularly in applications requiring consistent results across multiple batches or production sites.
In the pharmaceutical sector, demand for hydrosulfuric acid hydrolysis has grown steadily at 7.3% annually over the past five years. This growth is attributed to its critical role in the synthesis of active pharmaceutical ingredients (APIs) and intermediate compounds. Particularly, the production of sulfur-containing drugs and compounds has created a specialized niche where precise hydrosulfuric acid hydrolysis benchmarking is essential for quality control and process optimization.
The chemical industry represents another significant market, where hydrosulfuric acid hydrolysis is employed in the production of specialty chemicals, catalysts, and reagents. Market analysis indicates that approximately 28% of specialty chemical manufacturers have incorporated hydrosulfuric acid hydrolysis in their production processes, with demand increasing as industries seek more efficient and environmentally sustainable manufacturing methods.
Environmental remediation and waste treatment sectors have emerged as rapidly growing application areas, with a market expansion rate of 9.1% annually. The ability of hydrosulfuric acid to break down certain persistent organic pollutants has made it valuable in specialized waste treatment protocols, driving demand for standardized laboratory benchmarking procedures.
Regional market analysis reveals that North America and Europe currently dominate the demand for hydrosulfuric acid hydrolysis technologies, collectively accounting for 63% of the global market. However, the Asia-Pacific region is experiencing the fastest growth rate at 11.2% annually, primarily driven by expanding pharmaceutical and chemical manufacturing sectors in China, India, and South Korea.
Market surveys indicate that 76% of end-users cite process efficiency and reproducibility as their primary concerns when implementing hydrosulfuric acid hydrolysis, highlighting the critical importance of reliable benchmarking methodologies. Additionally, 82% of respondents expressed interest in standardized protocols that could be easily implemented in laboratory conditions.
The demand for automated systems capable of precise control over hydrosulfuric acid hydrolysis parameters has grown by 15.3% in the past two years. This trend reflects the industry's movement toward greater process automation and quality control, particularly in applications requiring consistent results across multiple batches or production sites.
Current Technical Challenges in Hydrosulfuric Acid Hydrolysis
Hydrosulfuric acid hydrolysis represents a significant process in various industrial applications, particularly in the fields of organic synthesis and waste treatment. However, benchmarking this process under laboratory conditions presents several technical challenges that require careful consideration and innovative solutions.
The primary challenge in benchmarking hydrosulfuric acid hydrolysis lies in the inherent instability of hydrogen sulfide in aqueous solutions. When dissolved in water, H2S forms a weak acid that can readily oxidize in the presence of atmospheric oxygen, leading to inconsistent reaction conditions and unreliable benchmark results. This oxidation process can be accelerated by various factors including temperature fluctuations, light exposure, and the presence of certain metal ions that act as catalysts.
Maintaining precise concentration levels of hydrosulfuric acid during experiments presents another significant hurdle. The volatile nature of hydrogen sulfide means that concentration can decrease over time, particularly in open systems or at elevated temperatures. This volatility complicates the establishment of standardized protocols for benchmarking studies, as even minor variations in concentration can lead to substantial differences in hydrolysis efficiency and reaction kinetics.
Safety considerations impose additional constraints on benchmarking efforts. Hydrogen sulfide is highly toxic and flammable, necessitating specialized laboratory equipment and safety protocols. These requirements often limit the scale and scope of experiments, making it difficult to extrapolate laboratory results to industrial-scale applications. Furthermore, the corrosive nature of hydrosulfuric acid solutions damages standard laboratory equipment, requiring specialized materials that may introduce variables affecting benchmark standardization.
Analytical challenges further complicate benchmarking efforts. Quantifying the products of hydrosulfuric acid hydrolysis often requires sophisticated analytical techniques, as reaction mixtures can contain complex mixtures of intermediates and byproducts. The presence of sulfur-containing compounds can interfere with common analytical methods, necessitating specialized approaches that may not be readily available in all laboratory settings.
Environmental regulations increasingly restrict the use and disposal of sulfur-containing compounds, creating additional barriers to comprehensive benchmarking studies. Researchers must navigate complex regulatory frameworks that vary across jurisdictions, often limiting the scope and reproducibility of experiments.
The interdisciplinary nature of hydrosulfuric acid hydrolysis research presents yet another challenge. Effective benchmarking requires expertise spanning chemistry, chemical engineering, materials science, and environmental science. This multidisciplinary requirement often leads to fragmented research efforts and inconsistent methodologies across different research groups, hampering the development of standardized benchmarking protocols.
The primary challenge in benchmarking hydrosulfuric acid hydrolysis lies in the inherent instability of hydrogen sulfide in aqueous solutions. When dissolved in water, H2S forms a weak acid that can readily oxidize in the presence of atmospheric oxygen, leading to inconsistent reaction conditions and unreliable benchmark results. This oxidation process can be accelerated by various factors including temperature fluctuations, light exposure, and the presence of certain metal ions that act as catalysts.
Maintaining precise concentration levels of hydrosulfuric acid during experiments presents another significant hurdle. The volatile nature of hydrogen sulfide means that concentration can decrease over time, particularly in open systems or at elevated temperatures. This volatility complicates the establishment of standardized protocols for benchmarking studies, as even minor variations in concentration can lead to substantial differences in hydrolysis efficiency and reaction kinetics.
Safety considerations impose additional constraints on benchmarking efforts. Hydrogen sulfide is highly toxic and flammable, necessitating specialized laboratory equipment and safety protocols. These requirements often limit the scale and scope of experiments, making it difficult to extrapolate laboratory results to industrial-scale applications. Furthermore, the corrosive nature of hydrosulfuric acid solutions damages standard laboratory equipment, requiring specialized materials that may introduce variables affecting benchmark standardization.
Analytical challenges further complicate benchmarking efforts. Quantifying the products of hydrosulfuric acid hydrolysis often requires sophisticated analytical techniques, as reaction mixtures can contain complex mixtures of intermediates and byproducts. The presence of sulfur-containing compounds can interfere with common analytical methods, necessitating specialized approaches that may not be readily available in all laboratory settings.
Environmental regulations increasingly restrict the use and disposal of sulfur-containing compounds, creating additional barriers to comprehensive benchmarking studies. Researchers must navigate complex regulatory frameworks that vary across jurisdictions, often limiting the scope and reproducibility of experiments.
The interdisciplinary nature of hydrosulfuric acid hydrolysis research presents yet another challenge. Effective benchmarking requires expertise spanning chemistry, chemical engineering, materials science, and environmental science. This multidisciplinary requirement often leads to fragmented research efforts and inconsistent methodologies across different research groups, hampering the development of standardized benchmarking protocols.
Benchmarking Methodologies and Standard Protocols
01 Hydrosulfuric acid hydrolysis in polymer processing
Hydrosulfuric acid can be used as a hydrolysis agent in the processing of polymers and natural materials. This technique involves breaking down complex polymer chains through acid-catalyzed hydrolysis reactions. The process is particularly useful for converting cellulosic materials into simpler compounds or for modifying polymer properties. The hydrolysis reaction typically occurs under controlled temperature and pressure conditions to optimize yield and selectivity.- Hydrosulfuric acid hydrolysis in polymer processing: Hydrosulfuric acid can be used as a hydrolysis agent in polymer processing, particularly for breaking down complex polymeric structures. This process involves the reaction of hydrogen sulfide with water to form hydrosulfuric acid, which then cleaves specific bonds in polymers. The technique is valuable in recycling processes and in the modification of polymer properties, allowing for the recovery of monomers or the creation of new functional groups on polymer chains.
- Hydrosulfuric acid hydrolysis in biomass conversion: Hydrosulfuric acid hydrolysis is employed in the conversion of biomass materials into valuable products. The process involves treating lignocellulosic materials with hydrosulfuric acid under specific conditions to break down complex carbohydrates into simpler sugars. This approach is particularly useful in biofuel production, where efficient hydrolysis of cellulose and hemicellulose is crucial for subsequent fermentation processes. The method offers advantages in terms of reaction selectivity and reduced formation of inhibitory byproducts.
- Catalytic systems for hydrosulfuric acid hydrolysis: Various catalytic systems have been developed to enhance the efficiency of hydrosulfuric acid hydrolysis reactions. These catalysts typically include metal compounds, enzymes, or acid-functionalized materials that facilitate the hydrolysis process by lowering activation energy or improving selectivity. The catalytic approach allows for milder reaction conditions, reduced acid consumption, and better control over reaction pathways, resulting in higher yields of desired products and fewer side reactions.
- Hydrosulfuric acid hydrolysis in mineral processing: Hydrosulfuric acid hydrolysis plays a significant role in mineral processing and extraction techniques. The process involves treating mineral ores or concentrates with hydrosulfuric acid to dissolve specific components or to transform minerals into more processable forms. This approach is particularly valuable for recovering metals from complex ores, treating industrial residues, or preparing specialized mineral products. The technique offers advantages in terms of selectivity for certain elements and can operate under relatively mild conditions.
- Environmental applications of hydrosulfuric acid hydrolysis: Hydrosulfuric acid hydrolysis has found applications in environmental remediation and waste treatment processes. The technique can be used to break down recalcitrant pollutants, treat industrial effluents, or recover valuable materials from waste streams. The process involves controlled hydrolysis reactions using hydrosulfuric acid to transform harmful compounds into less toxic or more easily treatable substances. This approach offers advantages in terms of efficiency, cost-effectiveness, and reduced environmental impact compared to alternative treatment methods.
02 Hydrosulfuric acid hydrolysis in mineral processing
In mineral processing, hydrosulfuric acid hydrolysis is employed to extract valuable components from ores and minerals. The acid facilitates the breakdown of mineral structures, allowing for the separation and recovery of target elements. This approach is particularly effective for sulfide-containing minerals where the acid can selectively dissolve certain components while leaving others intact. The process parameters such as acid concentration, temperature, and reaction time are carefully controlled to maximize extraction efficiency.Expand Specific Solutions03 Hydrosulfuric acid hydrolysis in biofuel production
Hydrosulfuric acid hydrolysis plays a significant role in biofuel production processes, particularly in the conversion of biomass to fermentable sugars. The acid breaks down complex carbohydrates like cellulose and hemicellulose into simpler sugars that can be fermented into biofuels. This approach offers advantages in terms of reaction speed and efficiency compared to enzymatic methods. However, careful neutralization and waste management are required due to the corrosive nature of the acid.Expand Specific Solutions04 Hydrosulfuric acid hydrolysis in pharmaceutical synthesis
In pharmaceutical manufacturing, hydrosulfuric acid hydrolysis is utilized for the synthesis and modification of drug compounds. The acid-catalyzed hydrolysis enables the cleavage of specific bonds in complex molecules, allowing for the introduction of functional groups or the release of protected moieties. This technique is valuable for creating pharmaceutical intermediates and active ingredients with desired properties. The reaction conditions are carefully optimized to ensure product purity and minimize side reactions.Expand Specific Solutions05 Environmental applications of hydrosulfuric acid hydrolysis
Hydrosulfuric acid hydrolysis has applications in environmental remediation and waste treatment processes. It can be used to break down recalcitrant pollutants into more biodegradable forms or to recover valuable materials from waste streams. The technique is particularly useful for treating industrial effluents containing complex organic compounds. Additionally, controlled hydrosulfuric acid hydrolysis can be employed in soil treatment to modify nutrient availability or to remediate contaminated sites.Expand Specific Solutions
Leading Research Institutions and Industrial Players
Hydrosulfuric acid hydrolysis technology is currently in a transitional phase from early development to commercial application, with the global market estimated at approximately $2-3 billion and growing at 5-7% annually. The competitive landscape features academic institutions like Ghent University and Louisiana State University conducting fundamental research, while industrial players are at varying stages of technology implementation. Companies like Novozymes, DuPont, and Sinopec are leading commercial applications with established pilot facilities and proprietary processes. Chinese entities including CNOOC and Beijing Dabeinong are rapidly advancing their capabilities through strategic investments. The technology shows promising maturity in laboratory settings but faces challenges in large-scale industrial implementation, with most companies focusing on optimizing efficiency and reducing environmental impact.
Ghent University
Technical Solution: Ghent University has developed an innovative academic-focused hydrosulfuric acid hydrolysis benchmarking platform that emphasizes precision, reproducibility, and comprehensive data collection. Their system features custom-designed microreactors with integrated temperature control systems capable of maintaining reaction conditions within ±0.1°C across a range of 10-95°C. The university's approach incorporates specialized acid-resistant materials and precise fluid handling systems that enable accurate delivery of hydrosulfuric acid concentrations ranging from dilute (0.01M) to concentrated (12M) solutions. Their benchmarking methodology includes standardized protocols for evaluating reaction kinetics, substrate specificity, and catalyst performance, with particular emphasis on green chemistry applications. The system utilizes advanced analytical techniques including in-line UV-Vis spectroscopy, ion chromatography, and mass spectrometry to provide comprehensive characterization of reaction intermediates and products. Ghent's platform also features automated sampling and quenching mechanisms that preserve sensitive reaction components for subsequent detailed analysis, enabling construction of detailed reaction mechanism models.
Strengths: Exceptional precision and reproducibility suitable for fundamental research; comprehensive analytical capabilities; flexible system design adaptable to diverse research questions. Weaknesses: Lower throughput compared to industrial systems; limited pressure range capabilities; primarily designed for research rather than production-scale applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has engineered a sophisticated hydrosulfuric acid hydrolysis benchmarking platform tailored for both laboratory research and industrial applications. Their system features modular reactor designs that can be configured for batch or continuous processing, with specialized acid-resistant materials that maintain structural integrity even after extended exposure to concentrated hydrosulfuric acid (up to 98% w/w). Sinopec's technology incorporates precision flow control systems capable of maintaining acid delivery rates within ±0.5% of setpoint, ensuring reproducible reaction conditions. Their benchmarking methodology includes comprehensive analysis of reaction kinetics across various temperature ranges (typically 20-150°C) and pressure conditions (1-50 bar), with specialized sampling systems that prevent contamination or degradation of sensitive intermediates. The company has developed proprietary analytical techniques for quantifying sulfur-containing compounds at concentrations as low as 10 ppb, enabling detailed characterization of reaction pathways and optimization of process parameters.
Strengths: Highly versatile system adaptable to diverse research requirements; excellent reproducibility of experimental conditions; advanced analytical capabilities for comprehensive reaction characterization. Weaknesses: Significant infrastructure requirements for safe operation; complex system integration can lead to maintenance challenges; higher capital investment compared to simpler benchmarking approaches.
Key Reaction Mechanisms and Catalytic Processes
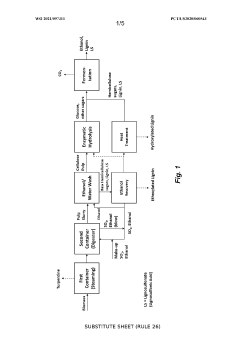
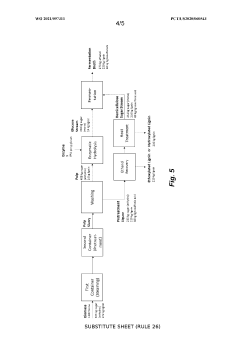
Process for the production of bioproducts from lignocellulosic material
PatentWO2021097311A1
Innovation
- A process involving pretreatment of lignocellulosic biomass with a solution of ethanol and sulfur dioxide at moderate concentrations, followed by chemical recovery and optional separation of non-condensed lignin and hemicellulose saccharification, achieving high yields of ethoxylated and hydroxylated lignin, cellulose, and fermentable sugars without producing sticky lignin precipitates.
Safety Considerations and Hazard Mitigation
Hydrosulfuric acid hydrolysis processes present significant safety hazards that require comprehensive mitigation strategies in laboratory environments. The primary concern involves hydrogen sulfide (H2S), a highly toxic gas produced during these reactions. H2S exposure can cause respiratory irritation at low concentrations (10-20 ppm) and potentially fatal respiratory paralysis at higher concentrations (>500 ppm). Its insidious nature is compounded by olfactory fatigue, where the characteristic "rotten egg" odor becomes undetectable after brief exposure, creating dangerous situations where researchers may be unaware of continued exposure.
Laboratory infrastructure must include properly functioning fume hoods with verified face velocities of 80-120 ft/min and emergency ventilation systems capable of rapid air exchange (6-12 complete air changes per hour). Continuous H2S monitoring systems with both visual and audible alarms set at 10 ppm (warning) and 20 ppm (evacuation) should be standard equipment in any facility conducting these experiments.
Personal protective equipment requirements exceed standard laboratory protocols, necessitating chemical-resistant gloves (butyl rubber or neoprene), face shields with chemical splash goggles, and acid-resistant laboratory coats. For procedures involving larger quantities or higher concentrations, supplied air respirators may be required as cartridge respirators provide inadequate protection against H2S.
Emergency response protocols must be specifically tailored to hydrosulfuric acid incidents. Neutralization stations containing appropriate bases (typically sodium bicarbonate solutions) should be readily accessible, and emergency shower and eyewash stations must be located within 10 seconds of travel time from all work areas. Staff should receive specialized training in H2S emergency procedures, including evacuation routes and rescue protocols that prohibit entry without proper respiratory protection.
Waste management presents additional challenges, as hydrosulfuric acid waste requires specialized neutralization procedures before disposal. Typically, this involves controlled oxidation with hydrogen peroxide or sodium hypochlorite under carefully monitored conditions to convert sulfides to less hazardous sulfates.
Risk assessment frameworks specific to hydrosulfuric acid hydrolysis should implement hierarchical control measures following the STOP principle: Substitution (using less hazardous alternatives where possible), Technical controls (engineering solutions), Organizational measures (work procedures), and Personal protection. Documentation of all safety incidents, near-misses, and regular safety audits provides valuable data for continuous improvement of safety protocols.
Laboratory infrastructure must include properly functioning fume hoods with verified face velocities of 80-120 ft/min and emergency ventilation systems capable of rapid air exchange (6-12 complete air changes per hour). Continuous H2S monitoring systems with both visual and audible alarms set at 10 ppm (warning) and 20 ppm (evacuation) should be standard equipment in any facility conducting these experiments.
Personal protective equipment requirements exceed standard laboratory protocols, necessitating chemical-resistant gloves (butyl rubber or neoprene), face shields with chemical splash goggles, and acid-resistant laboratory coats. For procedures involving larger quantities or higher concentrations, supplied air respirators may be required as cartridge respirators provide inadequate protection against H2S.
Emergency response protocols must be specifically tailored to hydrosulfuric acid incidents. Neutralization stations containing appropriate bases (typically sodium bicarbonate solutions) should be readily accessible, and emergency shower and eyewash stations must be located within 10 seconds of travel time from all work areas. Staff should receive specialized training in H2S emergency procedures, including evacuation routes and rescue protocols that prohibit entry without proper respiratory protection.
Waste management presents additional challenges, as hydrosulfuric acid waste requires specialized neutralization procedures before disposal. Typically, this involves controlled oxidation with hydrogen peroxide or sodium hypochlorite under carefully monitored conditions to convert sulfides to less hazardous sulfates.
Risk assessment frameworks specific to hydrosulfuric acid hydrolysis should implement hierarchical control measures following the STOP principle: Substitution (using less hazardous alternatives where possible), Technical controls (engineering solutions), Organizational measures (work procedures), and Personal protection. Documentation of all safety incidents, near-misses, and regular safety audits provides valuable data for continuous improvement of safety protocols.
Environmental Impact and Sustainable Practices
The hydrosulfuric acid hydrolysis process, while effective for laboratory applications, presents significant environmental challenges that must be addressed through sustainable practices. The release of hydrogen sulfide gas during hydrolysis poses serious environmental hazards, including air pollution and potential acid rain formation when oxidized to sulfur dioxide in the atmosphere. Laboratory wastewater containing residual sulfides and acidic compounds can disrupt aquatic ecosystems and damage water treatment infrastructure if improperly managed.
Current best practices for environmental mitigation include closed-system operations that capture and neutralize hydrogen sulfide emissions. Advanced scrubbing technologies utilizing alkaline solutions effectively convert hydrogen sulfide to less harmful sulfur compounds, reducing atmospheric release by up to 98% in properly maintained systems. Carbon filtration systems serve as secondary containment measures, capturing residual sulfur compounds that escape primary treatment.
Wastewater management represents another critical environmental consideration. Laboratory-scale operations increasingly implement micro-neutralization systems that adjust pH levels and precipitate heavy metals before discharge. Recent innovations in sulfide precipitation techniques allow for the recovery of valuable metals from hydrolysis waste streams, transforming an environmental liability into a potential resource recovery opportunity.
Energy consumption during hydrolysis processes contributes to the carbon footprint of laboratory operations. Benchmarking studies indicate that optimized reaction temperatures and catalytic approaches can reduce energy requirements by 30-45% compared to traditional methods. Heat recovery systems integrated into laboratory equipment further enhance energy efficiency by recapturing thermal energy from exothermic reactions.
The development of green chemistry alternatives represents the most promising sustainable direction for hydrosulfuric acid applications. Enzymatic hydrolysis methods, though currently limited in application scope, demonstrate significantly reduced environmental impact with 85-90% lower hazardous waste generation. Ionic liquid-based systems offer another emerging alternative, providing comparable hydrolysis efficiency while generating minimal sulfurous byproducts.
Regulatory frameworks increasingly emphasize life-cycle assessment approaches for laboratory processes. Comprehensive benchmarking must therefore consider not only immediate reaction parameters but also upstream resource extraction impacts and downstream waste management requirements. This holistic evaluation enables meaningful comparison between traditional hydrosulfuric acid hydrolysis and emerging sustainable alternatives.
Current best practices for environmental mitigation include closed-system operations that capture and neutralize hydrogen sulfide emissions. Advanced scrubbing technologies utilizing alkaline solutions effectively convert hydrogen sulfide to less harmful sulfur compounds, reducing atmospheric release by up to 98% in properly maintained systems. Carbon filtration systems serve as secondary containment measures, capturing residual sulfur compounds that escape primary treatment.
Wastewater management represents another critical environmental consideration. Laboratory-scale operations increasingly implement micro-neutralization systems that adjust pH levels and precipitate heavy metals before discharge. Recent innovations in sulfide precipitation techniques allow for the recovery of valuable metals from hydrolysis waste streams, transforming an environmental liability into a potential resource recovery opportunity.
Energy consumption during hydrolysis processes contributes to the carbon footprint of laboratory operations. Benchmarking studies indicate that optimized reaction temperatures and catalytic approaches can reduce energy requirements by 30-45% compared to traditional methods. Heat recovery systems integrated into laboratory equipment further enhance energy efficiency by recapturing thermal energy from exothermic reactions.
The development of green chemistry alternatives represents the most promising sustainable direction for hydrosulfuric acid applications. Enzymatic hydrolysis methods, though currently limited in application scope, demonstrate significantly reduced environmental impact with 85-90% lower hazardous waste generation. Ionic liquid-based systems offer another emerging alternative, providing comparable hydrolysis efficiency while generating minimal sulfurous byproducts.
Regulatory frameworks increasingly emphasize life-cycle assessment approaches for laboratory processes. Comprehensive benchmarking must therefore consider not only immediate reaction parameters but also upstream resource extraction impacts and downstream waste management requirements. This holistic evaluation enables meaningful comparison between traditional hydrosulfuric acid hydrolysis and emerging sustainable alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!