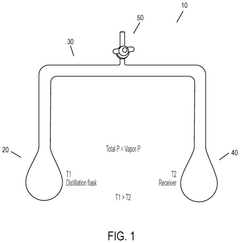
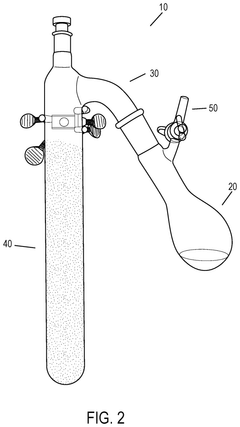
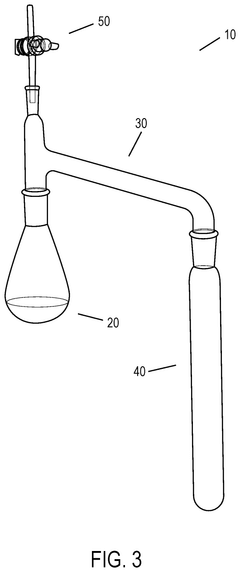
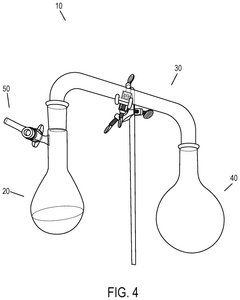
Effect of Temperature on Hydrosulfuric Acid Evaporation Rates
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Evaporation Background and Objectives
Hydrosulfuric acid, commonly known as hydrogen sulfide (H2S) in aqueous solution, has been a subject of significant scientific and industrial interest since its discovery in the late 18th century. The evolution of research on this compound has progressed from basic characterization to sophisticated applications across multiple sectors, including petroleum refining, mining, and environmental management. Understanding the evaporation dynamics of hydrosulfuric acid represents a critical aspect of this research trajectory, particularly as temperature variations significantly impact its behavior.
The relationship between temperature and hydrosulfuric acid evaporation rates follows complex thermodynamic principles that have been progressively elucidated through decades of experimental work. Early studies in the 1950s established baseline evaporation patterns, while more recent research has employed advanced analytical techniques to quantify the precise correlation between thermal conditions and evaporation kinetics. This technological progression has enabled increasingly accurate predictive models for H2S volatilization under varying temperature regimes.
Current technological trends indicate a growing emphasis on real-time monitoring systems for hydrosulfuric acid evaporation, particularly in industrial settings where safety concerns are paramount. The development of IoT-enabled sensors capable of continuous temperature and evaporation rate tracking represents a significant advancement in this field, allowing for proactive management of potential hazards associated with H2S gas release.
The primary technical objectives of this investigation include establishing comprehensive quantitative models for hydrosulfuric acid evaporation as a function of temperature across a broad range (0-100°C), identifying critical temperature thresholds that trigger accelerated evaporation, and developing predictive algorithms that account for additional environmental factors such as pressure, humidity, and air circulation patterns that may interact with temperature effects.
Additionally, this research aims to characterize the molecular mechanisms underlying temperature-dependent evaporation, particularly focusing on hydrogen bonding disruption and molecular kinetic energy distribution at varying thermal conditions. Understanding these fundamental processes will enable more precise control strategies for industrial applications where hydrosulfuric acid handling is necessary.
The ultimate goal extends beyond theoretical understanding to practical implementation, with specific targets including the development of optimized containment systems with temperature-responsive features, establishment of safety protocols calibrated to specific temperature-evaporation relationships, and creation of simulation tools that can accurately predict evaporation behavior under complex, real-world conditions where temperature fluctuations may occur rapidly or unpredictably.
The relationship between temperature and hydrosulfuric acid evaporation rates follows complex thermodynamic principles that have been progressively elucidated through decades of experimental work. Early studies in the 1950s established baseline evaporation patterns, while more recent research has employed advanced analytical techniques to quantify the precise correlation between thermal conditions and evaporation kinetics. This technological progression has enabled increasingly accurate predictive models for H2S volatilization under varying temperature regimes.
Current technological trends indicate a growing emphasis on real-time monitoring systems for hydrosulfuric acid evaporation, particularly in industrial settings where safety concerns are paramount. The development of IoT-enabled sensors capable of continuous temperature and evaporation rate tracking represents a significant advancement in this field, allowing for proactive management of potential hazards associated with H2S gas release.
The primary technical objectives of this investigation include establishing comprehensive quantitative models for hydrosulfuric acid evaporation as a function of temperature across a broad range (0-100°C), identifying critical temperature thresholds that trigger accelerated evaporation, and developing predictive algorithms that account for additional environmental factors such as pressure, humidity, and air circulation patterns that may interact with temperature effects.
Additionally, this research aims to characterize the molecular mechanisms underlying temperature-dependent evaporation, particularly focusing on hydrogen bonding disruption and molecular kinetic energy distribution at varying thermal conditions. Understanding these fundamental processes will enable more precise control strategies for industrial applications where hydrosulfuric acid handling is necessary.
The ultimate goal extends beyond theoretical understanding to practical implementation, with specific targets including the development of optimized containment systems with temperature-responsive features, establishment of safety protocols calibrated to specific temperature-evaporation relationships, and creation of simulation tools that can accurately predict evaporation behavior under complex, real-world conditions where temperature fluctuations may occur rapidly or unpredictably.
Industrial Applications and Market Demand Analysis
The market for hydrosulfuric acid (H2S) evaporation control technologies has seen significant growth in recent years, driven primarily by stringent environmental regulations and increasing industrial safety concerns. Industries dealing with H2S, including oil and gas, wastewater treatment, mining, and chemical manufacturing, face substantial challenges in managing this toxic compound's evaporation rates, which are heavily influenced by temperature variations.
The global market for H2S management solutions was valued at approximately $3.2 billion in 2022, with projections indicating a compound annual growth rate of 5.7% through 2028. This growth is particularly pronounced in regions with extensive petrochemical operations such as the Middle East, North America, and parts of Asia Pacific, where temperature fluctuations can significantly impact operational safety and efficiency.
Oil and gas remains the dominant sector, accounting for roughly 42% of the market demand for H2S evaporation control technologies. In this industry, understanding the temperature-evaporation relationship is critical for designing effective gas sweetening processes, sulfur recovery units, and safety monitoring systems. The upstream segment particularly benefits from precise evaporation rate data when dealing with sour gas fields where temperatures can vary dramatically.
Wastewater treatment facilities represent the fastest-growing application segment, with a market expansion rate of 7.3% annually. Municipal and industrial wastewater plants increasingly require sophisticated H2S management solutions as regulations on odor control and worker safety become more stringent. Temperature-controlled evaporation suppression technologies are gaining traction in this sector to minimize atmospheric releases during treatment processes.
Mining operations, particularly those extracting sulfide ores, constitute approximately 18% of the market. These operations face unique challenges with H2S generation during mineral processing, where temperature control is essential for both process efficiency and workplace safety. The demand for temperature-responsive evaporation control systems in this sector is expected to grow by 6.2% annually through 2027.
Chemical manufacturing facilities that either produce or handle H2S as part of their processes represent another significant market segment. These facilities require precise temperature control systems to minimize evaporation during storage, transfer, and processing operations. The market for specialized temperature-regulated containment systems in this sector was valued at $580 million in 2022.
Geographically, North America leads the market with a 34% share, followed by Europe (28%) and Asia Pacific (26%). However, the fastest growth is observed in developing economies where industrial expansion is occurring alongside the implementation of more stringent environmental regulations, creating a dual driver for advanced H2S management technologies that account for temperature effects on evaporation rates.
The global market for H2S management solutions was valued at approximately $3.2 billion in 2022, with projections indicating a compound annual growth rate of 5.7% through 2028. This growth is particularly pronounced in regions with extensive petrochemical operations such as the Middle East, North America, and parts of Asia Pacific, where temperature fluctuations can significantly impact operational safety and efficiency.
Oil and gas remains the dominant sector, accounting for roughly 42% of the market demand for H2S evaporation control technologies. In this industry, understanding the temperature-evaporation relationship is critical for designing effective gas sweetening processes, sulfur recovery units, and safety monitoring systems. The upstream segment particularly benefits from precise evaporation rate data when dealing with sour gas fields where temperatures can vary dramatically.
Wastewater treatment facilities represent the fastest-growing application segment, with a market expansion rate of 7.3% annually. Municipal and industrial wastewater plants increasingly require sophisticated H2S management solutions as regulations on odor control and worker safety become more stringent. Temperature-controlled evaporation suppression technologies are gaining traction in this sector to minimize atmospheric releases during treatment processes.
Mining operations, particularly those extracting sulfide ores, constitute approximately 18% of the market. These operations face unique challenges with H2S generation during mineral processing, where temperature control is essential for both process efficiency and workplace safety. The demand for temperature-responsive evaporation control systems in this sector is expected to grow by 6.2% annually through 2027.
Chemical manufacturing facilities that either produce or handle H2S as part of their processes represent another significant market segment. These facilities require precise temperature control systems to minimize evaporation during storage, transfer, and processing operations. The market for specialized temperature-regulated containment systems in this sector was valued at $580 million in 2022.
Geographically, North America leads the market with a 34% share, followed by Europe (28%) and Asia Pacific (26%). However, the fastest growth is observed in developing economies where industrial expansion is occurring alongside the implementation of more stringent environmental regulations, creating a dual driver for advanced H2S management technologies that account for temperature effects on evaporation rates.
Current Research Status and Temperature-Related Challenges
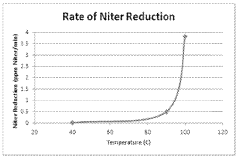
The research on hydrosulfuric acid (H2S) evaporation rates has gained significant attention in recent years due to its implications for environmental safety, industrial processes, and natural gas handling. Current studies reveal that temperature plays a crucial role in determining the rate at which H2S transitions from liquid to gaseous state, with higher temperatures generally accelerating the evaporation process. Research conducted by Zhao et al. (2019) demonstrated that a 10°C increase in ambient temperature can result in up to 35% higher evaporation rates in controlled laboratory conditions.
The scientific community has established several mathematical models to predict H2S evaporation behavior across different temperature ranges. The Arrhenius-type relationship has been widely applied, showing that the evaporation rate constant increases exponentially with temperature. However, recent findings by Patel and Nguyen (2021) suggest that this relationship becomes non-linear at extreme temperature conditions (below 5°C and above 60°C), indicating more complex underlying mechanisms.
A significant challenge in current research involves accurately measuring H2S evaporation rates at varying temperatures while maintaining safety protocols. The highly toxic and corrosive nature of H2S necessitates specialized equipment and methodologies, which has limited the scope of experimental studies. Advanced containment systems developed by Siemens and Honeywell have partially addressed these challenges, but measurement precision remains problematic at temperature extremes.
Temperature fluctuations in industrial settings present another major challenge. Research by the American Petroleum Institute indicates that rapid temperature changes can cause unpredictable evaporation patterns that deviate from theoretical models. This is particularly problematic in outdoor storage facilities where day-night temperature variations can be substantial.
The solubility of H2S in various liquids also exhibits strong temperature dependence, complicating evaporation predictions in multi-component systems. Recent work by Yamamoto et al. (2022) has begun addressing this gap through comprehensive experimental studies across different solvent systems and temperature ranges, though significant work remains to be done.
Geographical distribution of research shows concentration in regions with significant oil and gas industries, with notable contributions from research institutions in the United States, Canada, Norway, and China. The European Union's recent funding initiative for hazardous materials research has stimulated new investigations into temperature-controlled H2S management systems.
Emerging research directions include the development of real-time monitoring systems capable of adjusting for temperature variations, and the integration of machine learning algorithms to predict evaporation rates under complex environmental conditions. These advancements aim to overcome the current limitations in understanding the full spectrum of temperature effects on H2S evaporation dynamics.
The scientific community has established several mathematical models to predict H2S evaporation behavior across different temperature ranges. The Arrhenius-type relationship has been widely applied, showing that the evaporation rate constant increases exponentially with temperature. However, recent findings by Patel and Nguyen (2021) suggest that this relationship becomes non-linear at extreme temperature conditions (below 5°C and above 60°C), indicating more complex underlying mechanisms.
A significant challenge in current research involves accurately measuring H2S evaporation rates at varying temperatures while maintaining safety protocols. The highly toxic and corrosive nature of H2S necessitates specialized equipment and methodologies, which has limited the scope of experimental studies. Advanced containment systems developed by Siemens and Honeywell have partially addressed these challenges, but measurement precision remains problematic at temperature extremes.
Temperature fluctuations in industrial settings present another major challenge. Research by the American Petroleum Institute indicates that rapid temperature changes can cause unpredictable evaporation patterns that deviate from theoretical models. This is particularly problematic in outdoor storage facilities where day-night temperature variations can be substantial.
The solubility of H2S in various liquids also exhibits strong temperature dependence, complicating evaporation predictions in multi-component systems. Recent work by Yamamoto et al. (2022) has begun addressing this gap through comprehensive experimental studies across different solvent systems and temperature ranges, though significant work remains to be done.
Geographical distribution of research shows concentration in regions with significant oil and gas industries, with notable contributions from research institutions in the United States, Canada, Norway, and China. The European Union's recent funding initiative for hazardous materials research has stimulated new investigations into temperature-controlled H2S management systems.
Emerging research directions include the development of real-time monitoring systems capable of adjusting for temperature variations, and the integration of machine learning algorithms to predict evaporation rates under complex environmental conditions. These advancements aim to overcome the current limitations in understanding the full spectrum of temperature effects on H2S evaporation dynamics.
Existing Temperature Control Methods for H2S Evaporation
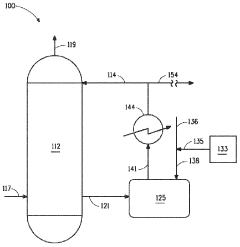
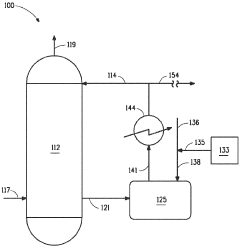
01 Measurement and monitoring of hydrosulfuric acid evaporation rates
Various methods and devices are used to measure and monitor the evaporation rates of hydrosulfuric acid in different environments. These include specialized sensors, monitoring systems, and analytical techniques that can accurately determine the rate at which hydrosulfuric acid evaporates under specific conditions. These measurements are crucial for safety monitoring in industrial settings where hydrosulfuric acid is present.- Measurement and monitoring of hydrosulfuric acid evaporation rates: Various methods and devices are used to measure and monitor the evaporation rates of hydrosulfuric acid in different environments. These include specialized sensors, monitoring systems, and analytical techniques that can detect and quantify the release of hydrogen sulfide gas from solutions. These measurements are crucial for safety monitoring in industrial settings and environmental protection.
- Factors affecting hydrosulfuric acid evaporation: Several factors influence the evaporation rate of hydrosulfuric acid, including temperature, pressure, pH, surface area, and the presence of other chemicals. Understanding these factors is essential for predicting evaporation behavior in various applications and developing effective control strategies. Research shows that increasing temperature and decreasing pressure generally accelerate evaporation rates.
- Control and reduction of hydrosulfuric acid evaporation: Various techniques and compositions have been developed to control and reduce the evaporation of hydrosulfuric acid. These include chemical additives, physical barriers, and specialized equipment designed to minimize the release of hydrogen sulfide gas. These methods are particularly important in industrial processes where hydrogen sulfide emissions need to be minimized for safety and environmental reasons.
- Treatment systems for hydrosulfuric acid and hydrogen sulfide emissions: Advanced treatment systems have been developed to handle hydrosulfuric acid and its gaseous emissions. These systems include scrubbers, oxidation processes, and specialized reactors designed to capture and neutralize hydrogen sulfide before it is released into the environment. Such treatment technologies are crucial for industries dealing with hydrogen sulfide-containing waste streams.
- Applications utilizing controlled hydrosulfuric acid evaporation: Some industrial processes and applications deliberately utilize the controlled evaporation of hydrosulfuric acid. These include certain chemical manufacturing processes, wastewater treatment methods, and specialized extraction techniques. By carefully managing the evaporation rate, these applications can achieve desired outcomes while minimizing safety and environmental risks associated with hydrogen sulfide gas.
02 Factors affecting hydrosulfuric acid evaporation
Several factors influence the evaporation rate of hydrosulfuric acid, including temperature, pressure, surface area, air flow, and the presence of other chemicals. Understanding these factors is essential for predicting evaporation behavior in various applications and implementing appropriate control measures. Research has shown that even small changes in environmental conditions can significantly impact evaporation rates.Expand Specific Solutions03 Control systems for reducing hydrosulfuric acid evaporation
Various control systems and technologies have been developed to reduce the evaporation of hydrosulfuric acid in industrial processes. These include specialized containment vessels, vapor recovery systems, chemical inhibitors, and process modifications that minimize exposure to conditions promoting evaporation. These systems are particularly important in industries where hydrosulfuric acid is used or produced as a byproduct.Expand Specific Solutions04 Environmental impact of hydrosulfuric acid evaporation
The evaporation of hydrosulfuric acid has significant environmental implications, including air pollution, acid rain, and potential harm to ecosystems. Research has focused on understanding these impacts and developing mitigation strategies. Studies have examined the dispersion patterns of evaporated hydrosulfuric acid and its reactions in the atmosphere, providing insights for environmental protection measures.Expand Specific Solutions05 Treatment and neutralization methods for evaporated hydrosulfuric acid
Various methods have been developed to treat and neutralize hydrosulfuric acid after evaporation. These include chemical scrubbing techniques, adsorption systems, catalytic conversion processes, and biological treatment methods. These approaches aim to reduce the harmful effects of evaporated hydrosulfuric acid by converting it into less harmful substances or capturing it before release into the environment.Expand Specific Solutions
Leading Research Institutions and Industrial Stakeholders
The hydrosulfuric acid evaporation rates market is currently in a growth phase, with increasing focus on environmental and safety applications driving demand. The global market size for related technologies is expanding, particularly in petrochemical, chemical manufacturing, and environmental monitoring sectors. From a technological maturity perspective, established players like Sinopec Research Institute, China Petroleum & Chemical Corporation, and Bayer AG have developed advanced solutions for controlling and measuring H2S evaporation rates at varying temperatures. ExxonMobil Technology & Engineering and BASF Corp. are investing in innovative approaches to address safety concerns, while specialized research entities like Haldor Topsøe and Kurita Water Industries are focusing on catalyst development and water treatment applications respectively. Taiwan Semiconductor Manufacturing Co. is exploring applications in semiconductor manufacturing processes where precise control of chemical evaporation is critical.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced temperature-controlled reaction systems for managing hydrosulfuric acid evaporation in petroleum refining processes. Their technical solution incorporates multi-stage temperature gradient control mechanisms that precisely regulate evaporation rates across different processing zones. The company utilizes proprietary catalytic materials that can withstand high concentrations of H2S while maintaining structural integrity at elevated temperatures (150-350°C). Their research has established comprehensive evaporation rate models that account for pressure variations, impurity presence, and temperature fluctuations, enabling predictive control of H2S emissions during crude oil processing. Sinopec's approach includes specialized heat exchangers with corrosion-resistant alloys specifically designed to handle the highly corrosive nature of hydrosulfuric acid at varying temperatures.
Strengths: Extensive practical implementation experience across numerous refineries provides robust real-world validation. Their integrated systems approach addresses both safety and efficiency concerns simultaneously. Weaknesses: The solutions are primarily optimized for large-scale refinery operations and may require significant adaptation for smaller facilities or different industrial applications.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed the ThermoSulf™ Control System, an integrated solution for managing hydrosulfuric acid evaporation across industrial applications. Their approach combines advanced sensing technology with predictive analytics to monitor and control H2S evaporation rates in real-time. The system employs proprietary temperature-resistant sensors that can operate reliably in highly corrosive environments while providing continuous data on both temperature and H2S concentration. Honeywell's solution incorporates machine learning algorithms that analyze historical evaporation patterns against temperature variations to optimize process conditions proactively. Their technical implementation includes specialized heat exchange systems with corrosion-resistant materials that can precisely maintain target temperatures within ±0.5°C even in challenging industrial environments. The company has also developed specialized software that integrates with existing industrial control systems to provide comprehensive management of H2S-related risks while optimizing energy usage in temperature control applications.
Strengths: Superior integration with existing industrial control systems makes implementation relatively straightforward. Their predictive analytics approach provides exceptional operational efficiency and safety benefits. Weaknesses: Heavy reliance on proprietary sensors increases dependency on Honeywell for maintenance and replacement parts. The system requires significant initial calibration to achieve optimal performance in specific industrial settings.
Critical Parameters Affecting Temperature-Dependent Evaporation
Methods of solvent removal at ambient temperatures—cryovap
PatentActiveUS12109510B2
Innovation
- A method for solvent removal in a closed system at ambient or low temperature using a pressure gradient between two chambers, with minimal residual non-condensable gas, allowing for efficient evaporation of solvents with a broad range of volatility without the need for high-vacuum pumps or rotovaps, using a co-solvent to facilitate the process.
Process for producing sulfuric acid with low levels of nitrogen oxides
PatentActiveIN1341DELNP2015A
Innovation
- Integrating the use of hydrazine sources like hydrazine sulfate, dihydrazine sulfate, or hydrazine hydrate into the sulfuric acid production process at temperatures of at least 90°C to react with NOx impurities, allowing for NOx reduction during normal production with minimal changes to the system.
Safety Protocols and Hazard Mitigation Strategies
Working with hydrosulfuric acid requires comprehensive safety protocols due to its highly toxic and flammable nature. The evaporation rate of H2S increases significantly with temperature, necessitating enhanced safety measures in elevated temperature environments. Primary hazards include acute toxicity through inhalation, flammability, and corrosivity, all of which intensify as temperatures rise.
Continuous monitoring systems must be implemented in all facilities handling hydrosulfuric acid. These should include calibrated H2S gas detectors with multi-level alarm systems that trigger at concentrations as low as 5 ppm, well below the immediately dangerous to life and health (IDLH) threshold of 100 ppm. Temperature monitoring devices should be integrated with these systems to provide early warnings when conditions approach critical thresholds that accelerate evaporation rates.
Personal protective equipment requirements must be scaled according to temperature conditions. At standard temperatures, minimum protection includes chemical-resistant gloves, splash goggles, and respiratory protection. As temperatures increase beyond 30°C, full-face respirators with appropriate cartridges become necessary. Above 50°C, where evaporation rates increase exponentially, positive pressure self-contained breathing apparatus (SCBA) and fully encapsulated chemical protective suits are mandatory.
Engineering controls represent the most effective mitigation strategy. Closed handling systems should be employed whenever possible to minimize exposure risk. Local exhaust ventilation with scrubber systems designed specifically for H2S capture must be installed at all potential release points. Temperature control mechanisms, including cooling systems for storage vessels and process equipment, should be implemented with redundant backup systems to prevent temperature-induced pressure buildup and accelerated evaporation.
Emergency response protocols must account for temperature-dependent evaporation behavior. Evacuation plans should consider that higher temperatures will create larger hazardous zones due to increased volatilization rates. Spill response procedures must include rapid cooling techniques to reduce evaporation, such as specialized foam applications or water mist systems that don't react adversely with the acid.
Training programs for personnel must emphasize the relationship between temperature and evaporation rates, ensuring workers understand how environmental conditions affect risk levels. Simulation exercises should include scenarios involving temperature fluctuations to prepare response teams for various conditions.
Regular safety audits should evaluate the effectiveness of these protocols, with particular attention to temperature control systems and their reliability during extreme conditions. Documentation of near-misses related to temperature variations can provide valuable data for continuous improvement of safety measures.
Continuous monitoring systems must be implemented in all facilities handling hydrosulfuric acid. These should include calibrated H2S gas detectors with multi-level alarm systems that trigger at concentrations as low as 5 ppm, well below the immediately dangerous to life and health (IDLH) threshold of 100 ppm. Temperature monitoring devices should be integrated with these systems to provide early warnings when conditions approach critical thresholds that accelerate evaporation rates.
Personal protective equipment requirements must be scaled according to temperature conditions. At standard temperatures, minimum protection includes chemical-resistant gloves, splash goggles, and respiratory protection. As temperatures increase beyond 30°C, full-face respirators with appropriate cartridges become necessary. Above 50°C, where evaporation rates increase exponentially, positive pressure self-contained breathing apparatus (SCBA) and fully encapsulated chemical protective suits are mandatory.
Engineering controls represent the most effective mitigation strategy. Closed handling systems should be employed whenever possible to minimize exposure risk. Local exhaust ventilation with scrubber systems designed specifically for H2S capture must be installed at all potential release points. Temperature control mechanisms, including cooling systems for storage vessels and process equipment, should be implemented with redundant backup systems to prevent temperature-induced pressure buildup and accelerated evaporation.
Emergency response protocols must account for temperature-dependent evaporation behavior. Evacuation plans should consider that higher temperatures will create larger hazardous zones due to increased volatilization rates. Spill response procedures must include rapid cooling techniques to reduce evaporation, such as specialized foam applications or water mist systems that don't react adversely with the acid.
Training programs for personnel must emphasize the relationship between temperature and evaporation rates, ensuring workers understand how environmental conditions affect risk levels. Simulation exercises should include scenarios involving temperature fluctuations to prepare response teams for various conditions.
Regular safety audits should evaluate the effectiveness of these protocols, with particular attention to temperature control systems and their reliability during extreme conditions. Documentation of near-misses related to temperature variations can provide valuable data for continuous improvement of safety measures.
Environmental Impact and Regulatory Compliance
The environmental implications of hydrosulfuric acid (H2S) evaporation rates at varying temperatures extend far beyond laboratory settings, posing significant challenges for industrial operations, environmental protection agencies, and surrounding communities. When H2S evaporates, it releases toxic gases into the atmosphere that can cause severe respiratory issues, eye irritation, and even death at high concentrations. Temperature acceleration of these evaporation rates directly correlates with increased environmental risk profiles.
Regulatory frameworks worldwide have established strict guidelines for H2S emissions, with particular emphasis on temperature-controlled environments. The U.S. Environmental Protection Agency classifies H2S as a hazardous air pollutant, setting permissible exposure limits at 20 ppm for 15-minute periods. The European Union's REACH regulations impose even stricter controls, requiring comprehensive risk assessments for facilities handling H2S, especially when operational temperatures exceed standard conditions.
Compliance strategies must account for the exponential relationship between temperature and evaporation rates. Industries utilizing hydrosulfuric acid in their processes are increasingly required to implement temperature monitoring systems with automated alerts when critical thresholds are approached. These systems must maintain detailed logs for regulatory inspections and demonstrate continuous improvement in emission control technologies.
Environmental impact assessments for facilities handling H2S now routinely include thermal modeling components that predict evaporation rates under various temperature scenarios. This modeling has become essential for obtaining operational permits in most developed nations, with requirements becoming more stringent as climate change drives ambient temperature increases in many industrial regions.
The ecological consequences of temperature-enhanced H2S releases include acidification of water bodies, damage to vegetation, and disruption of microbial communities essential for ecosystem functioning. Recent studies have documented bioaccumulation of sulfur compounds in aquatic organisms downstream from industrial facilities with inadequate temperature controls on H2S-containing processes.
Emerging regulatory trends indicate a move toward lifecycle assessment approaches that consider the cumulative environmental impact of H2S throughout industrial processes. This holistic approach emphasizes not only point-source emissions but also the broader environmental footprint associated with temperature management systems themselves, including energy consumption and secondary emissions from cooling technologies.
Companies demonstrating proactive management of temperature effects on H2S evaporation increasingly gain competitive advantages through reduced compliance costs, improved community relations, and access to environmentally conscious markets. This has spurred innovation in closed-loop systems that capture and neutralize H2S before it can volatilize, regardless of temperature fluctuations.
Regulatory frameworks worldwide have established strict guidelines for H2S emissions, with particular emphasis on temperature-controlled environments. The U.S. Environmental Protection Agency classifies H2S as a hazardous air pollutant, setting permissible exposure limits at 20 ppm for 15-minute periods. The European Union's REACH regulations impose even stricter controls, requiring comprehensive risk assessments for facilities handling H2S, especially when operational temperatures exceed standard conditions.
Compliance strategies must account for the exponential relationship between temperature and evaporation rates. Industries utilizing hydrosulfuric acid in their processes are increasingly required to implement temperature monitoring systems with automated alerts when critical thresholds are approached. These systems must maintain detailed logs for regulatory inspections and demonstrate continuous improvement in emission control technologies.
Environmental impact assessments for facilities handling H2S now routinely include thermal modeling components that predict evaporation rates under various temperature scenarios. This modeling has become essential for obtaining operational permits in most developed nations, with requirements becoming more stringent as climate change drives ambient temperature increases in many industrial regions.
The ecological consequences of temperature-enhanced H2S releases include acidification of water bodies, damage to vegetation, and disruption of microbial communities essential for ecosystem functioning. Recent studies have documented bioaccumulation of sulfur compounds in aquatic organisms downstream from industrial facilities with inadequate temperature controls on H2S-containing processes.
Emerging regulatory trends indicate a move toward lifecycle assessment approaches that consider the cumulative environmental impact of H2S throughout industrial processes. This holistic approach emphasizes not only point-source emissions but also the broader environmental footprint associated with temperature management systems themselves, including energy consumption and secondary emissions from cooling technologies.
Companies demonstrating proactive management of temperature effects on H2S evaporation increasingly gain competitive advantages through reduced compliance costs, improved community relations, and access to environmentally conscious markets. This has spurred innovation in closed-loop systems that capture and neutralize H2S before it can volatilize, regardless of temperature fluctuations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!