Comparing Hydrosulfuric Acid and Hydrochloric Acid Effects
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Chemistry Background and Research Objectives
Acids have been fundamental to chemical research and industrial applications since the dawn of modern chemistry. Hydrosulfuric acid (H₂S in aqueous solution) and hydrochloric acid (HCl in aqueous solution) represent two distinct classes of acids with varying properties, applications, and environmental impacts. The historical development of acid chemistry traces back to the alchemical era, with significant advancements occurring during the Industrial Revolution when mass production of acids became essential for manufacturing processes.
Hydrochloric acid, discovered in the early medieval period by alchemists as "spirit of salt," has evolved into one of the most widely used industrial acids. Its production methods have progressed from rudimentary salt decomposition techniques to modern chlor-alkali processes. Hydrosulfuric acid, while less commonly used in pure form, has been recognized since ancient times by its characteristic rotten egg odor, with its chemistry becoming better understood through the work of Carl Wilhelm Scheele in the 18th century.
The technological evolution in acid production and application has been driven by industrial needs across sectors including metallurgy, chemical synthesis, petroleum refining, and environmental management. Recent advancements in analytical techniques have enabled more precise understanding of acid-base interactions at molecular levels, particularly in biological systems where pH regulation is critical.
Current research trends indicate growing interest in comparing these acids' effects across multiple dimensions: reaction kinetics, corrosion mechanisms, environmental persistence, and biological interactions. The differential behaviors of these acids in various media present both challenges and opportunities for specialized applications. Hydrochloric acid's strong mineral acid properties contrast with hydrosulfuric acid's weaker acidity but higher reactivity with metals and unique sulfur chemistry.
This technical research aims to systematically evaluate and compare the effects of hydrosulfuric acid and hydrochloric acid across multiple parameters including: reaction mechanisms with various substrates, corrosion patterns on different materials, environmental fate and transport characteristics, biological interaction pathways, and industrial application efficacy. The comparative analysis will focus particularly on identifying conditional advantages of each acid in specific application scenarios.
The research objectives include developing predictive models for acid behavior in complex systems, establishing comprehensive safety protocols based on comparative hazard profiles, identifying novel application areas leveraging the unique properties of each acid, and formulating hybrid approaches that might combine beneficial aspects of both acids for specialized applications. Additionally, we aim to explore emerging green chemistry alternatives that might eventually replace these traditional acids in environmentally sensitive applications.
By establishing a thorough understanding of the comparative effects of these acids, this research seeks to optimize acid selection processes across industries, enhance safety protocols, reduce environmental impacts, and potentially discover novel applications at the intersection of these distinct acid chemistries.
Hydrochloric acid, discovered in the early medieval period by alchemists as "spirit of salt," has evolved into one of the most widely used industrial acids. Its production methods have progressed from rudimentary salt decomposition techniques to modern chlor-alkali processes. Hydrosulfuric acid, while less commonly used in pure form, has been recognized since ancient times by its characteristic rotten egg odor, with its chemistry becoming better understood through the work of Carl Wilhelm Scheele in the 18th century.
The technological evolution in acid production and application has been driven by industrial needs across sectors including metallurgy, chemical synthesis, petroleum refining, and environmental management. Recent advancements in analytical techniques have enabled more precise understanding of acid-base interactions at molecular levels, particularly in biological systems where pH regulation is critical.
Current research trends indicate growing interest in comparing these acids' effects across multiple dimensions: reaction kinetics, corrosion mechanisms, environmental persistence, and biological interactions. The differential behaviors of these acids in various media present both challenges and opportunities for specialized applications. Hydrochloric acid's strong mineral acid properties contrast with hydrosulfuric acid's weaker acidity but higher reactivity with metals and unique sulfur chemistry.
This technical research aims to systematically evaluate and compare the effects of hydrosulfuric acid and hydrochloric acid across multiple parameters including: reaction mechanisms with various substrates, corrosion patterns on different materials, environmental fate and transport characteristics, biological interaction pathways, and industrial application efficacy. The comparative analysis will focus particularly on identifying conditional advantages of each acid in specific application scenarios.
The research objectives include developing predictive models for acid behavior in complex systems, establishing comprehensive safety protocols based on comparative hazard profiles, identifying novel application areas leveraging the unique properties of each acid, and formulating hybrid approaches that might combine beneficial aspects of both acids for specialized applications. Additionally, we aim to explore emerging green chemistry alternatives that might eventually replace these traditional acids in environmentally sensitive applications.
By establishing a thorough understanding of the comparative effects of these acids, this research seeks to optimize acid selection processes across industries, enhance safety protocols, reduce environmental impacts, and potentially discover novel applications at the intersection of these distinct acid chemistries.
Market Applications and Demand Analysis
The global market for acids is experiencing significant growth, with hydrochloric acid (HCl) and hydrosulfuric acid (H2S) playing distinct roles across various industries. The current market size for hydrochloric acid is estimated at 4 billion USD, with a compound annual growth rate of 6.2% projected through 2028, primarily driven by chemical manufacturing, metal processing, and water treatment applications.
Hydrochloric acid dominates industrial applications due to its stability, cost-effectiveness, and versatile chemical properties. The metal processing sector accounts for approximately 28% of global HCl consumption, where it serves as a critical agent for metal pickling, particularly in steel manufacturing. The oil and gas industry represents another significant market, utilizing HCl for well acidizing and scale removal operations, with demand closely tied to drilling activities.
In contrast, hydrosulfuric acid (often handled as hydrogen sulfide gas dissolved in solution) has a more specialized market profile. Its primary commercial applications center around analytical chemistry, sulfide precipitation reactions, and as a reagent in certain manufacturing processes. The market for H2S solutions remains relatively niche compared to HCl, with an estimated global value of 320 million USD.
Regional demand patterns show notable variations. Asia-Pacific, particularly China and India, leads hydrochloric acid consumption due to rapid industrialization and expanding manufacturing sectors. North America maintains steady demand driven by oil and gas operations, while European markets focus on specialty chemical applications with stricter environmental regulations influencing usage patterns.
Environmental regulations are increasingly shaping market dynamics for both acids. Hydrochloric acid faces growing regulatory scrutiny regarding emissions and disposal, though its essential role in many industrial processes ensures continued demand. Hydrosulfuric acid faces even stricter controls due to its extreme toxicity and environmental hazards, limiting its widespread application despite certain technical advantages in specific processes.
Emerging applications are creating new market opportunities. Green technology sectors are exploring hydrochloric acid in lithium extraction for batteries, while certain biofuel production processes utilize controlled applications of both acids. Additionally, water treatment applications continue to expand as global water quality concerns intensify, with HCl playing a crucial role in pH adjustment and contaminant removal systems.
Price sensitivity varies significantly between the two acids. Hydrochloric acid maintains relatively stable pricing due to established production methods and widespread availability, while hydrosulfuric acid solutions command premium prices due to handling complexities, safety requirements, and specialized production needs.
Hydrochloric acid dominates industrial applications due to its stability, cost-effectiveness, and versatile chemical properties. The metal processing sector accounts for approximately 28% of global HCl consumption, where it serves as a critical agent for metal pickling, particularly in steel manufacturing. The oil and gas industry represents another significant market, utilizing HCl for well acidizing and scale removal operations, with demand closely tied to drilling activities.
In contrast, hydrosulfuric acid (often handled as hydrogen sulfide gas dissolved in solution) has a more specialized market profile. Its primary commercial applications center around analytical chemistry, sulfide precipitation reactions, and as a reagent in certain manufacturing processes. The market for H2S solutions remains relatively niche compared to HCl, with an estimated global value of 320 million USD.
Regional demand patterns show notable variations. Asia-Pacific, particularly China and India, leads hydrochloric acid consumption due to rapid industrialization and expanding manufacturing sectors. North America maintains steady demand driven by oil and gas operations, while European markets focus on specialty chemical applications with stricter environmental regulations influencing usage patterns.
Environmental regulations are increasingly shaping market dynamics for both acids. Hydrochloric acid faces growing regulatory scrutiny regarding emissions and disposal, though its essential role in many industrial processes ensures continued demand. Hydrosulfuric acid faces even stricter controls due to its extreme toxicity and environmental hazards, limiting its widespread application despite certain technical advantages in specific processes.
Emerging applications are creating new market opportunities. Green technology sectors are exploring hydrochloric acid in lithium extraction for batteries, while certain biofuel production processes utilize controlled applications of both acids. Additionally, water treatment applications continue to expand as global water quality concerns intensify, with HCl playing a crucial role in pH adjustment and contaminant removal systems.
Price sensitivity varies significantly between the two acids. Hydrochloric acid maintains relatively stable pricing due to established production methods and widespread availability, while hydrosulfuric acid solutions command premium prices due to handling complexities, safety requirements, and specialized production needs.
Current Technical Challenges in Acid Comparison
The comparison of hydrosulfuric acid (H₂S in aqueous solution) and hydrochloric acid (HCl) presents several significant technical challenges that impede comprehensive analysis and application optimization. One primary challenge lies in the accurate measurement of reaction kinetics under varying environmental conditions. While hydrochloric acid demonstrates relatively predictable behavior across different temperatures and concentrations, hydrosulfuric acid exhibits complex phase-dependent reactivity that changes dramatically with slight environmental variations, making standardized comparison methodologies difficult to establish.
Safety considerations create substantial barriers to direct comparative research. Hydrosulfuric acid releases highly toxic hydrogen sulfide gas, which poses immediate life-threatening risks at concentrations as low as 100 ppm. This necessitates specialized containment systems and safety protocols that are not uniformly available across research facilities, resulting in fragmented and sometimes incomparable research outcomes.
Material compatibility testing represents another significant challenge. The corrosive mechanisms of these acids differ fundamentally - hydrochloric acid primarily causes uniform corrosion through direct hydrogen ion attack, while hydrosulfuric acid often induces more complex sulfide stress cracking and hydrogen embrittlement in metals. Current testing protocols are predominantly optimized for hydrochloric acid effects, creating systematic bias in comparative analyses.
Analytical instrumentation limitations further complicate accurate comparisons. Many standard pH measurement electrodes and sensors experience interference or degradation when exposed to sulfide species, leading to measurement drift and unreliable data when analyzing hydrosulfuric acid solutions. This technical limitation has resulted in fewer quantitative studies on hydrosulfuric acid compared to the extensively documented hydrochloric acid.
Environmental impact assessment methodologies lack standardization across these different acid types. While chloride monitoring in wastewater and environmental samples is well-established, sulfide species detection requires more complex analytical approaches with higher detection limits. This disparity creates challenges in comparing the true environmental footprints of processes utilizing these different acids.
The industrial scalability gap presents perhaps the most significant practical challenge. Hydrochloric acid benefits from decades of industrial handling experience and established infrastructure, while hydrosulfuric acid applications face substantial engineering barriers related to materials selection, worker safety, and process control. This imbalance in technological readiness levels makes direct cost-benefit comparisons between the acids inherently problematic for industrial applications.
Safety considerations create substantial barriers to direct comparative research. Hydrosulfuric acid releases highly toxic hydrogen sulfide gas, which poses immediate life-threatening risks at concentrations as low as 100 ppm. This necessitates specialized containment systems and safety protocols that are not uniformly available across research facilities, resulting in fragmented and sometimes incomparable research outcomes.
Material compatibility testing represents another significant challenge. The corrosive mechanisms of these acids differ fundamentally - hydrochloric acid primarily causes uniform corrosion through direct hydrogen ion attack, while hydrosulfuric acid often induces more complex sulfide stress cracking and hydrogen embrittlement in metals. Current testing protocols are predominantly optimized for hydrochloric acid effects, creating systematic bias in comparative analyses.
Analytical instrumentation limitations further complicate accurate comparisons. Many standard pH measurement electrodes and sensors experience interference or degradation when exposed to sulfide species, leading to measurement drift and unreliable data when analyzing hydrosulfuric acid solutions. This technical limitation has resulted in fewer quantitative studies on hydrosulfuric acid compared to the extensively documented hydrochloric acid.
Environmental impact assessment methodologies lack standardization across these different acid types. While chloride monitoring in wastewater and environmental samples is well-established, sulfide species detection requires more complex analytical approaches with higher detection limits. This disparity creates challenges in comparing the true environmental footprints of processes utilizing these different acids.
The industrial scalability gap presents perhaps the most significant practical challenge. Hydrochloric acid benefits from decades of industrial handling experience and established infrastructure, while hydrosulfuric acid applications face substantial engineering barriers related to materials selection, worker safety, and process control. This imbalance in technological readiness levels makes direct cost-benefit comparisons between the acids inherently problematic for industrial applications.
Methodologies for Comparative Acid Analysis
01 Corrosion effects and prevention methods
Hydrosulfuric acid and hydrochloric acid are highly corrosive substances that can damage various materials, particularly metals. These acids can cause severe corrosion in industrial equipment, pipelines, and storage facilities. Various prevention methods have been developed, including protective coatings, corrosion inhibitors, and specialized materials that resist acid attack. These solutions help extend the lifespan of equipment exposed to these aggressive acids and reduce maintenance costs in industrial settings.- Corrosion effects and prevention methods: Hydrosulfuric acid and hydrochloric acid are highly corrosive substances that can damage various materials, particularly metals. These acids can cause severe corrosion in industrial equipment, pipelines, and storage containers. Prevention methods include using corrosion-resistant materials, protective coatings, and implementing regular maintenance protocols. Specialized equipment and treatment processes have been developed to mitigate the corrosive effects of these acids in industrial applications.
- Treatment and neutralization processes: Various methods have been developed to treat and neutralize hydrosulfuric acid and hydrochloric acid in industrial waste streams. These processes include chemical neutralization, precipitation reactions, and advanced oxidation techniques. Treatment systems often incorporate multiple stages to effectively handle these acids and convert them into less harmful substances. The neutralization processes help in reducing environmental pollution and making the waste safe for disposal.
- Environmental impact and emission control: Hydrosulfuric acid and hydrochloric acid emissions can have significant environmental impacts, including air pollution, water contamination, and harm to ecosystems. Various technologies have been developed to control and reduce these emissions from industrial processes. These include scrubbing systems, absorption techniques, and catalytic conversion methods. Monitoring systems are also employed to detect and measure acid concentrations in emissions to ensure compliance with environmental regulations.
- Industrial applications and production processes: Despite their corrosive nature, hydrosulfuric acid and hydrochloric acid have important applications in various industries. They are used in chemical manufacturing, metal processing, oil refining, and wastewater treatment. Specialized equipment and processes have been developed for the safe production, handling, and application of these acids in industrial settings. These include reactor designs, catalytic processes, and separation techniques that optimize the use of these acids while minimizing risks.
- Safety measures and handling protocols: Due to the hazardous nature of hydrosulfuric acid and hydrochloric acid, specific safety measures and handling protocols are essential. These include specialized containment systems, detection equipment for gas leaks, emergency neutralization procedures, and personal protective equipment requirements. Advanced monitoring systems have been developed to continuously track acid concentrations in work environments. Training programs and safety protocols are implemented to ensure proper handling and response to potential accidents involving these acids.
02 Treatment and neutralization processes
Various processes have been developed to treat and neutralize hydrosulfuric acid and hydrochloric acid in industrial waste streams. These processes include chemical neutralization using alkaline substances, adsorption techniques, and specialized filtration systems. The treatment methods aim to reduce the acidity and harmful effects of these acids before discharge into the environment, ensuring compliance with environmental regulations and minimizing ecological impact.Expand Specific Solutions03 Industrial applications and production methods
Hydrosulfuric acid and hydrochloric acid have numerous industrial applications despite their corrosive nature. They are used in chemical manufacturing, metal processing, oil refining, and mineral extraction. Various production methods have been developed to synthesize these acids efficiently and safely. The patents describe innovative approaches to utilizing these acids in industrial processes while minimizing their hazardous effects on equipment and personnel.Expand Specific Solutions04 Environmental impact and emission control
The release of hydrosulfuric acid and hydrochloric acid into the environment can have significant negative impacts on ecosystems and air quality. These acids contribute to acid rain, water pollution, and can harm plant and animal life. Various technologies have been developed to control and reduce emissions of these acids from industrial processes. These include scrubbing systems, catalytic converters, and advanced monitoring equipment that help industries comply with increasingly stringent environmental regulations.Expand Specific Solutions05 Safety systems and detection methods
Due to the hazardous nature of hydrosulfuric acid and hydrochloric acid, specialized safety systems and detection methods have been developed. These include gas detection sensors, emergency neutralization systems, and personal protective equipment. Early detection of acid leaks or dangerous concentration levels is crucial for preventing workplace accidents and protecting personnel. The innovations in this area focus on improving response time, accuracy of detection, and overall safety in environments where these acids are present.Expand Specific Solutions
Leading Institutions and Companies in Acid Research
The hydrosulfuric and hydrochloric acid market is in a mature growth phase with established applications across multiple industries. The global market size for these acids is estimated at several billion dollars annually, with hydrochloric acid dominating due to its wider industrial applications. Technologically, both acids have well-established production methods, though innovation continues in safer handling and environmental impact reduction. Leading companies like Fluid Energy Group and LANXESS Deutschland have developed specialized formulations for oil and gas applications, while PetroChina, ExxonMobil, and Sinopec leverage these acids in their petrochemical operations. Research institutions such as Southwest Petroleum University and Council of Scientific & Industrial Research are advancing new applications, particularly in environmentally-friendly alternatives and specialized industrial processes.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has developed proprietary acid systems that compare hydrosulfuric acid and hydrochloric acid effects in industrial applications. Their technology, known as Complex Acid™, offers a safer alternative to traditional strong mineral acids like HCl while maintaining comparable efficacy. The company's research demonstrates that their modified acid formulations can achieve similar dissolution rates to hydrochloric acid but with significantly reduced corrosivity and harmful emissions. Their comparative studies show that while hydrosulfuric acid (H2S in solution) presents severe toxicity concerns, their engineered acid systems provide the reactivity benefits without the associated health and environmental risks. The company has extensively documented how their acid systems perform across various pH ranges and temperatures compared to both traditional acids, allowing for optimized application in oil and gas, mining, and industrial cleaning sectors.
Strengths: Provides safer handling characteristics than both traditional acids with reduced corrosion rates on metallurgy; environmentally superior with biodegradable components. Weaknesses: May require higher concentrations than HCl for equivalent reaction rates in some applications; potentially higher production costs compared to commodity acids.
PetroChina Co., Ltd.
Technical Solution: PetroChina has developed sophisticated acid management technologies specifically focused on comparing hydrosulfuric acid and hydrochloric acid effects in challenging oil and gas production environments. Their research encompasses both laboratory studies and extensive field trials across diverse geological formations. PetroChina's technical documentation demonstrates that while hydrochloric acid provides superior dissolution rates for carbonate formations, their engineered acid systems incorporating controlled hydrogen sulfide precursors offer significant advantages in high-temperature, high-pressure wells where conventional HCl may react too rapidly. Their comparative analysis shows that properly inhibited H2S-generating systems can provide more uniform penetration into the formation while reducing the risk of precipitate formation that often plagues HCl treatments. PetroChina has also pioneered specialized corrosion testing protocols that quantify the differential effects of these acids on various metallurgies common in oil and gas production, with their data showing that certain alloys exhibit dramatically different corrosion rates between the two acid types, sometimes by factors exceeding 10x under identical concentration and temperature conditions.
Strengths: Highly specialized acid formulations for ultra-deep, high-temperature wells where traditional acids show limitations; extensive field validation data across diverse geological formations in Asia. Weaknesses: More complex handling and mixing procedures required for their advanced acid systems; higher initial costs though often justified by improved production outcomes and reduced equipment damage.
Key Scientific Findings on H2S vs HCl Effects
Composition for pH control
PatentActiveUS12091338B2
Innovation
- A composition comprising sodium bisulphate, aluminium sulphate, copper sulphate, and a chelating agent, which maintains pH control while also addressing water clarity, phosphate removal, and algae control in a single application, minimizing precipitation and corrosion risks, and including a calcium scale inhibitor to prevent scale formation.
Hydrogen sulfide removal from reverse osmosis product water
PatentInactiveUS5096589A
Innovation
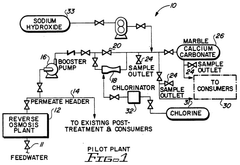
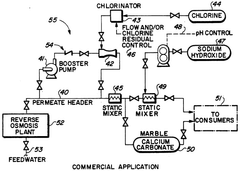
- A method involving demineralization of reverse osmosis product water followed by chlorination to oxidize hydrogen sulfide to sulfuric and hydrochloric acids, avoiding elemental sulfur formation, and subsequent neutralization using a marble contact bed or sodium hydroxide to adjust pH and hardness.
Environmental Impact Assessment
The environmental impact of acids is a critical consideration in both industrial applications and accidental releases. Hydrosulfuric acid (H2S in aqueous solution) and hydrochloric acid (HCl) present distinctly different environmental hazards that require thorough assessment for responsible management.
Hydrosulfuric acid poses significant atmospheric pollution concerns due to its gaseous form (hydrogen sulfide) readily escaping from solution. When released into the atmosphere, it contributes to acid rain formation and can travel considerable distances, affecting ecosystems remote from the source. The characteristic "rotten egg" odor becomes detectable at concentrations as low as 0.5 ppb, serving as an early warning system, though olfactory fatigue quickly renders this protection mechanism ineffective.
Water ecosystems suffer particularly severe impacts from hydrosulfuric acid contamination. Even at concentrations of 0.5 mg/L, it can cause mass mortality in aquatic organisms, with fish being especially vulnerable. The acid depletes dissolved oxygen through chemical reactions and disrupts the respiratory functions of aquatic life. Additionally, it can mobilize toxic metals from sediments, creating secondary contamination issues that persist long after the initial release.
Hydrochloric acid, while highly corrosive, presents a different environmental profile. Its environmental persistence is generally lower than hydrosulfuric acid, as it tends to be neutralized through reactions with environmental carbonates and other buffering compounds. However, concentrated releases can dramatically lower the pH of water bodies, causing immediate and severe damage to aquatic ecosystems, with effects potentially lasting for years in poorly buffered systems.
Soil contamination patterns also differ significantly between these acids. Hydrochloric acid primarily affects surface soil chemistry through pH alteration, potentially increasing the bioavailability of toxic metals. In contrast, hydrosulfuric acid can penetrate deeper soil layers due to its gaseous component, affecting soil microbial communities and plant root systems at greater depths, with recovery times often exceeding a decade.
Remediation approaches must be tailored to these distinct environmental behaviors. Hydrochloric acid spills typically require neutralization with alkaline compounds, followed by dilution when appropriate. Hydrosulfuric acid contamination often necessitates more complex treatment strategies, including chemical oxidation to convert sulfides to less harmful sulfates, and potentially long-term monitoring of affected ecosystems.
Regulatory frameworks increasingly recognize these differences, with hydrosulfuric acid facing stricter emission controls in many jurisdictions due to its acute toxicity and persistent environmental effects. Both acids are subject to comprehensive reporting requirements under various environmental protection regulations, though threshold quantities for mandatory reporting are typically lower for hydrosulfuric acid.
Hydrosulfuric acid poses significant atmospheric pollution concerns due to its gaseous form (hydrogen sulfide) readily escaping from solution. When released into the atmosphere, it contributes to acid rain formation and can travel considerable distances, affecting ecosystems remote from the source. The characteristic "rotten egg" odor becomes detectable at concentrations as low as 0.5 ppb, serving as an early warning system, though olfactory fatigue quickly renders this protection mechanism ineffective.
Water ecosystems suffer particularly severe impacts from hydrosulfuric acid contamination. Even at concentrations of 0.5 mg/L, it can cause mass mortality in aquatic organisms, with fish being especially vulnerable. The acid depletes dissolved oxygen through chemical reactions and disrupts the respiratory functions of aquatic life. Additionally, it can mobilize toxic metals from sediments, creating secondary contamination issues that persist long after the initial release.
Hydrochloric acid, while highly corrosive, presents a different environmental profile. Its environmental persistence is generally lower than hydrosulfuric acid, as it tends to be neutralized through reactions with environmental carbonates and other buffering compounds. However, concentrated releases can dramatically lower the pH of water bodies, causing immediate and severe damage to aquatic ecosystems, with effects potentially lasting for years in poorly buffered systems.
Soil contamination patterns also differ significantly between these acids. Hydrochloric acid primarily affects surface soil chemistry through pH alteration, potentially increasing the bioavailability of toxic metals. In contrast, hydrosulfuric acid can penetrate deeper soil layers due to its gaseous component, affecting soil microbial communities and plant root systems at greater depths, with recovery times often exceeding a decade.
Remediation approaches must be tailored to these distinct environmental behaviors. Hydrochloric acid spills typically require neutralization with alkaline compounds, followed by dilution when appropriate. Hydrosulfuric acid contamination often necessitates more complex treatment strategies, including chemical oxidation to convert sulfides to less harmful sulfates, and potentially long-term monitoring of affected ecosystems.
Regulatory frameworks increasingly recognize these differences, with hydrosulfuric acid facing stricter emission controls in many jurisdictions due to its acute toxicity and persistent environmental effects. Both acids are subject to comprehensive reporting requirements under various environmental protection regulations, though threshold quantities for mandatory reporting are typically lower for hydrosulfuric acid.
Safety Protocols and Handling Regulations
The handling of hydrosulfuric acid (H2S in aqueous solution) and hydrochloric acid (HCl) requires strict adherence to comprehensive safety protocols due to their hazardous properties. Both acids present significant risks including corrosivity, toxicity, and potential for chemical burns, with hydrosulfuric acid posing the additional danger of releasing highly toxic hydrogen sulfide gas.
Personal protective equipment (PPE) requirements differ slightly between these acids. For both, chemical-resistant gloves (neoprene or butyl rubber), face shields, chemical splash goggles, and acid-resistant aprons are mandatory. However, when handling hydrosulfuric acid, respiratory protection with appropriate gas filters is essential due to the potential release of H2S gas, which can cause respiratory paralysis at concentrations as low as 500-700 ppm.
Storage regulations mandate segregation of these acids from incompatible substances, particularly bases, active metals, and oxidizers. Hydrosulfuric acid requires additional precautions including specialized ventilation systems with H2S detectors and pressure-relief mechanisms for containers. Both acids must be stored in corrosion-resistant containers in cool, well-ventilated areas away from direct sunlight.
Emergency response protocols for both acids include immediate flushing of affected areas with water for at least 15 minutes following skin or eye contact. However, hydrosulfuric acid exposures may require additional medical intervention due to potential systemic toxicity. Spill management procedures differ significantly: hydrochloric acid spills can be neutralized with sodium bicarbonate or lime, while hydrosulfuric acid spills require specialized absorbents and potentially evacuation of the area due to gas hazards.
Regulatory frameworks governing these acids include OSHA's Permissible Exposure Limits (PEL of 5 ppm ceiling for HCl; 10 ppm ceiling for H2S), EPA regulations for disposal, and Department of Transportation (DOT) requirements for transport. Hydrosulfuric acid is subject to additional reporting requirements under CERCLA and EPCRA due to its higher environmental impact potential.
Training requirements for personnel handling these acids must include acid-specific hazard communication, proper use of PPE, spill response procedures, and first aid measures. For hydrosulfuric acid, additional training on gas monitoring equipment operation and evacuation procedures is necessary. Documentation and regular auditing of safety practices are required for both acids, with more frequent reviews recommended for facilities handling hydrosulfuric acid due to its higher risk profile.
Personal protective equipment (PPE) requirements differ slightly between these acids. For both, chemical-resistant gloves (neoprene or butyl rubber), face shields, chemical splash goggles, and acid-resistant aprons are mandatory. However, when handling hydrosulfuric acid, respiratory protection with appropriate gas filters is essential due to the potential release of H2S gas, which can cause respiratory paralysis at concentrations as low as 500-700 ppm.
Storage regulations mandate segregation of these acids from incompatible substances, particularly bases, active metals, and oxidizers. Hydrosulfuric acid requires additional precautions including specialized ventilation systems with H2S detectors and pressure-relief mechanisms for containers. Both acids must be stored in corrosion-resistant containers in cool, well-ventilated areas away from direct sunlight.
Emergency response protocols for both acids include immediate flushing of affected areas with water for at least 15 minutes following skin or eye contact. However, hydrosulfuric acid exposures may require additional medical intervention due to potential systemic toxicity. Spill management procedures differ significantly: hydrochloric acid spills can be neutralized with sodium bicarbonate or lime, while hydrosulfuric acid spills require specialized absorbents and potentially evacuation of the area due to gas hazards.
Regulatory frameworks governing these acids include OSHA's Permissible Exposure Limits (PEL of 5 ppm ceiling for HCl; 10 ppm ceiling for H2S), EPA regulations for disposal, and Department of Transportation (DOT) requirements for transport. Hydrosulfuric acid is subject to additional reporting requirements under CERCLA and EPCRA due to its higher environmental impact potential.
Training requirements for personnel handling these acids must include acid-specific hazard communication, proper use of PPE, spill response procedures, and first aid measures. For hydrosulfuric acid, additional training on gas monitoring equipment operation and evacuation procedures is necessary. Documentation and regular auditing of safety practices are required for both acids, with more frequent reviews recommended for facilities handling hydrosulfuric acid due to its higher risk profile.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!