Emission Control Strategies for Hydrosulfuric Acid in Industries
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Emission Control Background and Objectives
Hydrogen sulfide (H2S) emissions represent a significant environmental and health concern across multiple industries. The evolution of emission control technologies for this toxic compound has been driven by increasingly stringent regulatory frameworks and growing awareness of its detrimental effects. Historically, H2S control methods have progressed from basic chemical scrubbing techniques in the early 20th century to sophisticated biological treatment systems and advanced catalytic conversion processes in recent decades.
The industrial sources of H2S emissions are diverse, with petroleum refining, natural gas processing, wastewater treatment, and pulp and paper manufacturing being primary contributors. Each sector presents unique challenges for emission control due to varying H2S concentrations, presence of other contaminants, and operational conditions. This technological diversity has necessitated the development of specialized control strategies tailored to specific industrial applications.
Recent technological trends indicate a shift toward more sustainable and cost-effective H2S control methods. Innovations in biotechnology have enabled the development of biofilters and bioscrubbers that utilize microorganisms to convert H2S into less harmful compounds. Simultaneously, advancements in material science have led to improved catalysts and adsorbents with enhanced selectivity and capacity for H2S removal.
The global regulatory landscape for H2S emissions continues to evolve, with many countries implementing progressively lower emission thresholds. This regulatory pressure, coupled with corporate sustainability initiatives, has accelerated research and development efforts in this field. The World Health Organization and various environmental protection agencies have established guidelines for H2S exposure limits, further emphasizing the importance of effective control strategies.
The primary objectives of current H2S emission control research include developing technologies that achieve higher removal efficiencies while minimizing energy consumption and operational costs. There is also a growing focus on creating integrated systems that can simultaneously address multiple pollutants, including H2S, in industrial exhaust streams. Additionally, researchers aim to design more robust control systems capable of handling fluctuations in H2S concentrations and operating conditions.
Another critical goal is the development of real-time monitoring technologies that can provide accurate measurements of H2S concentrations, enabling more responsive and efficient control strategies. This aspect has gained importance as industries move toward automated and intelligent emission management systems that can optimize performance based on continuous data analysis.
Looking forward, the trajectory of H2S emission control technology is expected to align with broader industrial sustainability goals, including carbon neutrality and circular economy principles. This alignment will likely drive innovation toward solutions that not only remove H2S but also potentially recover valuable byproducts or energy from the treatment process.
The industrial sources of H2S emissions are diverse, with petroleum refining, natural gas processing, wastewater treatment, and pulp and paper manufacturing being primary contributors. Each sector presents unique challenges for emission control due to varying H2S concentrations, presence of other contaminants, and operational conditions. This technological diversity has necessitated the development of specialized control strategies tailored to specific industrial applications.
Recent technological trends indicate a shift toward more sustainable and cost-effective H2S control methods. Innovations in biotechnology have enabled the development of biofilters and bioscrubbers that utilize microorganisms to convert H2S into less harmful compounds. Simultaneously, advancements in material science have led to improved catalysts and adsorbents with enhanced selectivity and capacity for H2S removal.
The global regulatory landscape for H2S emissions continues to evolve, with many countries implementing progressively lower emission thresholds. This regulatory pressure, coupled with corporate sustainability initiatives, has accelerated research and development efforts in this field. The World Health Organization and various environmental protection agencies have established guidelines for H2S exposure limits, further emphasizing the importance of effective control strategies.
The primary objectives of current H2S emission control research include developing technologies that achieve higher removal efficiencies while minimizing energy consumption and operational costs. There is also a growing focus on creating integrated systems that can simultaneously address multiple pollutants, including H2S, in industrial exhaust streams. Additionally, researchers aim to design more robust control systems capable of handling fluctuations in H2S concentrations and operating conditions.
Another critical goal is the development of real-time monitoring technologies that can provide accurate measurements of H2S concentrations, enabling more responsive and efficient control strategies. This aspect has gained importance as industries move toward automated and intelligent emission management systems that can optimize performance based on continuous data analysis.
Looking forward, the trajectory of H2S emission control technology is expected to align with broader industrial sustainability goals, including carbon neutrality and circular economy principles. This alignment will likely drive innovation toward solutions that not only remove H2S but also potentially recover valuable byproducts or energy from the treatment process.
Market Demand for H2S Abatement Technologies
The global market for hydrogen sulfide (H2S) abatement technologies has been experiencing significant growth, driven by increasingly stringent environmental regulations and heightened awareness of the health hazards associated with H2S emissions. Current market assessments value the global H2S removal technology market at approximately 1.5 billion USD, with projections indicating a compound annual growth rate of 5-7% through 2030.
Primary demand stems from oil and gas processing facilities, which contribute nearly 40% of the total market share. These operations frequently encounter high concentrations of H2S in natural gas streams and refinery processes, necessitating effective removal technologies to meet product specifications and environmental standards. The petroleum refining sector specifically requires solutions capable of handling variable H2S concentrations while maintaining operational efficiency.
Wastewater treatment represents the second-largest market segment, accounting for roughly 25% of demand. Municipal facilities and industrial operations generating sulfide-rich effluent require specialized treatment systems to prevent atmospheric releases and protect infrastructure from corrosion damage. This sector shows particular sensitivity to cost-effectiveness and operational simplicity in abatement solutions.
Mining operations, particularly those extracting sulfide ores, constitute approximately 15% of the market. These facilities face unique challenges with intermittent but potentially high-concentration H2S releases during extraction and processing activities. The remaining market share is distributed among chemical manufacturing, pulp and paper production, and food processing industries.
Geographically, North America and Europe currently dominate market demand, collectively representing over 60% of global expenditure on H2S abatement technologies. However, the most rapid growth is occurring in Asia-Pacific regions, particularly China and India, where industrial expansion coincides with evolving environmental regulatory frameworks. Market analysts project this region will overtake traditional markets within the next decade.
Customer requirements are increasingly focused on integrated solutions that address not only H2S removal but also recovery of valuable sulfur compounds, energy efficiency, and reduced chemical consumption. Technologies demonstrating lower operational costs and smaller environmental footprints are gaining market share over traditional approaches, despite potentially higher initial capital investments.
The market exhibits growing demand for modular, scalable systems that can accommodate fluctuating H2S concentrations and varying process conditions. Additionally, there is increasing interest in real-time monitoring and control systems that optimize abatement processes while providing compliance documentation for regulatory purposes.
Primary demand stems from oil and gas processing facilities, which contribute nearly 40% of the total market share. These operations frequently encounter high concentrations of H2S in natural gas streams and refinery processes, necessitating effective removal technologies to meet product specifications and environmental standards. The petroleum refining sector specifically requires solutions capable of handling variable H2S concentrations while maintaining operational efficiency.
Wastewater treatment represents the second-largest market segment, accounting for roughly 25% of demand. Municipal facilities and industrial operations generating sulfide-rich effluent require specialized treatment systems to prevent atmospheric releases and protect infrastructure from corrosion damage. This sector shows particular sensitivity to cost-effectiveness and operational simplicity in abatement solutions.
Mining operations, particularly those extracting sulfide ores, constitute approximately 15% of the market. These facilities face unique challenges with intermittent but potentially high-concentration H2S releases during extraction and processing activities. The remaining market share is distributed among chemical manufacturing, pulp and paper production, and food processing industries.
Geographically, North America and Europe currently dominate market demand, collectively representing over 60% of global expenditure on H2S abatement technologies. However, the most rapid growth is occurring in Asia-Pacific regions, particularly China and India, where industrial expansion coincides with evolving environmental regulatory frameworks. Market analysts project this region will overtake traditional markets within the next decade.
Customer requirements are increasingly focused on integrated solutions that address not only H2S removal but also recovery of valuable sulfur compounds, energy efficiency, and reduced chemical consumption. Technologies demonstrating lower operational costs and smaller environmental footprints are gaining market share over traditional approaches, despite potentially higher initial capital investments.
The market exhibits growing demand for modular, scalable systems that can accommodate fluctuating H2S concentrations and varying process conditions. Additionally, there is increasing interest in real-time monitoring and control systems that optimize abatement processes while providing compliance documentation for regulatory purposes.
Current H2S Control Technologies and Challenges
Hydrogen sulfide (H2S) emission control in industrial settings currently employs several established technologies, each with specific advantages and limitations. Physical-chemical methods remain the most widely implemented approach, with absorption techniques using alkaline solutions such as sodium hydroxide achieving removal efficiencies of 90-99% in optimal conditions. These systems are relatively cost-effective for medium-scale operations but face challenges with solution regeneration and waste disposal.
Adsorption technologies utilizing activated carbon, zeolites, and metal oxides demonstrate high efficiency for low-concentration H2S streams (below 500 ppm). Recent advancements in modified adsorbents have improved capacity by 30-40%, though these materials suffer from saturation issues and require regular replacement or regeneration, creating operational disruptions in continuous processes.
Biological treatment methods have gained significant attention in the past decade, with biofilters and biotrickling filters showing promising results for low to medium concentration applications. These systems leverage microorganisms like Thiobacillus species to convert H2S to elemental sulfur or sulfate. While environmentally friendly and operating at ambient conditions, biological methods face challenges with sensitivity to pH fluctuations, temperature variations, and nutrient requirements.
Thermal and catalytic oxidation technologies offer near-complete destruction of H2S (>99.9%) by converting it to SO2 and subsequently to sulfuric acid or elemental sulfur. These systems handle high concentrations effectively but require significant energy input and face corrosion issues from acid formation. The capital expenditure for thermal systems remains prohibitively high for small to medium enterprises.
Membrane separation technologies represent an emerging solution, with selective membranes demonstrating H2S removal efficiencies of 80-95% in laboratory settings. However, industrial-scale implementation faces challenges with membrane fouling, limited lifespan in harsh industrial environments, and high replacement costs.
A critical challenge across all technologies is the trade-off between removal efficiency and economic viability. High-efficiency systems typically demand greater capital investment and operational costs, creating barriers for widespread adoption, particularly in developing economies. Additionally, most technologies generate secondary waste streams requiring further treatment, complicating the overall environmental impact assessment.
Monitoring and control systems present another significant challenge, as H2S's corrosive nature damages conventional sensors, leading to reliability issues in continuous monitoring. Recent developments in optical sensing technologies show promise but remain costly for widespread deployment.
Regulatory compliance adds another layer of complexity, with increasingly stringent emission standards worldwide requiring industries to achieve higher removal efficiencies, often necessitating combinations of different control technologies to meet these standards.
Adsorption technologies utilizing activated carbon, zeolites, and metal oxides demonstrate high efficiency for low-concentration H2S streams (below 500 ppm). Recent advancements in modified adsorbents have improved capacity by 30-40%, though these materials suffer from saturation issues and require regular replacement or regeneration, creating operational disruptions in continuous processes.
Biological treatment methods have gained significant attention in the past decade, with biofilters and biotrickling filters showing promising results for low to medium concentration applications. These systems leverage microorganisms like Thiobacillus species to convert H2S to elemental sulfur or sulfate. While environmentally friendly and operating at ambient conditions, biological methods face challenges with sensitivity to pH fluctuations, temperature variations, and nutrient requirements.
Thermal and catalytic oxidation technologies offer near-complete destruction of H2S (>99.9%) by converting it to SO2 and subsequently to sulfuric acid or elemental sulfur. These systems handle high concentrations effectively but require significant energy input and face corrosion issues from acid formation. The capital expenditure for thermal systems remains prohibitively high for small to medium enterprises.
Membrane separation technologies represent an emerging solution, with selective membranes demonstrating H2S removal efficiencies of 80-95% in laboratory settings. However, industrial-scale implementation faces challenges with membrane fouling, limited lifespan in harsh industrial environments, and high replacement costs.
A critical challenge across all technologies is the trade-off between removal efficiency and economic viability. High-efficiency systems typically demand greater capital investment and operational costs, creating barriers for widespread adoption, particularly in developing economies. Additionally, most technologies generate secondary waste streams requiring further treatment, complicating the overall environmental impact assessment.
Monitoring and control systems present another significant challenge, as H2S's corrosive nature damages conventional sensors, leading to reliability issues in continuous monitoring. Recent developments in optical sensing technologies show promise but remain costly for widespread deployment.
Regulatory compliance adds another layer of complexity, with increasingly stringent emission standards worldwide requiring industries to achieve higher removal efficiencies, often necessitating combinations of different control technologies to meet these standards.
Mainstream H2S Removal Technologies Analysis
01 Chemical absorption and oxidation methods
Chemical absorption and oxidation methods involve the use of specific chemicals to absorb H2S from gas streams and subsequently oxidize it to less harmful compounds. These methods typically employ alkaline solutions or metal-based oxidants that react with H2S to form sulfur compounds that can be more easily handled or disposed of. The processes can be designed as wet scrubbers, packed bed reactors, or multi-stage treatment systems to efficiently remove H2S from industrial emissions.- Chemical absorption and oxidation methods: Chemical absorption and oxidation methods involve the use of specific chemicals to absorb H2S from gas streams and subsequently oxidize it to less harmful compounds. These methods typically employ alkaline solutions or metal-based oxidants that react with H2S to form sulfur compounds that can be more easily handled or disposed of. The process can be optimized by controlling parameters such as pH, temperature, and residence time to achieve high removal efficiencies.
- Biological treatment systems: Biological treatment systems utilize microorganisms to convert H2S into elemental sulfur or sulfate. These systems typically employ specialized bacteria that can metabolize sulfur compounds under controlled conditions. Biofiltration, biotrickling filters, and bioscrubbers are common biological treatment technologies used for H2S emission control. These systems offer advantages such as low operating costs, minimal chemical usage, and environmental friendliness compared to chemical methods.
- Adsorption using solid media: Adsorption technologies employ solid media such as activated carbon, zeolites, metal oxides, or specialized polymers to capture H2S molecules from gas streams. These materials have high surface areas and specific chemical properties that allow them to selectively bind with H2S. The adsorption capacity can be enhanced by impregnating the media with chemicals that promote the conversion of H2S to elemental sulfur. Once saturated, the media can either be regenerated or replaced.
- Membrane separation technology: Membrane separation technology utilizes selective permeable barriers to separate H2S from gas streams. These membranes are designed with specific pore sizes and chemical properties that allow H2S molecules to pass through while blocking other components. Various membrane materials including polymers, ceramics, and composite materials can be employed depending on the gas composition and operating conditions. This technology offers advantages such as continuous operation, compact design, and the ability to handle varying concentrations of H2S.
- Integrated monitoring and control systems: Integrated monitoring and control systems combine sensors, analyzers, and automated control mechanisms to effectively manage H2S emissions. These systems continuously monitor H2S concentrations in real-time, allowing for immediate response to concentration changes. Advanced control algorithms optimize the operation of treatment systems based on input parameters such as gas flow rate, H2S concentration, and ambient conditions. These integrated approaches enhance the efficiency of emission control while minimizing resource consumption and operational costs.
02 Biological treatment systems
Biological treatment systems utilize microorganisms to convert H2S into elemental sulfur or sulfate compounds. These systems typically employ specialized bacteria that can metabolize hydrogen sulfide under controlled conditions. Biofiltration, biotrickling filters, and bioscrubbers are common configurations used in these systems. The biological approach offers advantages of lower chemical consumption, reduced secondary waste generation, and the ability to treat varying concentrations of H2S in a sustainable manner.Expand Specific Solutions03 Adsorption-based removal technologies
Adsorption-based technologies use solid adsorbents such as activated carbon, zeolites, metal oxides, or specialized polymers to capture H2S molecules from gas streams. These materials have high surface areas and specific affinity for hydrogen sulfide. The adsorption process can be designed as fixed beds, fluidized beds, or moving bed systems. Many adsorption technologies incorporate regeneration capabilities to extend the life of the adsorbent material and improve the overall economics of the H2S removal process.Expand Specific Solutions04 Membrane separation techniques
Membrane separation techniques employ selective membranes that allow certain components of a gas mixture to pass through while retaining others. For H2S control, specialized membranes with high selectivity for hydrogen sulfide are used to separate it from other gases. These systems can operate at various pressures and temperatures depending on the membrane material and process requirements. Membrane technologies offer advantages such as continuous operation, compact design, and the ability to handle varying gas compositions without significant process modifications.Expand Specific Solutions05 Integrated monitoring and control systems
Integrated monitoring and control systems combine sensors, analyzers, and automated control mechanisms to detect H2S levels and manage emission control equipment in real-time. These systems typically include H2S detectors, data acquisition units, control algorithms, and response protocols to ensure safe operation and regulatory compliance. Advanced systems may incorporate predictive analytics, remote monitoring capabilities, and integration with broader facility management systems to optimize H2S control strategies and minimize operational disruptions.Expand Specific Solutions
Key Industry Players in H2S Treatment Solutions
The hydrogen sulfide emission control market is in a growth phase, characterized by increasing regulatory pressures and technological advancements. The global market size for H2S control technologies is expanding significantly due to stricter environmental regulations across industrial sectors. Leading players include major oil and gas corporations like Sinopec, PetroChina, and Saudi Aramco, who are investing heavily in advanced desulfurization technologies. Technology maturity varies across solutions, with traditional chemical scrubbing being well-established while newer catalytic oxidation methods are emerging. Companies like Haldor Topsøe, Johnson Matthey, and BASF are at the forefront of catalyst technology development, while specialized firms such as Auterra and New Sky Energy offer innovative approaches to H2S treatment. Academic-industry collaborations with institutions like Tianjin University and King Fahd University of Petroleum & Minerals are accelerating technological advancement in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced sulfur recovery technologies for hydrosulfuric acid emission control. Their primary approach utilizes modified Claus process with oxygen enrichment, achieving sulfur recovery rates of up to 99.5%[1]. The company has implemented a three-stage tail gas treatment system that combines selective catalytic reduction with proprietary amine absorption technology, significantly reducing H2S emissions to below 10ppm in exhaust gases[3]. Sinopec has also pioneered low-temperature plasma technology for treating H2S in refinery gases, which can decompose over 95% of hydrosulfuric acid without generating secondary pollutants[5]. Their integrated emission control strategy includes continuous monitoring systems with real-time data analytics to optimize process parameters and prevent breakthrough emissions during operational fluctuations.
Strengths: Comprehensive integration of multiple technologies allowing for flexible application across different refinery configurations; proprietary catalysts with enhanced sulfur conversion efficiency; extensive operational experience across diverse processing conditions. Weaknesses: High capital investment requirements; complex implementation requiring specialized expertise; some technologies remain energy-intensive despite efficiency improvements.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has developed the SNOX™ process, an integrated solution for simultaneous removal of sulfur compounds (including H2S), NOx, and particulates from flue gases. Their technology converts hydrosulfuric acid to elemental sulfur through a proprietary catalytic process achieving removal efficiencies exceeding 98%[2]. The company's TiGAS™ (Topsøe Improved Gasification) technology incorporates advanced H2S removal systems for syngas cleaning, utilizing metal oxide sorbents that can operate at elevated temperatures (300-500°C), significantly improving energy efficiency[4]. Topsøe has also commercialized the WSA (Wet Sulfuric Acid) process that converts H2S directly to commercial-grade sulfuric acid, providing both environmental and economic benefits with recovery rates up to 99.7%[6]. Their ClearView™ digital platform enhances emission control by providing predictive maintenance and real-time optimization of catalyst performance, reducing operational variability and extending catalyst lifetime by up to 30%.
Strengths: Highly efficient sulfur recovery with valuable by-product generation; lower energy consumption compared to conventional technologies; robust catalyst formulations with extended operational lifetimes; integrated digital solutions for process optimization. Weaknesses: Higher initial capital costs; requires specialized operational expertise; performance can be affected by feed gas composition fluctuations; some technologies require significant plant modifications for retrofitting.
Critical Patents and Innovations in H2S Control
Methods to control H2S and arsine emissions
PatentInactiveUS6800259B2
Innovation
- Adding a copper compound, such as copper oxide or copper sulfate, to the ore during the digestion process to effectively reduce hydrogen sulfide and arsine emissions, with the copper compound being easily incorporated into the existing process without affecting tantalum recovery or increasing ore residue.
System and method for removing hydrogen sulfide from an emissions stream
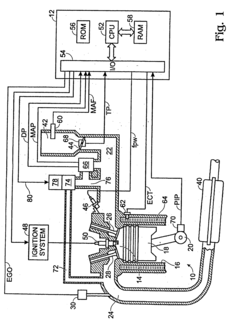
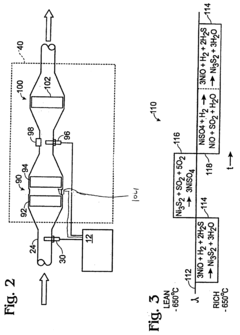
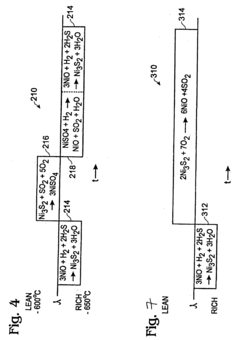
PatentInactiveUS20050163690A1
Innovation
- A system and method involving a hydrogen sulfide converter with a metal oxide catalyst that adsorbs and chemically transforms hydrogen sulfide, adjusting the air-fuel ratio based on exhaust temperature to desulfate NOx traps while minimizing hydrogen sulfide emissions across a wide temperature range.
Environmental Regulations and Compliance Standards
The regulatory landscape governing hydrogen sulfide emissions has evolved significantly over the past decades, with increasingly stringent standards being implemented worldwide. In the United States, the Environmental Protection Agency (EPA) has established National Ambient Air Quality Standards (NAAQS) that indirectly affect H2S emissions, while the Occupational Safety and Health Administration (OSHA) has set specific workplace exposure limits at 10 ppm for 8-hour periods and 15 ppm for short-term exposure. These standards are complemented by the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), which requires reporting of H2S releases exceeding 100 pounds within a 24-hour period.
The European Union has implemented more comprehensive frameworks through the Industrial Emissions Directive (IED) and the Best Available Techniques Reference Documents (BREFs), which establish emission limit values for various industrial sectors. These documents specifically address hydrogen sulfide emissions in industries such as oil refining, wastewater treatment, and pulp and paper manufacturing, with typical limits ranging from 5-15 mg/Nm³ depending on the sector.
Emerging economies have also strengthened their regulatory approaches. China's recent environmental protection laws have established a national standard (GB 14554-93) for odorous pollutants including H2S, with emission limits of 0.06-0.10 mg/m³ at facility boundaries. India's Central Pollution Control Board has implemented similar standards under the Air (Prevention and Control of Pollution) Act, with specific provisions for industries known to generate significant H2S emissions.
International agreements have further shaped compliance requirements. The Stockholm Convention on Persistent Organic Pollutants and the Minamata Convention on Mercury both contain provisions that indirectly affect hydrogen sulfide management practices, particularly regarding waste treatment and disposal methods that might generate H2S as a byproduct.
Compliance monitoring technologies have advanced considerably, with continuous emission monitoring systems (CEMS) becoming standard requirements in many jurisdictions. These systems must meet specific performance criteria for accuracy, reliability, and data reporting. Many regulatory frameworks now mandate real-time data transmission to environmental authorities, enabling more effective oversight and enforcement.
The financial implications of non-compliance have increased substantially, with penalties ranging from tens of thousands to millions of dollars depending on the severity and duration of violations. Beyond direct fines, companies face potential criminal liability, mandatory facility upgrades, and reputational damage that can affect market position and stakeholder relations.
The European Union has implemented more comprehensive frameworks through the Industrial Emissions Directive (IED) and the Best Available Techniques Reference Documents (BREFs), which establish emission limit values for various industrial sectors. These documents specifically address hydrogen sulfide emissions in industries such as oil refining, wastewater treatment, and pulp and paper manufacturing, with typical limits ranging from 5-15 mg/Nm³ depending on the sector.
Emerging economies have also strengthened their regulatory approaches. China's recent environmental protection laws have established a national standard (GB 14554-93) for odorous pollutants including H2S, with emission limits of 0.06-0.10 mg/m³ at facility boundaries. India's Central Pollution Control Board has implemented similar standards under the Air (Prevention and Control of Pollution) Act, with specific provisions for industries known to generate significant H2S emissions.
International agreements have further shaped compliance requirements. The Stockholm Convention on Persistent Organic Pollutants and the Minamata Convention on Mercury both contain provisions that indirectly affect hydrogen sulfide management practices, particularly regarding waste treatment and disposal methods that might generate H2S as a byproduct.
Compliance monitoring technologies have advanced considerably, with continuous emission monitoring systems (CEMS) becoming standard requirements in many jurisdictions. These systems must meet specific performance criteria for accuracy, reliability, and data reporting. Many regulatory frameworks now mandate real-time data transmission to environmental authorities, enabling more effective oversight and enforcement.
The financial implications of non-compliance have increased substantially, with penalties ranging from tens of thousands to millions of dollars depending on the severity and duration of violations. Beyond direct fines, companies face potential criminal liability, mandatory facility upgrades, and reputational damage that can affect market position and stakeholder relations.
Health and Safety Implications of H2S Exposure
Hydrogen sulfide (H2S) exposure presents significant health and safety risks in industrial settings, with both acute and chronic effects that vary based on concentration levels and exposure duration. At low concentrations (0.00047 ppm), H2S is detectable by its characteristic rotten egg odor, but olfactory fatigue occurs rapidly at higher concentrations, eliminating this warning mechanism and increasing danger to workers.
Acute exposure to H2S at concentrations between 50-100 ppm causes eye irritation, respiratory tract inflammation, and headaches. Concentrations of 100-300 ppm lead to olfactory paralysis, while exposure to 300-500 ppm can cause pulmonary edema and potentially life-threatening respiratory distress. Concentrations exceeding 700 ppm may result in immediate collapse, respiratory paralysis, and death within minutes, classifying H2S as a chemical asphyxiant.
Chronic low-level exposure presents equally concerning health implications, including persistent neurological effects, chronic respiratory conditions, and cardiovascular abnormalities. Research indicates potential links between prolonged H2S exposure and increased incidence of certain cancers, though more epidemiological studies are needed to establish definitive causal relationships.
Industry-specific risk profiles vary significantly. Oil and gas operations, wastewater treatment facilities, and paper manufacturing plants typically experience the highest exposure risks. Confined spaces in these industries present particularly hazardous conditions, with numerous fatalities documented annually from H2S accumulation in tanks, pipelines, and maintenance chambers.
Current regulatory frameworks establish varying permissible exposure limits (PELs). OSHA sets the general industry ceiling at 20 ppm, with a 10-minute maximum peak of 50 ppm. NIOSH recommends a more conservative 10-minute ceiling of 10 ppm. The ACGIH threshold limit value (TLV) stands at 1 ppm for an 8-hour time-weighted average, with a 5 ppm short-term exposure limit.
Comprehensive safety management protocols must include continuous monitoring systems with multi-level alarms, mandatory respiratory protection equipment, and emergency response procedures. Advanced detection technologies utilizing electrochemical sensors and infrared spectroscopy have improved early warning capabilities. Personal H2S monitors with vibration and audible alarms represent the current best practice for worker protection.
Training programs focusing on recognition of exposure symptoms, proper use of personal protective equipment, and emergency evacuation procedures remain essential components of industrial safety protocols. Regular simulation drills significantly improve survival rates during actual H2S release incidents, underscoring the importance of preparedness in high-risk industrial environments.
Acute exposure to H2S at concentrations between 50-100 ppm causes eye irritation, respiratory tract inflammation, and headaches. Concentrations of 100-300 ppm lead to olfactory paralysis, while exposure to 300-500 ppm can cause pulmonary edema and potentially life-threatening respiratory distress. Concentrations exceeding 700 ppm may result in immediate collapse, respiratory paralysis, and death within minutes, classifying H2S as a chemical asphyxiant.
Chronic low-level exposure presents equally concerning health implications, including persistent neurological effects, chronic respiratory conditions, and cardiovascular abnormalities. Research indicates potential links between prolonged H2S exposure and increased incidence of certain cancers, though more epidemiological studies are needed to establish definitive causal relationships.
Industry-specific risk profiles vary significantly. Oil and gas operations, wastewater treatment facilities, and paper manufacturing plants typically experience the highest exposure risks. Confined spaces in these industries present particularly hazardous conditions, with numerous fatalities documented annually from H2S accumulation in tanks, pipelines, and maintenance chambers.
Current regulatory frameworks establish varying permissible exposure limits (PELs). OSHA sets the general industry ceiling at 20 ppm, with a 10-minute maximum peak of 50 ppm. NIOSH recommends a more conservative 10-minute ceiling of 10 ppm. The ACGIH threshold limit value (TLV) stands at 1 ppm for an 8-hour time-weighted average, with a 5 ppm short-term exposure limit.
Comprehensive safety management protocols must include continuous monitoring systems with multi-level alarms, mandatory respiratory protection equipment, and emergency response procedures. Advanced detection technologies utilizing electrochemical sensors and infrared spectroscopy have improved early warning capabilities. Personal H2S monitors with vibration and audible alarms represent the current best practice for worker protection.
Training programs focusing on recognition of exposure symptoms, proper use of personal protective equipment, and emergency evacuation procedures remain essential components of industrial safety protocols. Regular simulation drills significantly improve survival rates during actual H2S release incidents, underscoring the importance of preparedness in high-risk industrial environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!