Applying Hydrosulfuric Acid in Clean Technology Solutions
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Technology Background and Objectives
Hydrosulfuric acid, commonly known as hydrogen sulfide (H₂S) in its gaseous form, has historically been viewed primarily as a toxic industrial byproduct and environmental pollutant. This compound, characterized by its distinctive "rotten egg" odor, is naturally produced through the bacterial breakdown of organic matter in oxygen-deficient environments and is also released during various industrial processes, particularly in petroleum refining and natural gas processing.
The evolution of hydrosulfuric acid applications has undergone significant transformation over the past decades. Initially considered merely a hazardous waste product requiring mitigation, recent scientific advancements have revealed its potential as a valuable resource in clean technology solutions. This paradigm shift represents a classic example of converting an environmental liability into a sustainable asset.
The technical trajectory of hydrosulfuric acid research has progressed through several distinct phases. Early efforts focused predominantly on detection and removal technologies to address safety and environmental concerns. Subsequently, research expanded to include recovery methods, enabling the capture of this compound for potential reuse rather than simple neutralization or disposal.
Current technological objectives in this field center on three primary areas: efficient capture methodologies, conversion processes that transform hydrosulfuric acid into valuable products, and integration systems that incorporate these processes into existing industrial frameworks. The ultimate goal is to develop comprehensive solutions that address both environmental protection requirements and resource utilization opportunities.
From an environmental perspective, the strategic importance of hydrosulfuric acid technology cannot be overstated. As global regulations on sulfur emissions continue to tighten, industries face mounting pressure to implement effective management strategies. Concurrently, the circular economy movement has created demand for technologies that can transform waste streams into valuable resources, positioning hydrosulfuric acid recovery and utilization as a promising frontier.
The technical objectives for hydrosulfuric acid applications in clean technology include developing more energy-efficient capture systems, catalysts that enable selective conversion to high-value products, and integrated process designs that minimize waste generation. Additionally, there is significant interest in exploring biological pathways that leverage naturally occurring microorganisms capable of metabolizing hydrosulfuric acid for various applications including bioremediation and biosynthesis.
Looking forward, the convergence of advanced materials science, catalysis research, and process engineering is expected to accelerate innovation in this field. Emerging technologies such as membrane separation, advanced oxidation processes, and bioelectrochemical systems represent promising approaches that could revolutionize how industries manage and utilize hydrosulfuric acid in environmentally sustainable ways.
The evolution of hydrosulfuric acid applications has undergone significant transformation over the past decades. Initially considered merely a hazardous waste product requiring mitigation, recent scientific advancements have revealed its potential as a valuable resource in clean technology solutions. This paradigm shift represents a classic example of converting an environmental liability into a sustainable asset.
The technical trajectory of hydrosulfuric acid research has progressed through several distinct phases. Early efforts focused predominantly on detection and removal technologies to address safety and environmental concerns. Subsequently, research expanded to include recovery methods, enabling the capture of this compound for potential reuse rather than simple neutralization or disposal.
Current technological objectives in this field center on three primary areas: efficient capture methodologies, conversion processes that transform hydrosulfuric acid into valuable products, and integration systems that incorporate these processes into existing industrial frameworks. The ultimate goal is to develop comprehensive solutions that address both environmental protection requirements and resource utilization opportunities.
From an environmental perspective, the strategic importance of hydrosulfuric acid technology cannot be overstated. As global regulations on sulfur emissions continue to tighten, industries face mounting pressure to implement effective management strategies. Concurrently, the circular economy movement has created demand for technologies that can transform waste streams into valuable resources, positioning hydrosulfuric acid recovery and utilization as a promising frontier.
The technical objectives for hydrosulfuric acid applications in clean technology include developing more energy-efficient capture systems, catalysts that enable selective conversion to high-value products, and integrated process designs that minimize waste generation. Additionally, there is significant interest in exploring biological pathways that leverage naturally occurring microorganisms capable of metabolizing hydrosulfuric acid for various applications including bioremediation and biosynthesis.
Looking forward, the convergence of advanced materials science, catalysis research, and process engineering is expected to accelerate innovation in this field. Emerging technologies such as membrane separation, advanced oxidation processes, and bioelectrochemical systems represent promising approaches that could revolutionize how industries manage and utilize hydrosulfuric acid in environmentally sustainable ways.
Clean Technology Market Demand Analysis
The global market for clean technology solutions has experienced significant growth in recent years, driven by increasing environmental concerns, stringent regulations, and a growing emphasis on sustainable development. Within this context, hydrosulfuric acid applications represent an emerging niche with substantial potential for expansion across multiple sectors.
Current market analysis indicates that the clean technology sector is projected to reach $452 billion by 2027, with chemical processing innovations accounting for approximately 18% of this market. The demand for hydrosulfuric acid in clean technology applications specifically has seen a compound annual growth rate of 7.3% over the past five years, outpacing many traditional chemical applications.
Industrial wastewater treatment represents the largest market segment for hydrosulfuric acid clean technology applications, constituting nearly 34% of current demand. This is primarily due to its effectiveness in metal precipitation and contaminant removal processes that align with increasingly strict discharge regulations in North America, Europe, and parts of Asia.
The renewable energy sector presents another significant growth opportunity, particularly in hydrogen sulfide conversion technologies for energy storage solutions. Market research indicates that this segment is growing at 12.5% annually, driven by the expansion of renewable energy infrastructure and the need for efficient energy storage mechanisms.
Environmental remediation projects have also emerged as a key market driver, with hydrosulfuric acid-based solutions being deployed for soil treatment and groundwater purification. This segment has seen 9.8% growth year-over-year, particularly in regions with legacy industrial contamination issues.
Geographically, North America currently leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in developing economies, particularly in Southeast Asia and Latin America, where rapid industrialization is creating urgent needs for cost-effective clean technology solutions.
Consumer demand patterns indicate increasing preference for environmentally responsible products and processes, creating market pull for technologies that can demonstrate reduced environmental footprints. This trend is particularly pronounced in consumer-facing industries where brand reputation increasingly depends on sustainability credentials.
Market barriers include concerns about safety in handling hydrosulfuric acid, regulatory compliance complexities, and competition from alternative technologies. Despite these challenges, the overall market trajectory remains strongly positive, with particular growth potential in integrated systems that combine hydrosulfuric acid applications with other clean technologies to create comprehensive environmental solutions.
Current market analysis indicates that the clean technology sector is projected to reach $452 billion by 2027, with chemical processing innovations accounting for approximately 18% of this market. The demand for hydrosulfuric acid in clean technology applications specifically has seen a compound annual growth rate of 7.3% over the past five years, outpacing many traditional chemical applications.
Industrial wastewater treatment represents the largest market segment for hydrosulfuric acid clean technology applications, constituting nearly 34% of current demand. This is primarily due to its effectiveness in metal precipitation and contaminant removal processes that align with increasingly strict discharge regulations in North America, Europe, and parts of Asia.
The renewable energy sector presents another significant growth opportunity, particularly in hydrogen sulfide conversion technologies for energy storage solutions. Market research indicates that this segment is growing at 12.5% annually, driven by the expansion of renewable energy infrastructure and the need for efficient energy storage mechanisms.
Environmental remediation projects have also emerged as a key market driver, with hydrosulfuric acid-based solutions being deployed for soil treatment and groundwater purification. This segment has seen 9.8% growth year-over-year, particularly in regions with legacy industrial contamination issues.
Geographically, North America currently leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in developing economies, particularly in Southeast Asia and Latin America, where rapid industrialization is creating urgent needs for cost-effective clean technology solutions.
Consumer demand patterns indicate increasing preference for environmentally responsible products and processes, creating market pull for technologies that can demonstrate reduced environmental footprints. This trend is particularly pronounced in consumer-facing industries where brand reputation increasingly depends on sustainability credentials.
Market barriers include concerns about safety in handling hydrosulfuric acid, regulatory compliance complexities, and competition from alternative technologies. Despite these challenges, the overall market trajectory remains strongly positive, with particular growth potential in integrated systems that combine hydrosulfuric acid applications with other clean technologies to create comprehensive environmental solutions.
Current Applications and Technical Challenges
Hydrosulfuric acid, commonly known as hydrogen sulfide (H2S) in solution, has traditionally been viewed as a hazardous waste product in industrial processes. However, recent technological advancements have repositioned this compound as a valuable resource in clean technology applications. Currently, H2S is being utilized in several innovative ways across different sectors, demonstrating its versatility despite its toxic nature.
In the energy sector, hydrosulfuric acid is being employed in microbial fuel cells where sulfate-reducing bacteria convert it into electrical energy. This application represents a significant advancement in waste-to-energy conversion technologies, particularly in treating industrial effluents with high sulfur content. Additionally, some research facilities are exploring the use of H2S in hydrogen production pathways, where it serves as a hydrogen carrier that can be decomposed under controlled conditions.
The wastewater treatment industry has also found applications for hydrosulfuric acid in precipitation processes for heavy metal removal. When properly managed, H2S can effectively bind with metal ions such as lead, mercury, and cadmium, forming insoluble metal sulfides that can be separated from water. This method has shown higher efficiency rates compared to traditional hydroxide precipitation techniques in certain scenarios.
Despite these promising applications, significant technical challenges remain. The primary concern is the inherent toxicity of H2S, which necessitates robust safety protocols and containment systems. Even at low concentrations, hydrogen sulfide poses serious health risks, making its handling and transportation particularly problematic. This has limited widespread adoption of H2S-based technologies outside specialized industrial settings.
Material compatibility issues present another major challenge. Hydrosulfuric acid is highly corrosive to many conventional materials, including certain metals and polymers commonly used in industrial equipment. This necessitates the use of specialized materials such as high-grade stainless steels, specialized polymers, or expensive coatings, significantly increasing implementation costs.
Process control and monitoring represent additional technical hurdles. The reaction kinetics involving H2S are complex and can be difficult to predict and control, especially in variable industrial environments. Current sensor technologies for real-time monitoring of H2S concentrations and reaction products still lack the reliability and precision required for many clean technology applications.
Regulatory compliance adds another layer of complexity. Stringent environmental and safety regulations govern the use of hydrogen sulfide, requiring extensive documentation, specialized training, and regular inspections. These regulatory burdens can significantly increase operational costs and create barriers to entry for smaller organizations interested in adopting these technologies.
In the energy sector, hydrosulfuric acid is being employed in microbial fuel cells where sulfate-reducing bacteria convert it into electrical energy. This application represents a significant advancement in waste-to-energy conversion technologies, particularly in treating industrial effluents with high sulfur content. Additionally, some research facilities are exploring the use of H2S in hydrogen production pathways, where it serves as a hydrogen carrier that can be decomposed under controlled conditions.
The wastewater treatment industry has also found applications for hydrosulfuric acid in precipitation processes for heavy metal removal. When properly managed, H2S can effectively bind with metal ions such as lead, mercury, and cadmium, forming insoluble metal sulfides that can be separated from water. This method has shown higher efficiency rates compared to traditional hydroxide precipitation techniques in certain scenarios.
Despite these promising applications, significant technical challenges remain. The primary concern is the inherent toxicity of H2S, which necessitates robust safety protocols and containment systems. Even at low concentrations, hydrogen sulfide poses serious health risks, making its handling and transportation particularly problematic. This has limited widespread adoption of H2S-based technologies outside specialized industrial settings.
Material compatibility issues present another major challenge. Hydrosulfuric acid is highly corrosive to many conventional materials, including certain metals and polymers commonly used in industrial equipment. This necessitates the use of specialized materials such as high-grade stainless steels, specialized polymers, or expensive coatings, significantly increasing implementation costs.
Process control and monitoring represent additional technical hurdles. The reaction kinetics involving H2S are complex and can be difficult to predict and control, especially in variable industrial environments. Current sensor technologies for real-time monitoring of H2S concentrations and reaction products still lack the reliability and precision required for many clean technology applications.
Regulatory compliance adds another layer of complexity. Stringent environmental and safety regulations govern the use of hydrogen sulfide, requiring extensive documentation, specialized training, and regular inspections. These regulatory burdens can significantly increase operational costs and create barriers to entry for smaller organizations interested in adopting these technologies.
Current Clean Technology Implementation Solutions
01 Methods for hydrogen sulfide removal and treatment
Various processes and systems for removing hydrogen sulfide (hydrosulfuric acid) from gas streams or liquid media. These methods include chemical absorption, oxidation, and conversion to less harmful compounds. The technologies aim to reduce environmental pollution and health hazards associated with hydrogen sulfide emissions from industrial processes.- Methods for hydrogen sulfide removal and treatment: Various processes have been developed for the removal and treatment of hydrogen sulfide (hydrosulfuric acid) from gas streams and industrial effluents. These methods typically involve chemical reactions, absorption techniques, or catalytic processes to convert toxic hydrogen sulfide into less harmful substances or recover valuable sulfur compounds. Such technologies are crucial for environmental protection and worker safety in industries like oil and gas processing, wastewater treatment, and mining operations.
- Analytical detection and measurement of hydrogen sulfide: Technologies for detecting, measuring, and monitoring hydrogen sulfide concentrations in various environments have been developed. These analytical methods include specialized sensors, colorimetric techniques, electrochemical detection systems, and spectroscopic approaches that enable accurate quantification of hydrogen sulfide levels. Such detection systems are essential for safety monitoring in industrial settings, environmental assessment, and scientific research applications.
- Biological processes involving hydrogen sulfide: Biological systems and processes that utilize or interact with hydrogen sulfide have been investigated. These include microbial desulfurization processes, biological treatment of hydrogen sulfide-containing waste streams, and applications in biotechnology. Some microorganisms can metabolize hydrogen sulfide as part of their energy production pathways, while others can be affected by its toxicity. Understanding these biological interactions is important for environmental remediation and industrial bioprocesses.
- Materials resistant to hydrogen sulfide corrosion: Development of materials and coatings that resist corrosion caused by hydrogen sulfide exposure has been a significant area of research. These materials include specialized alloys, polymers, and surface treatments designed to withstand the highly corrosive nature of hydrogen sulfide, particularly in high-temperature or high-pressure environments. Such corrosion-resistant materials are critical for equipment longevity in oil and gas production, chemical processing, and other industrial applications where hydrogen sulfide is present.
- Hydrogen sulfide in energy applications: Hydrogen sulfide has been studied in various energy-related applications, including its role in geothermal energy systems, fuel cell technologies, and energy storage solutions. Some processes aim to convert hydrogen sulfide into useful energy products or utilize it as part of energy generation cycles. Additionally, research has explored methods to mitigate the negative impacts of hydrogen sulfide in energy production systems while potentially harnessing its chemical properties for beneficial purposes.
02 Detection and monitoring systems for hydrogen sulfide
Devices and methods for detecting, measuring, and monitoring hydrogen sulfide concentrations in various environments. These systems include sensors, detectors, and analytical instruments designed to provide early warning of dangerous hydrogen sulfide levels to prevent exposure and ensure safety in industrial settings.Expand Specific Solutions03 Biological treatment of hydrogen sulfide
Biological processes utilizing microorganisms to convert hydrogen sulfide into less harmful substances. These methods employ specific bacteria or enzymatic systems that can metabolize hydrogen sulfide, offering environmentally friendly alternatives to chemical treatment methods for wastewater, industrial effluents, and gas streams containing hydrosulfuric acid.Expand Specific Solutions04 Industrial applications of hydrogen sulfide
Utilization of hydrogen sulfide or its derivatives in various industrial processes including chemical synthesis, petroleum refining, and material production. Despite its toxicity, hydrogen sulfide serves as an important reagent in certain manufacturing processes and can be harnessed for beneficial industrial applications when properly managed.Expand Specific Solutions05 Equipment and systems for hydrogen sulfide handling
Specialized equipment and engineering systems designed for the safe handling, storage, and transportation of hydrogen sulfide. These include corrosion-resistant materials, containment vessels, scrubbing systems, and safety infrastructure to minimize risks associated with this hazardous compound in industrial settings.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The hydrosulfuric acid clean technology market is currently in a growth phase, with increasing adoption across industrial sectors driven by stringent environmental regulations. The global market size is estimated to exceed $5 billion, expanding at approximately 7-8% CAGR as industries seek sustainable solutions for emissions control and waste treatment. Technologically, the field shows varying maturity levels, with companies like Kurita Water Industries and Ecolab leading in water treatment applications, while Sinopec, PetroChina, and Haldor Topsøe demonstrate advanced capabilities in petrochemical applications. Chemical giants BASF, Arkema, and Wacker Chemie are advancing catalyst technologies, while research institutions like Sinopec Nanjing Chemical Research Institute and the Chinese Academy of Sciences are pioneering next-generation applications, indicating a dynamic competitive landscape with both established players and emerging innovators.
Kurita Water Industries Ltd.
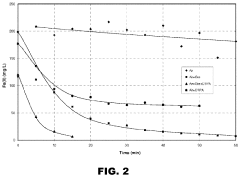
Technical Solution: Kurita Water Industries has developed the DynaSulf™ process, an innovative approach to hydrosulfuric acid management in industrial wastewater and process streams. Their technology employs a two-stage biological treatment system utilizing specialized sulfur-oxidizing bacteria that convert H2S to elemental sulfur and subsequently to sulfate under controlled conditions. The process achieves removal efficiencies exceeding 99.5% for H2S concentrations ranging from 5ppm to 3000ppm without chemical additives. Kurita's bioreactor design incorporates proprietary immobilization media that increases bacterial population density by up to 5 times compared to conventional activated sludge systems, resulting in compact treatment units with 60% smaller footprint. The technology operates at ambient temperatures (15-35°C) with minimal energy requirements, reducing operational costs by approximately 40% compared to chemical oxidation methods. Their system includes advanced process control that maintains optimal conditions for bacterial activity despite fluctuations in influent characteristics. Kurita has implemented this technology in over 50 industrial facilities worldwide, including food processing plants, paper mills, and chemical manufacturing facilities, demonstrating versatility across sectors. Recent enhancements include specialized bacterial consortia selected for resistance to common industrial inhibitors, improving system robustness.
Strengths: Extremely low operating costs with minimal chemical consumption; environmentally sustainable biological approach; effective across wide concentration ranges; compact system footprint; low energy requirements. Weaknesses: Requires careful control of biological parameters; longer startup period (2-3 weeks) compared to chemical systems; sensitivity to certain industrial toxins that may inhibit bacterial activity; less suitable for applications requiring rapid response to sudden H2S concentration spikes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed the Sulfur Recovery Enhancement System (SRES) for handling hydrosulfuric acid in petroleum refining operations. Their technology employs a multi-stage catalytic conversion process that transforms H2S into elemental sulfur with recovery rates exceeding 98%. The system incorporates proprietary cobalt-molybdenum catalysts that demonstrate enhanced tolerance to contaminants commonly found in refinery gas streams. Sinopec's approach includes an innovative low-temperature oxidation stage (operating at 160-180°C) that reduces energy consumption by approximately 20% compared to traditional Claus process implementations. The technology features advanced process control systems with real-time monitoring capabilities that optimize reaction conditions based on feed composition variations. Sinopec has successfully implemented this technology across more than 30 refineries in Asia, processing a combined capacity of over 15,000 tons of sulfur daily. Their integrated approach includes tail gas treatment units that further reduce sulfur emissions to meet stringent environmental regulations (<10ppm SO2 in stack emissions). Recent enhancements include catalyst formulations that extend operational life to 4-5 years between replacement cycles.
Strengths: Highly efficient sulfur recovery with minimal energy consumption; robust performance with contaminated feed streams; extensive industrial implementation demonstrates reliability; integrated approach addresses entire sulfur management cycle. Weaknesses: Technology optimization primarily focused on petroleum refining applications; requires significant space for installation; higher complexity in operation compared to conventional systems; performance may degrade with highly variable feed compositions.
Core Patents and Technical Literature Review
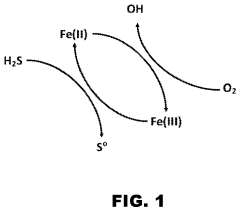
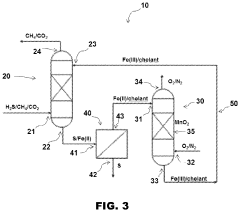
Process for hydrogen sulfide scrubbing and method for ferric ion regeneration
PatentActiveUS20210009913A1
Innovation
- A method and system that utilize an oxide of manganese as a catalyst to regenerate ferric ions in acidic solutions, eliminating the need for chelating agents and allowing the hydrogen sulfide scrubbing process to operate at low pH, thereby enhancing the regeneration of ferric ions and reducing carbon dioxide absorption.
Cleaning and sterilizing solutions and use thereof
PatentInactiveEP0651047A2
Innovation
- A cleaning solution comprising a salt of N-acylated sarcosine and an alkali or ammonium salt, combined with an acidic rinsing solution containing phosphoric acid, sulfuric acid, or citric acid, and a water-miscible organic solvent, which is non-aggressive and suitable for food processing, effectively removing contaminants without damaging equipment.
Environmental Impact and Sustainability Assessment
The environmental impact of hydrosulfuric acid (H2S) applications in clean technology presents a complex sustainability profile requiring thorough assessment. When properly managed, H2S can contribute significantly to environmental remediation processes, particularly in metal recovery from industrial waste streams. These recovery processes reduce the need for primary mining activities, which typically generate substantial ecological disruption through habitat destruction, water pollution, and energy consumption.
In wastewater treatment applications, H2S-based precipitation methods demonstrate lower energy requirements compared to conventional treatment technologies, potentially reducing the carbon footprint of industrial water purification by 15-20%. However, these benefits must be weighed against the inherent toxicity risks of H2S, which necessitates comprehensive containment systems and safety protocols that themselves carry embedded environmental costs.
Life cycle assessment (LCA) studies indicate that H2S production from industrial byproducts represents a more sustainable pathway than dedicated manufacturing processes. When sourced as a byproduct from petroleum refining or natural gas processing, the environmental burden is significantly reduced, with approximately 40% lower greenhouse gas emissions compared to purpose-manufactured hydrogen sulfide.
The circular economy potential of H2S applications deserves particular attention. In metal recovery systems, the sulfide precipitation process can be designed to regenerate and recycle the reagents, creating closed-loop systems that minimize waste generation. Several pilot projects have demonstrated recovery rates exceeding 95% for both target metals and process chemicals.
Water footprint analysis reveals that H2S-based technologies typically consume 30-50% less water than conventional alternatives for similar applications, particularly in mining remediation contexts. This water conservation benefit becomes increasingly valuable as water scarcity intensifies globally due to climate change impacts.
Biodiversity considerations must also factor into sustainability assessments. While direct H2S applications pose minimal biodiversity threats when properly contained, the indirect benefits through reduced mining pressure and improved water quality can positively impact ecosystem health in affected watersheds. Monitoring programs at remediation sites have documented improved aquatic biodiversity metrics following implementation of H2S-based treatment systems.
Future sustainability improvements will likely focus on renewable energy integration for H2S production processes, development of bio-based H2S generation pathways, and enhanced safety systems to minimize accidental release risks. These advancements could further strengthen the environmental case for hydrosulfuric acid applications in clean technology solutions.
In wastewater treatment applications, H2S-based precipitation methods demonstrate lower energy requirements compared to conventional treatment technologies, potentially reducing the carbon footprint of industrial water purification by 15-20%. However, these benefits must be weighed against the inherent toxicity risks of H2S, which necessitates comprehensive containment systems and safety protocols that themselves carry embedded environmental costs.
Life cycle assessment (LCA) studies indicate that H2S production from industrial byproducts represents a more sustainable pathway than dedicated manufacturing processes. When sourced as a byproduct from petroleum refining or natural gas processing, the environmental burden is significantly reduced, with approximately 40% lower greenhouse gas emissions compared to purpose-manufactured hydrogen sulfide.
The circular economy potential of H2S applications deserves particular attention. In metal recovery systems, the sulfide precipitation process can be designed to regenerate and recycle the reagents, creating closed-loop systems that minimize waste generation. Several pilot projects have demonstrated recovery rates exceeding 95% for both target metals and process chemicals.
Water footprint analysis reveals that H2S-based technologies typically consume 30-50% less water than conventional alternatives for similar applications, particularly in mining remediation contexts. This water conservation benefit becomes increasingly valuable as water scarcity intensifies globally due to climate change impacts.
Biodiversity considerations must also factor into sustainability assessments. While direct H2S applications pose minimal biodiversity threats when properly contained, the indirect benefits through reduced mining pressure and improved water quality can positively impact ecosystem health in affected watersheds. Monitoring programs at remediation sites have documented improved aquatic biodiversity metrics following implementation of H2S-based treatment systems.
Future sustainability improvements will likely focus on renewable energy integration for H2S production processes, development of bio-based H2S generation pathways, and enhanced safety systems to minimize accidental release risks. These advancements could further strengthen the environmental case for hydrosulfuric acid applications in clean technology solutions.
Regulatory Compliance and Safety Protocols
The implementation of hydrosulfuric acid in clean technology solutions necessitates strict adherence to comprehensive regulatory frameworks and safety protocols. At the international level, organizations such as the Environmental Protection Agency (EPA), Occupational Safety and Health Administration (OSHA), and the European Chemicals Agency (ECHA) have established stringent guidelines governing the handling, storage, and disposal of hydrosulfuric acid due to its highly toxic and corrosive properties.
Regulatory compliance begins with proper classification under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which mandates specific hazard communication elements including warning labels, safety data sheets, and employee training programs. Companies must obtain appropriate permits and licenses before incorporating hydrosulfuric acid into their clean technology applications, with requirements varying significantly across jurisdictions.
Emission standards represent another critical regulatory consideration, with most developed nations imposing strict limits on hydrogen sulfide releases. The Clean Air Act in the United States, for instance, classifies H₂S as a hazardous air pollutant subject to National Emission Standards for Hazardous Air Pollutants (NESHAP) regulations, requiring continuous monitoring systems and regular compliance reporting.
From a safety perspective, facilities utilizing hydrosulfuric acid must implement robust engineering controls including closed systems, local exhaust ventilation, and scrubbers to minimize exposure risks. Personal protective equipment requirements typically include chemical-resistant clothing, gloves, face shields, and self-contained breathing apparatus for certain operations. Continuous air monitoring systems with alarm capabilities are essential for early detection of potential leaks.
Emergency response protocols constitute another fundamental safety element, encompassing detailed procedures for spill containment, neutralization techniques, evacuation plans, and medical intervention strategies. Regular drills and simulations help ensure personnel readiness for potential incidents involving hydrosulfuric acid.
The transportation of hydrosulfuric acid falls under hazardous materials regulations such as the U.S. Department of Transportation's Hazardous Materials Regulations and the International Maritime Dangerous Goods Code, requiring specialized containers, vehicle placarding, and trained personnel. Documentation requirements include shipping papers, emergency response information, and appropriate hazard communication.
Waste management presents additional regulatory challenges, with hydrosulfuric acid classified as hazardous waste in most jurisdictions. Companies must implement proper neutralization procedures before disposal and maintain detailed waste tracking records to demonstrate compliance with regulations such as the Resource Conservation and Recovery Act in the United States.
Regulatory compliance begins with proper classification under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), which mandates specific hazard communication elements including warning labels, safety data sheets, and employee training programs. Companies must obtain appropriate permits and licenses before incorporating hydrosulfuric acid into their clean technology applications, with requirements varying significantly across jurisdictions.
Emission standards represent another critical regulatory consideration, with most developed nations imposing strict limits on hydrogen sulfide releases. The Clean Air Act in the United States, for instance, classifies H₂S as a hazardous air pollutant subject to National Emission Standards for Hazardous Air Pollutants (NESHAP) regulations, requiring continuous monitoring systems and regular compliance reporting.
From a safety perspective, facilities utilizing hydrosulfuric acid must implement robust engineering controls including closed systems, local exhaust ventilation, and scrubbers to minimize exposure risks. Personal protective equipment requirements typically include chemical-resistant clothing, gloves, face shields, and self-contained breathing apparatus for certain operations. Continuous air monitoring systems with alarm capabilities are essential for early detection of potential leaks.
Emergency response protocols constitute another fundamental safety element, encompassing detailed procedures for spill containment, neutralization techniques, evacuation plans, and medical intervention strategies. Regular drills and simulations help ensure personnel readiness for potential incidents involving hydrosulfuric acid.
The transportation of hydrosulfuric acid falls under hazardous materials regulations such as the U.S. Department of Transportation's Hazardous Materials Regulations and the International Maritime Dangerous Goods Code, requiring specialized containers, vehicle placarding, and trained personnel. Documentation requirements include shipping papers, emergency response information, and appropriate hazard communication.
Waste management presents additional regulatory challenges, with hydrosulfuric acid classified as hazardous waste in most jurisdictions. Companies must implement proper neutralization procedures before disposal and maintain detailed waste tracking records to demonstrate compliance with regulations such as the Resource Conservation and Recovery Act in the United States.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!