Evaluate Absorbent Materials for Hydrosulfuric Acid Control
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Absorption Technology Background and Objectives
Hydrogen sulfide (H2S) absorption technology has evolved significantly over the past century, with major advancements occurring in response to industrial needs, environmental regulations, and technological innovations. Initially developed for natural gas sweetening operations in the early 20th century, H2S removal technologies have expanded to address challenges in petroleum refining, wastewater treatment, biogas purification, and various industrial processes where this toxic and corrosive gas poses operational and safety hazards.
The evolution of absorbent materials for H2S control reflects a progressive shift from simple chemical reactions to sophisticated engineered solutions. Early approaches relied primarily on iron oxide-based materials and basic amine solutions. The 1950s and 1960s saw the introduction of more efficient alkanolamine systems, while the 1970s and 1980s brought advancements in physical solvents and hybrid absorption systems. Recent decades have witnessed remarkable progress in nanomaterial-based absorbents, metal-organic frameworks (MOFs), and functionalized porous materials that offer unprecedented selectivity and capacity.
Current technological trends indicate a growing emphasis on developing absorbent materials that combine high H2S removal efficiency with reduced energy requirements, minimal environmental impact, and economic viability at industrial scales. Research is increasingly focused on materials that can operate effectively across diverse conditions, including varying H2S concentrations, presence of contaminants, and fluctuating temperature and pressure environments.
The primary objectives of this technical pre-research report are to comprehensively evaluate the current landscape of absorbent materials for H2S control, identify promising emerging technologies, and assess their potential for industrial implementation. Specifically, we aim to analyze the performance characteristics of various absorbent materials, including removal efficiency, selectivity, regeneration capabilities, operational stability, and cost-effectiveness.
Additionally, this report seeks to identify critical technological gaps and challenges that must be addressed to advance H2S absorption technologies. These include improving absorbent capacity and kinetics, enhancing resistance to degradation and poisoning, reducing regeneration energy requirements, and developing materials compatible with existing industrial infrastructure.
The ultimate goal is to provide strategic insights that can guide future research and development efforts, inform investment decisions, and support the formulation of long-term technological roadmaps for H2S control solutions that meet increasingly stringent environmental regulations while maintaining operational efficiency and economic feasibility.
The evolution of absorbent materials for H2S control reflects a progressive shift from simple chemical reactions to sophisticated engineered solutions. Early approaches relied primarily on iron oxide-based materials and basic amine solutions. The 1950s and 1960s saw the introduction of more efficient alkanolamine systems, while the 1970s and 1980s brought advancements in physical solvents and hybrid absorption systems. Recent decades have witnessed remarkable progress in nanomaterial-based absorbents, metal-organic frameworks (MOFs), and functionalized porous materials that offer unprecedented selectivity and capacity.
Current technological trends indicate a growing emphasis on developing absorbent materials that combine high H2S removal efficiency with reduced energy requirements, minimal environmental impact, and economic viability at industrial scales. Research is increasingly focused on materials that can operate effectively across diverse conditions, including varying H2S concentrations, presence of contaminants, and fluctuating temperature and pressure environments.
The primary objectives of this technical pre-research report are to comprehensively evaluate the current landscape of absorbent materials for H2S control, identify promising emerging technologies, and assess their potential for industrial implementation. Specifically, we aim to analyze the performance characteristics of various absorbent materials, including removal efficiency, selectivity, regeneration capabilities, operational stability, and cost-effectiveness.
Additionally, this report seeks to identify critical technological gaps and challenges that must be addressed to advance H2S absorption technologies. These include improving absorbent capacity and kinetics, enhancing resistance to degradation and poisoning, reducing regeneration energy requirements, and developing materials compatible with existing industrial infrastructure.
The ultimate goal is to provide strategic insights that can guide future research and development efforts, inform investment decisions, and support the formulation of long-term technological roadmaps for H2S control solutions that meet increasingly stringent environmental regulations while maintaining operational efficiency and economic feasibility.
Market Analysis for H2S Control Solutions
The global market for hydrogen sulfide (H2S) control solutions has been experiencing steady growth, primarily driven by stringent environmental regulations and increasing awareness about workplace safety. The market size for H2S control technologies was valued at approximately $1.5 billion in 2022 and is projected to reach $2.3 billion by 2028, representing a compound annual growth rate of 7.4%. This growth trajectory is supported by expanding industrial activities in oil and gas, wastewater treatment, and chemical manufacturing sectors.
North America currently dominates the market with a 35% share, followed by Europe at 28% and Asia-Pacific at 24%. The remaining 13% is distributed across Latin America, Middle East, and Africa. The oil and gas industry remains the largest end-user segment, accounting for nearly 45% of the total market demand, followed by wastewater treatment at 25% and chemical processing at 15%.
Within the absorbent materials segment, activated carbon holds the largest market share at 32%, followed by metal oxides at 28% and specialized polymers at 22%. Zeolites and other novel materials constitute the remaining 18%. The preference for these materials varies significantly across different applications and regions, influenced by factors such as removal efficiency, cost-effectiveness, and operational conditions.
Key market drivers include increasingly strict emission standards worldwide, growing concerns about worker safety in H2S-exposed environments, and the expansion of industries where H2S is a byproduct. The United States Environmental Protection Agency's regulations limiting H2S emissions to 10 ppm for prolonged exposure have particularly influenced market dynamics in North America.
Market challenges primarily revolve around the high initial investment costs for advanced H2S control systems and the technical complexities associated with implementing these solutions in existing industrial setups. Additionally, the varying regulatory standards across different regions create compliance challenges for multinational corporations.
Customer preferences are shifting toward integrated solutions that offer not only H2S removal but also comprehensive gas treatment capabilities. There is also a growing demand for sustainable and regenerable absorbent materials that reduce waste generation and operational costs over time. The market is witnessing increased interest in smart monitoring systems that can be integrated with H2S control solutions to provide real-time data on gas concentrations and absorbent material performance.
North America currently dominates the market with a 35% share, followed by Europe at 28% and Asia-Pacific at 24%. The remaining 13% is distributed across Latin America, Middle East, and Africa. The oil and gas industry remains the largest end-user segment, accounting for nearly 45% of the total market demand, followed by wastewater treatment at 25% and chemical processing at 15%.
Within the absorbent materials segment, activated carbon holds the largest market share at 32%, followed by metal oxides at 28% and specialized polymers at 22%. Zeolites and other novel materials constitute the remaining 18%. The preference for these materials varies significantly across different applications and regions, influenced by factors such as removal efficiency, cost-effectiveness, and operational conditions.
Key market drivers include increasingly strict emission standards worldwide, growing concerns about worker safety in H2S-exposed environments, and the expansion of industries where H2S is a byproduct. The United States Environmental Protection Agency's regulations limiting H2S emissions to 10 ppm for prolonged exposure have particularly influenced market dynamics in North America.
Market challenges primarily revolve around the high initial investment costs for advanced H2S control systems and the technical complexities associated with implementing these solutions in existing industrial setups. Additionally, the varying regulatory standards across different regions create compliance challenges for multinational corporations.
Customer preferences are shifting toward integrated solutions that offer not only H2S removal but also comprehensive gas treatment capabilities. There is also a growing demand for sustainable and regenerable absorbent materials that reduce waste generation and operational costs over time. The market is witnessing increased interest in smart monitoring systems that can be integrated with H2S control solutions to provide real-time data on gas concentrations and absorbent material performance.
Current Absorbent Materials and Technical Challenges
The current landscape of absorbent materials for hydrogen sulfide control encompasses several established technologies with varying degrees of effectiveness. Activated carbon remains the most widely utilized absorbent due to its high surface area and versatile modification capabilities. Impregnated activated carbons, particularly those treated with metal oxides such as copper oxide, zinc oxide, and iron oxide, demonstrate enhanced H2S removal efficiency through chemisorption mechanisms. These materials typically achieve removal capacities ranging from 0.05 to 0.20 g H2S per gram of absorbent under standard conditions.
Metal oxide-based absorbents represent another significant category, with zinc oxide sorbents showing particular promise for high-temperature applications (200-400°C). These materials can achieve theoretical absorption capacities approaching 0.35 g H2S per gram, though practical implementations often realize only 60-70% of this potential due to diffusion limitations and incomplete conversion.
Alkaline materials including lime (CaO), caustic soda (NaOH), and sodium carbonate (Na2CO3) are employed in wet scrubbing systems, offering cost-effective solutions for high-concentration H2S streams. However, these systems generate substantial secondary waste requiring further treatment and disposal.
Despite these advances, significant technical challenges persist in H2S absorption technology. Moisture sensitivity remains a critical issue, as many absorbents experience dramatically reduced performance in humid conditions due to competitive adsorption between water vapor and H2S molecules. This is particularly problematic for activated carbon-based materials without hydrophobic treatments.
Thermal stability limitations constrain application scenarios, with many organic-based absorbents degrading at temperatures above 150°C, while some metal oxide systems require elevated temperatures to maintain optimal performance, creating energy efficiency concerns.
Regeneration efficiency presents another substantial challenge, as most current absorbents experience 10-25% capacity loss per regeneration cycle, necessitating frequent replacement and increasing operational costs. This is compounded by the formation of elemental sulfur and other compounds that permanently block active sites.
Selectivity issues arise in complex gas streams containing multiple contaminants, where competitive adsorption can significantly reduce H2S removal efficiency. Most current materials demonstrate poor performance in the presence of carbon dioxide, ammonia, or volatile organic compounds.
Cost-effectiveness remains a persistent barrier to widespread implementation, with high-performance materials like silver-impregnated carbons and specialized metal-organic frameworks commanding prices exceeding $50-100 per kilogram, limiting their application to niche scenarios where conventional alternatives are inadequate.
Metal oxide-based absorbents represent another significant category, with zinc oxide sorbents showing particular promise for high-temperature applications (200-400°C). These materials can achieve theoretical absorption capacities approaching 0.35 g H2S per gram, though practical implementations often realize only 60-70% of this potential due to diffusion limitations and incomplete conversion.
Alkaline materials including lime (CaO), caustic soda (NaOH), and sodium carbonate (Na2CO3) are employed in wet scrubbing systems, offering cost-effective solutions for high-concentration H2S streams. However, these systems generate substantial secondary waste requiring further treatment and disposal.
Despite these advances, significant technical challenges persist in H2S absorption technology. Moisture sensitivity remains a critical issue, as many absorbents experience dramatically reduced performance in humid conditions due to competitive adsorption between water vapor and H2S molecules. This is particularly problematic for activated carbon-based materials without hydrophobic treatments.
Thermal stability limitations constrain application scenarios, with many organic-based absorbents degrading at temperatures above 150°C, while some metal oxide systems require elevated temperatures to maintain optimal performance, creating energy efficiency concerns.
Regeneration efficiency presents another substantial challenge, as most current absorbents experience 10-25% capacity loss per regeneration cycle, necessitating frequent replacement and increasing operational costs. This is compounded by the formation of elemental sulfur and other compounds that permanently block active sites.
Selectivity issues arise in complex gas streams containing multiple contaminants, where competitive adsorption can significantly reduce H2S removal efficiency. Most current materials demonstrate poor performance in the presence of carbon dioxide, ammonia, or volatile organic compounds.
Cost-effectiveness remains a persistent barrier to widespread implementation, with high-performance materials like silver-impregnated carbons and specialized metal-organic frameworks commanding prices exceeding $50-100 per kilogram, limiting their application to niche scenarios where conventional alternatives are inadequate.
Existing H2S Absorbent Material Solutions
01 Superabsorbent polymers for enhanced absorption capacity
Superabsorbent polymers (SAPs) are materials that can absorb and retain extremely large amounts of liquid relative to their own mass. These polymers, typically based on acrylic acid and its salts, can be incorporated into absorbent materials to significantly increase their absorption capacity. The cross-linked structure of these polymers allows them to swell and form a gel when in contact with liquids, making them ideal for applications requiring high fluid retention.- Superabsorbent polymers for enhanced absorption capacity: Superabsorbent polymers (SAPs) are materials that can absorb and retain extremely large amounts of liquid relative to their own mass. These polymers, typically based on sodium polyacrylate or polyacrylamide copolymers, can absorb hundreds of times their weight in water. They are commonly used in absorbent products to significantly increase absorption capacity and fluid retention under pressure. The cross-linking density and particle size distribution of these polymers can be optimized to achieve desired absorption rates and capacities.
- Cellulosic fibers and their modifications for absorbent applications: Cellulosic fibers, derived from wood pulp or other plant sources, serve as the foundation for many absorbent materials. These fibers can be chemically or mechanically modified to enhance their absorption properties. Treatments include crosslinking, surface modification, or mercerization to improve fluid uptake and retention. The combination of cellulosic fibers with synthetic materials creates composite structures that optimize absorption capacity while maintaining structural integrity. The fiber morphology, including length, curl, and coarseness, significantly impacts the overall absorption performance.
- Multilayer absorbent structures for improved fluid management: Multilayer absorbent structures incorporate different materials in layers to optimize fluid acquisition, distribution, and storage. These structures typically include a rapid acquisition layer that quickly takes in fluid, a distribution layer that spreads fluid horizontally, and a storage layer containing high-capacity absorbent materials. This layered approach prevents localized saturation and utilizes the full capacity of the absorbent core. The interfaces between layers are designed to maintain optimal fluid transfer while preventing backflow, enhancing the overall absorption efficiency of the system.
- Nanomaterials and advanced composites for absorption enhancement: Nanomaterials and advanced composites represent cutting-edge developments in absorbent technology. Materials such as nanocellulose, graphene oxide, and nanofiber networks provide exceptional surface area-to-volume ratios, creating numerous sites for fluid interaction. These materials can be incorporated into traditional absorbent structures to dramatically increase absorption capacity without significantly increasing bulk. Advanced composites may combine organic and inorganic components to create synergistic absorption properties that exceed the capabilities of individual materials, while maintaining structural stability even when saturated.
- Absorption capacity testing methods and performance metrics: Standardized testing methods are essential for evaluating and comparing the absorption capacity of different materials. Common tests include free swell capacity, centrifuge retention capacity, and absorption under load, which simulate various real-world conditions. Performance metrics consider not only maximum fluid uptake but also absorption rate, retention under pressure, and rewet properties. These comprehensive evaluations help in designing materials with optimal absorption characteristics for specific applications, ensuring that laboratory performance translates to effective real-world functionality.
02 Multilayer absorbent structures for improved fluid management
Multilayer absorbent structures combine different materials in layers to optimize absorption capacity and fluid distribution. These structures typically include an acquisition layer that quickly takes in fluid, a distribution layer that spreads the fluid horizontally, and a storage layer containing superabsorbent materials that lock away the fluid. This layered approach prevents leakage and ensures efficient use of the absorbent materials throughout the product, enhancing overall absorption capacity and user comfort.Expand Specific Solutions03 Natural fiber-based absorbent materials
Natural fibers such as cellulose, cotton, and other plant-based materials can be processed to create highly absorbent materials. These fibers can be treated or modified to enhance their natural absorption properties. The porous structure of these natural fibers provides excellent capillary action for fluid uptake. Additionally, these materials are biodegradable and environmentally friendly, making them suitable for sustainable absorbent products while maintaining effective absorption capacity.Expand Specific Solutions04 Surface modification techniques for improved absorption
Various surface modification techniques can be applied to absorbent materials to enhance their absorption capacity. These include chemical treatments, plasma treatments, and grafting of hydrophilic groups onto the material surface. Such modifications can increase the wettability of the material, improve the rate of fluid uptake, and enhance the overall absorption capacity. These techniques are particularly useful for synthetic fibers that may not naturally have good absorption properties.Expand Specific Solutions05 Composite absorbent materials with nanostructures
Incorporating nanostructures such as nanofibers, nanoparticles, or nanoporous materials into absorbent products can dramatically increase absorption capacity. These nanostructures provide an extremely high surface area-to-volume ratio, creating numerous sites for fluid interaction and retention. Composite materials combining conventional absorbents with these nanostructures can achieve superior absorption performance while maintaining structural integrity even when saturated with fluid.Expand Specific Solutions
Leading Companies in H2S Control Industry
The hydrosulfuric acid control market is in a growth phase, driven by increasing environmental regulations and industrial safety requirements. The global market size is estimated to exceed $2 billion, with projected annual growth of 5-7%. Major oil corporations like China Petroleum & Chemical Corp., CNOOC, ExxonMobil, and Shell lead technological innovation in this field, with specialized companies such as Air Products & Chemicals and BASF providing complementary solutions. The technology landscape shows varying maturity levels, with traditional absorption methods well-established while novel nanomaterial-based absorbents from research institutions like Tsinghua University and King Fahd University of Petroleum & Minerals represent emerging approaches. The competitive environment features strategic partnerships between industrial players and research institutions to develop more efficient and cost-effective absorbent materials.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced metal oxide-based absorbent materials for hydrosulfuric acid control, particularly focusing on iron oxide-based sorbents. Their proprietary technology utilizes porous iron oxide matrices with enhanced surface area (>200 m²/g) and modified with promoters such as zinc and copper oxides to improve H2S removal efficiency. The technology employs a regenerable absorption process where the spent sorbent undergoes controlled oxidation at 400-500°C, allowing for multiple absorption-regeneration cycles. Sinopec has implemented this technology across their refineries, achieving H2S removal efficiencies exceeding 99.5% and reducing sulfur content in gas streams to below 1 ppm in optimal conditions.
Strengths: High absorption capacity (>15 wt% sulfur loading), excellent regenerability (>10 cycles without significant degradation), and cost-effectiveness due to the use of abundant raw materials. Weaknesses: Performance degradation in high-moisture environments and potential for thermal sintering during regeneration that reduces long-term efficiency.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil Technology & Engineering Co. has developed the FLEXSORB® technology platform for hydrosulfuric acid control, representing a significant advancement in selective gas treating. Their approach utilizes proprietary hindered amine absorbents that demonstrate exceptional selectivity for H2S over CO2, allowing for precise sulfur management in complex gas streams. The technology employs a regenerable absorption process with specialized solvent formulations that resist degradation even after thousands of absorption-regeneration cycles. ExxonMobil's latest generation FLEXSORB SE Plus technology achieves H2S removal to sub-ppm levels while minimizing energy consumption through optimized process integration. The company has implemented this technology in over 150 installations worldwide, demonstrating its versatility across natural gas processing, refinery operations, and LNG production facilities.
Strengths: Superior selectivity for H2S in the presence of CO2 (up to 8:1 selectivity ratio), excellent resistance to thermal and chemical degradation, and lower energy requirements compared to conventional amine systems. Weaknesses: Higher initial solvent cost compared to generic amines and more complex process control requirements to maintain optimal performance.
Key Innovations in H2S Absorption Materials
Purification process
PatentWO1993023159A1
Innovation
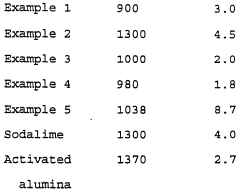
- A solid absorbent material comprising sodium hydroxide, alumina, zinc oxide, lime, and copper II oxide, formulated into a particulate form for efficient removal of acidic contaminants from hydrocarbons, with a preferred composition range and method of formation, including a two-bed treatment process for extended usage.
Absorbent, process for producing an absorbent, and process and device for separating off hydrogen sulphide from an acidic gas
PatentWO2014170047A1
Innovation
- An absorbent comprising an amino acid salt and a metal salt, with the amino acid salt concentration between 5-50% by weight and metal salt less than 3% by weight, selectively absorbs H2S and CO2, allowing reversible oxidation of H2S to sulfur or sulfate ions, and is regenerated using oxidizing agents, reducing heating steam requirements and solvent degradation.
Environmental Impact Assessment
The environmental implications of hydrogen sulfide (H2S) control technologies are significant and multifaceted. When evaluating absorbent materials for H2S control, their environmental footprint must be thoroughly assessed across the entire lifecycle. Traditional absorbent materials such as activated carbon, metal oxides, and zeolites generate substantial waste streams after saturation, often containing toxic sulfur compounds that require specialized disposal methods to prevent secondary pollution.
Water consumption represents another critical environmental concern, particularly for wet scrubbing systems utilizing liquid absorbents. These systems can consume significant quantities of water, potentially straining local resources in water-scarce regions. Additionally, the regeneration processes for many absorbent materials demand considerable energy inputs, contributing to greenhouse gas emissions when powered by fossil fuel sources.
The manufacturing of advanced absorbent materials frequently involves energy-intensive processes and potentially hazardous chemicals. Metal-organic frameworks (MOFs) and functionalized polymers, while highly effective for H2S removal, may incorporate rare earth elements or synthetic compounds with their own environmental extraction and production impacts. A comprehensive life cycle assessment reveals that the environmental benefits of H2S removal must be weighed against these production-related environmental costs.
Biodegradability and end-of-life management present ongoing challenges. Many high-performance absorbent materials are non-biodegradable, creating long-term waste management issues. Recent innovations in bio-based absorbents derived from agricultural waste, modified cellulose, and chitosan offer promising alternatives with reduced environmental impacts, though often with performance trade-offs that must be carefully evaluated.
Regulatory compliance across different jurisdictions adds complexity to environmental impact assessments. Materials must meet increasingly stringent standards for leachability, toxicity, and disposal. The potential for absorbent materials to release captured sulfur compounds under changing environmental conditions (temperature fluctuations, pH changes) must be thoroughly characterized to prevent unintended environmental releases.
The most environmentally sustainable H2S control strategies often involve integrated approaches that combine multiple technologies to minimize overall environmental impact. This includes coupling absorption with biological treatment systems that convert captured sulfur compounds into less harmful forms, thereby reducing waste generation and creating potential for resource recovery in the form of elemental sulfur or sulfate compounds.
Water consumption represents another critical environmental concern, particularly for wet scrubbing systems utilizing liquid absorbents. These systems can consume significant quantities of water, potentially straining local resources in water-scarce regions. Additionally, the regeneration processes for many absorbent materials demand considerable energy inputs, contributing to greenhouse gas emissions when powered by fossil fuel sources.
The manufacturing of advanced absorbent materials frequently involves energy-intensive processes and potentially hazardous chemicals. Metal-organic frameworks (MOFs) and functionalized polymers, while highly effective for H2S removal, may incorporate rare earth elements or synthetic compounds with their own environmental extraction and production impacts. A comprehensive life cycle assessment reveals that the environmental benefits of H2S removal must be weighed against these production-related environmental costs.
Biodegradability and end-of-life management present ongoing challenges. Many high-performance absorbent materials are non-biodegradable, creating long-term waste management issues. Recent innovations in bio-based absorbents derived from agricultural waste, modified cellulose, and chitosan offer promising alternatives with reduced environmental impacts, though often with performance trade-offs that must be carefully evaluated.
Regulatory compliance across different jurisdictions adds complexity to environmental impact assessments. Materials must meet increasingly stringent standards for leachability, toxicity, and disposal. The potential for absorbent materials to release captured sulfur compounds under changing environmental conditions (temperature fluctuations, pH changes) must be thoroughly characterized to prevent unintended environmental releases.
The most environmentally sustainable H2S control strategies often involve integrated approaches that combine multiple technologies to minimize overall environmental impact. This includes coupling absorption with biological treatment systems that convert captured sulfur compounds into less harmful forms, thereby reducing waste generation and creating potential for resource recovery in the form of elemental sulfur or sulfate compounds.
Safety Standards and Compliance Requirements
The regulatory landscape for hydrosulfuric acid (H2S) control materials encompasses multiple layers of standards and compliance requirements that must be carefully navigated by organizations implementing absorption technologies. OSHA's Permissible Exposure Limit (PEL) establishes a ceiling of 20 ppm for H2S with a 50 ppm peak allowance for 10 minutes, while NIOSH recommends a more stringent 10-minute ceiling of 10 ppm. These thresholds directly influence the performance requirements for absorbent materials, which must demonstrate capacity to reduce H2S concentrations below these regulatory limits.
EPA regulations under the Clean Air Act classify H2S as a hazardous air pollutant, requiring facilities to implement Maximum Achievable Control Technology (MACT) standards. For absorbent materials, this translates to documented removal efficiencies typically exceeding 95% under specified operating conditions. Additionally, the Resource Conservation and Recovery Act (RCRA) governs the disposal of spent absorbent materials, with particular attention to those that may release H2S during decomposition or contain heavy metals from catalytic components.
International standards provide further guidance, with ISO 14001 environmental management systems requiring organizations to establish procedures for identifying environmental aspects of H2S control. The European Union's REACH regulation imposes additional requirements for registration and safety assessment of chemical absorbents used in H2S control applications, particularly those containing metal oxides or organic compounds.
Industry-specific standards also apply, with API RP 55 providing guidelines for oil and gas operations, and NFPA 704 requiring proper hazard identification for absorbent materials. The American Society for Testing and Materials (ASTM) has developed standardized test methods for evaluating absorbent performance, including ASTM D5504 for sulfur compound analysis and ASTM E679 for odor threshold determinations.
Compliance documentation requirements include maintaining Safety Data Sheets (SDS) for all absorbent materials, conducting regular performance testing with calibrated equipment, and implementing a comprehensive management of change process for absorbent material modifications. Many jurisdictions also mandate employee training programs specific to H2S hazards and control measures, with refresher training typically required annually.
Emerging regulatory trends indicate increasing scrutiny of the environmental footprint of absorbent materials, with growing emphasis on biodegradability, regeneration capabilities, and reduced waste generation. Several jurisdictions are developing more stringent requirements for continuous monitoring systems that verify ongoing performance of H2S control technologies, potentially necessitating absorbent materials with integrated monitoring capabilities or predictable breakthrough characteristics.
EPA regulations under the Clean Air Act classify H2S as a hazardous air pollutant, requiring facilities to implement Maximum Achievable Control Technology (MACT) standards. For absorbent materials, this translates to documented removal efficiencies typically exceeding 95% under specified operating conditions. Additionally, the Resource Conservation and Recovery Act (RCRA) governs the disposal of spent absorbent materials, with particular attention to those that may release H2S during decomposition or contain heavy metals from catalytic components.
International standards provide further guidance, with ISO 14001 environmental management systems requiring organizations to establish procedures for identifying environmental aspects of H2S control. The European Union's REACH regulation imposes additional requirements for registration and safety assessment of chemical absorbents used in H2S control applications, particularly those containing metal oxides or organic compounds.
Industry-specific standards also apply, with API RP 55 providing guidelines for oil and gas operations, and NFPA 704 requiring proper hazard identification for absorbent materials. The American Society for Testing and Materials (ASTM) has developed standardized test methods for evaluating absorbent performance, including ASTM D5504 for sulfur compound analysis and ASTM E679 for odor threshold determinations.
Compliance documentation requirements include maintaining Safety Data Sheets (SDS) for all absorbent materials, conducting regular performance testing with calibrated equipment, and implementing a comprehensive management of change process for absorbent material modifications. Many jurisdictions also mandate employee training programs specific to H2S hazards and control measures, with refresher training typically required annually.
Emerging regulatory trends indicate increasing scrutiny of the environmental footprint of absorbent materials, with growing emphasis on biodegradability, regeneration capabilities, and reduced waste generation. Several jurisdictions are developing more stringent requirements for continuous monitoring systems that verify ongoing performance of H2S control technologies, potentially necessitating absorbent materials with integrated monitoring capabilities or predictable breakthrough characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!