Design Parameters for Hydrosulfuric Acid Absorption Towers
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Absorption Technology Background and Objectives
Hydrogen sulfide (H2S) absorption technology has evolved significantly over the past century, with its origins dating back to the early 1900s when the first industrial gas treatment processes were developed. Initially, simple water scrubbing was employed, but this proved inefficient for high-concentration H2S removal. The 1930s saw the introduction of chemical absorption using alkaline solutions, marking a significant advancement in the field. By the 1950s, amine-based absorption systems became prevalent, offering improved selectivity and regeneration capabilities.
The evolution of H2S absorption technology has been primarily driven by increasingly stringent environmental regulations and the growing need for cleaner energy sources. As natural gas and petroleum processing expanded globally, the demand for effective H2S removal systems intensified, leading to continuous technological improvements. The 1970s environmental movement catalyzed further advancements, with regulatory frameworks like the Clean Air Act in the United States establishing strict limits on sulfur emissions.
Current technological trends in H2S absorption focus on enhancing efficiency while reducing operational costs and environmental impact. Advanced solvent formulations with higher absorption capacities and lower regeneration energy requirements represent a significant area of development. Simultaneously, hybrid systems combining chemical and physical absorption mechanisms are gaining traction for their versatility across varying H2S concentrations and operating conditions.
The integration of process intensification techniques, such as structured packing and novel contactor designs, has substantially improved mass transfer efficiency in absorption towers. These innovations have enabled smaller equipment footprints and reduced capital expenditures. Additionally, the incorporation of digital technologies for real-time monitoring and automated control has optimized operational parameters, resulting in more stable and efficient absorption processes.
The primary technical objectives for modern H2S absorption towers include maximizing removal efficiency while minimizing energy consumption, reducing solvent degradation and makeup requirements, and ensuring operational flexibility to handle fluctuating feed compositions. Design parameters must balance these sometimes competing objectives while maintaining compliance with increasingly stringent emission standards across global markets.
Future development trajectories point toward bio-based absorption media, advanced materials for tower internals resistant to corrosion, and integration with carbon capture technologies to address multiple environmental concerns simultaneously. The ultimate goal remains developing absorption systems that achieve near-complete H2S removal with minimal environmental footprint and operational complexity, supporting the transition toward cleaner industrial processes and energy systems.
The evolution of H2S absorption technology has been primarily driven by increasingly stringent environmental regulations and the growing need for cleaner energy sources. As natural gas and petroleum processing expanded globally, the demand for effective H2S removal systems intensified, leading to continuous technological improvements. The 1970s environmental movement catalyzed further advancements, with regulatory frameworks like the Clean Air Act in the United States establishing strict limits on sulfur emissions.
Current technological trends in H2S absorption focus on enhancing efficiency while reducing operational costs and environmental impact. Advanced solvent formulations with higher absorption capacities and lower regeneration energy requirements represent a significant area of development. Simultaneously, hybrid systems combining chemical and physical absorption mechanisms are gaining traction for their versatility across varying H2S concentrations and operating conditions.
The integration of process intensification techniques, such as structured packing and novel contactor designs, has substantially improved mass transfer efficiency in absorption towers. These innovations have enabled smaller equipment footprints and reduced capital expenditures. Additionally, the incorporation of digital technologies for real-time monitoring and automated control has optimized operational parameters, resulting in more stable and efficient absorption processes.
The primary technical objectives for modern H2S absorption towers include maximizing removal efficiency while minimizing energy consumption, reducing solvent degradation and makeup requirements, and ensuring operational flexibility to handle fluctuating feed compositions. Design parameters must balance these sometimes competing objectives while maintaining compliance with increasingly stringent emission standards across global markets.
Future development trajectories point toward bio-based absorption media, advanced materials for tower internals resistant to corrosion, and integration with carbon capture technologies to address multiple environmental concerns simultaneously. The ultimate goal remains developing absorption systems that achieve near-complete H2S removal with minimal environmental footprint and operational complexity, supporting the transition toward cleaner industrial processes and energy systems.
Market Analysis for H2S Removal Systems
The global market for hydrogen sulfide (H2S) removal systems has been experiencing steady growth, primarily driven by stringent environmental regulations and increasing industrial activities in oil and gas, wastewater treatment, and chemical processing sectors. The market size for H2S removal technologies was valued at approximately 1.8 billion USD in 2022, with projections indicating a compound annual growth rate (CAGR) of 5.7% through 2030.
The oil and gas industry remains the dominant end-user segment, accounting for over 45% of the total market share. This is attributed to the high concentration of H2S in natural gas streams and petroleum refining processes, necessitating efficient removal systems to meet product specifications and environmental standards. The natural gas processing sector, in particular, has shown increased demand for advanced H2S absorption technologies due to the exploitation of sour gas reserves globally.
Regionally, North America holds the largest market share at approximately 32%, followed by Asia-Pacific at 28% and Europe at 24%. The Middle East and Africa region, though currently representing a smaller portion at 12%, is expected to witness the fastest growth rate of 7.2% annually due to expanding oil and gas activities and increasing environmental awareness.
The market is segmented by technology type into amine-based systems, iron sponge processes, caustic washing, and advanced oxidation processes. Amine-based absorption towers continue to dominate with approximately 38% market share due to their high efficiency and established operational protocols. However, newer technologies utilizing specialized catalysts and hybrid systems are gaining traction, particularly in applications requiring lower energy consumption and reduced operational costs.
Customer demand is increasingly focused on absorption towers that offer higher removal efficiency, lower energy consumption, reduced chemical usage, and minimal environmental impact. End-users are willing to pay premium prices for systems that demonstrate lower total cost of ownership over the equipment lifecycle, despite higher initial capital expenditure.
Market challenges include the high capital and operational costs associated with advanced H2S removal systems, technical complexities in handling varying H2S concentrations, and the need for specialized expertise in system design and operation. Additionally, the market faces pressure from emerging alternative technologies such as membrane separation and biological treatment methods, which are gaining attention for specific applications.
The competitive landscape features established players like Schlumberger, Honeywell UOP, and Siemens, alongside specialized technology providers such as Pall Corporation and Veolia Water Technologies. Recent market trends indicate increasing consolidation through mergers and acquisitions, as companies seek to expand their technological portfolios and geographical presence.
The oil and gas industry remains the dominant end-user segment, accounting for over 45% of the total market share. This is attributed to the high concentration of H2S in natural gas streams and petroleum refining processes, necessitating efficient removal systems to meet product specifications and environmental standards. The natural gas processing sector, in particular, has shown increased demand for advanced H2S absorption technologies due to the exploitation of sour gas reserves globally.
Regionally, North America holds the largest market share at approximately 32%, followed by Asia-Pacific at 28% and Europe at 24%. The Middle East and Africa region, though currently representing a smaller portion at 12%, is expected to witness the fastest growth rate of 7.2% annually due to expanding oil and gas activities and increasing environmental awareness.
The market is segmented by technology type into amine-based systems, iron sponge processes, caustic washing, and advanced oxidation processes. Amine-based absorption towers continue to dominate with approximately 38% market share due to their high efficiency and established operational protocols. However, newer technologies utilizing specialized catalysts and hybrid systems are gaining traction, particularly in applications requiring lower energy consumption and reduced operational costs.
Customer demand is increasingly focused on absorption towers that offer higher removal efficiency, lower energy consumption, reduced chemical usage, and minimal environmental impact. End-users are willing to pay premium prices for systems that demonstrate lower total cost of ownership over the equipment lifecycle, despite higher initial capital expenditure.
Market challenges include the high capital and operational costs associated with advanced H2S removal systems, technical complexities in handling varying H2S concentrations, and the need for specialized expertise in system design and operation. Additionally, the market faces pressure from emerging alternative technologies such as membrane separation and biological treatment methods, which are gaining attention for specific applications.
The competitive landscape features established players like Schlumberger, Honeywell UOP, and Siemens, alongside specialized technology providers such as Pall Corporation and Veolia Water Technologies. Recent market trends indicate increasing consolidation through mergers and acquisitions, as companies seek to expand their technological portfolios and geographical presence.
Current Challenges in Hydrosulfuric Acid Absorption
Despite significant advancements in hydrosulfuric acid absorption technology, several critical challenges continue to impede optimal tower design and operation. Material degradation remains a primary concern, as H2S creates highly corrosive environments that aggressively attack conventional construction materials. Even specialized alloys and coatings experience accelerated deterioration, leading to increased maintenance frequency and operational costs. This corrosion challenge is particularly pronounced at higher concentrations and temperatures, creating a complex design constraint that limits operational parameters.
Efficiency limitations present another significant hurdle, with current absorption systems typically achieving only 85-95% removal efficiency. This performance gap becomes particularly problematic when regulatory requirements demand higher purification levels, often necessitating multi-stage treatment systems that increase both capital and operational expenditures. The efficiency challenge is compounded by the difficulty in maintaining optimal gas-liquid contact across varying operational conditions.
Process control complexity represents a substantial operational challenge. The absorption process is highly sensitive to temperature fluctuations, pressure variations, and flow rate inconsistencies. Minor deviations from optimal parameters can trigger significant performance degradation or safety concerns. Current monitoring systems often struggle to provide the real-time precision required for truly adaptive control, particularly in facilities processing variable feedstock compositions.
Energy consumption remains disproportionately high in conventional absorption tower designs. The pumping requirements for circulation of absorption solutions, coupled with heating needs for regeneration processes, create substantial energy footprints. This challenge is exacerbated by the inherent trade-off between absorption efficiency and energy utilization, with more thorough removal typically demanding exponentially higher energy inputs.
Scale formation and fouling constitute persistent operational challenges that reduce heat transfer efficiency and increase pressure drops across absorption systems. The chemical interactions between H2S, absorption media, and trace contaminants frequently produce solid precipitates that accumulate on internal surfaces. Current prevention strategies rely heavily on chemical additives that introduce additional operational complexities and environmental considerations.
Safety concerns remain paramount in H2S handling, with its high toxicity demanding robust containment systems and comprehensive monitoring networks. The risk of breakthrough events or system failures necessitates redundant safety systems that add complexity and cost to tower designs. These safety requirements often conflict with design objectives for efficiency and compactness, creating challenging engineering trade-offs.
Efficiency limitations present another significant hurdle, with current absorption systems typically achieving only 85-95% removal efficiency. This performance gap becomes particularly problematic when regulatory requirements demand higher purification levels, often necessitating multi-stage treatment systems that increase both capital and operational expenditures. The efficiency challenge is compounded by the difficulty in maintaining optimal gas-liquid contact across varying operational conditions.
Process control complexity represents a substantial operational challenge. The absorption process is highly sensitive to temperature fluctuations, pressure variations, and flow rate inconsistencies. Minor deviations from optimal parameters can trigger significant performance degradation or safety concerns. Current monitoring systems often struggle to provide the real-time precision required for truly adaptive control, particularly in facilities processing variable feedstock compositions.
Energy consumption remains disproportionately high in conventional absorption tower designs. The pumping requirements for circulation of absorption solutions, coupled with heating needs for regeneration processes, create substantial energy footprints. This challenge is exacerbated by the inherent trade-off between absorption efficiency and energy utilization, with more thorough removal typically demanding exponentially higher energy inputs.
Scale formation and fouling constitute persistent operational challenges that reduce heat transfer efficiency and increase pressure drops across absorption systems. The chemical interactions between H2S, absorption media, and trace contaminants frequently produce solid precipitates that accumulate on internal surfaces. Current prevention strategies rely heavily on chemical additives that introduce additional operational complexities and environmental considerations.
Safety concerns remain paramount in H2S handling, with its high toxicity demanding robust containment systems and comprehensive monitoring networks. The risk of breakthrough events or system failures necessitates redundant safety systems that add complexity and cost to tower designs. These safety requirements often conflict with design objectives for efficiency and compactness, creating challenging engineering trade-offs.
State-of-the-Art Absorption Tower Solutions
01 Tower Structure and Configuration
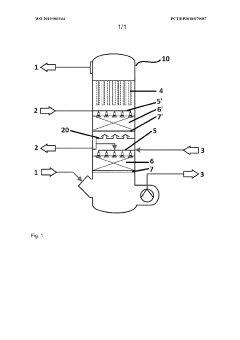
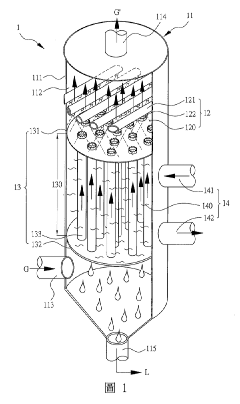
The design of hydrosulfuric acid absorption towers focuses on structural elements that optimize gas-liquid contact and enhance absorption efficiency. Key structural parameters include tower height-to-diameter ratio, internal configuration, and material selection for corrosion resistance. Multi-stage absorption systems with specific sectional designs improve the overall absorption capacity while maintaining operational stability under varying process conditions.- Tower Structure and Material Selection: The design of hydrosulfuric acid absorption towers requires careful consideration of structural elements and materials to withstand corrosive environments. Key parameters include the selection of corrosion-resistant materials such as specialized alloys or coated steel, tower height-to-diameter ratios for optimal gas-liquid contact, and structural reinforcements to ensure mechanical stability under operating conditions. The internal structure typically includes support grids, liquid distributors, and mist eliminators to enhance absorption efficiency while maintaining structural integrity.
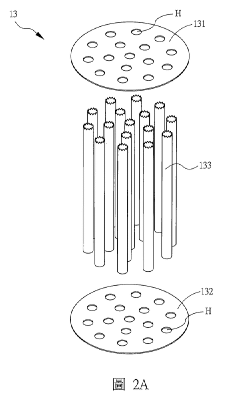
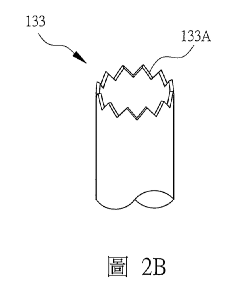
- Packing and Internals Design: The selection and arrangement of tower packing materials significantly impact absorption efficiency. Design parameters include packing type (structured vs. random), specific surface area, void fraction, and material compatibility with hydrosulfuric acid. Tower internals such as liquid distributors, redistributors, and collectors must be designed to ensure uniform liquid distribution across the packing, preventing channeling and ensuring maximum contact between the gas and liquid phases. The packing height and density are critical parameters that determine the overall mass transfer efficiency.
- Operating Parameters and Process Control: Key operating parameters for hydrosulfuric acid absorption towers include gas and liquid flow rates, temperature profiles, pressure drop across the tower, and concentration gradients. The liquid-to-gas ratio must be optimized to achieve the desired absorption efficiency while minimizing energy consumption. Process control systems monitor and adjust these parameters in real-time, maintaining optimal absorption conditions despite variations in inlet gas composition or flow rate. Temperature control is particularly important as absorption reactions are typically exothermic.
- Absorption Solution Chemistry and Regeneration: The selection of absorption solution chemistry is critical for efficient hydrosulfuric acid removal. Design parameters include solution concentration, pH control, chemical additives for enhanced absorption, and solution stability under operating conditions. Regeneration systems must be integrated into the overall design to allow for continuous operation, with parameters such as regeneration temperature, pressure, and residence time carefully optimized. The balance between absorption capacity and regeneration energy requirements is a key design consideration.
- Safety and Environmental Considerations: Hydrosulfuric acid absorption tower design must incorporate robust safety and environmental protection features. Parameters include emergency relief systems, containment measures for potential leaks, monitoring systems for toxic gas detection, and materials selection for long-term reliability. Environmental considerations include treatment of waste streams, minimization of secondary pollutants, and energy efficiency measures. Design parameters must account for regulatory compliance requirements and incorporate sufficient margins of safety for all operating scenarios.
02 Packing Materials and Internal Components
Selection of appropriate packing materials significantly impacts the absorption efficiency of hydrosulfuric acid towers. Advanced packing designs provide increased surface area for gas-liquid contact while minimizing pressure drop. Internal components such as liquid distributors, gas distributors, and support grids must be precisely engineered to ensure uniform flow distribution throughout the tower. Ceramic, plastic, and specialized metal alloy packings offer different advantages depending on specific process requirements.Expand Specific Solutions03 Operating Parameters and Process Control
Critical operating parameters for hydrosulfuric acid absorption towers include liquid-to-gas ratio, temperature profiles, pressure gradients, and residence time. Maintaining optimal pH levels in the absorption solution enhances removal efficiency. Advanced control systems monitor and adjust these parameters in real-time to accommodate variations in inlet gas composition and flow rates, ensuring consistent performance while preventing operational issues such as flooding or channeling.Expand Specific Solutions04 Absorption Solution Chemistry
The chemical composition of absorption solutions plays a crucial role in hydrosulfuric acid removal efficiency. Alkaline solutions containing specific additives enhance absorption capacity and reaction kinetics. Oxidizing agents can be incorporated to convert absorbed hydrogen sulfide into elemental sulfur or sulfates. Solution regeneration systems extend the useful life of absorption media while reducing operational costs and waste generation.Expand Specific Solutions05 Energy Efficiency and Environmental Considerations
Modern hydrosulfuric acid absorption tower designs incorporate energy recovery systems to minimize operational costs. Heat exchangers capture and reuse thermal energy from exothermic absorption reactions. Advanced designs focus on reducing environmental impact through minimizing secondary pollutants and optimizing resource utilization. Integrated monitoring systems ensure compliance with emission standards while providing data for continuous process improvement.Expand Specific Solutions
Leading Manufacturers and Technology Providers
The hydrosulfuric acid absorption tower technology market is in a growth phase, with increasing demand driven by stringent environmental regulations across petrochemical, energy, and manufacturing sectors. The global market size for sulfur recovery technologies is expanding at approximately 6-8% annually, reaching multi-billion dollar valuations. From a technical maturity perspective, the field shows varied development levels. Industry leaders like China Petroleum & Chemical Corp. (Sinopec) and Air Products & Chemicals have established mature absorption tower designs, while companies such as Haldor Topsøe, ThyssenKrupp, and Jinchuan Group are advancing specialized applications with enhanced efficiency parameters. Korean players (KEPCO, KIER) and Japanese firms (Mitsubishi Heavy Industries, IBIDEN) are focusing on integration with clean energy systems, indicating the technology's evolution toward sustainability applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced hydrosulfuric acid absorption tower designs featuring multi-stage absorption systems with optimized packing materials. Their technology employs a dual-flow countercurrent absorption process with specialized alkaline solutions that achieve H2S removal efficiencies exceeding 99.5%. The absorption towers incorporate proprietary structured packing that maximizes gas-liquid contact area while minimizing pressure drop. Sinopec's design includes temperature-controlled absorption zones that maintain optimal operating conditions between 35-45°C, enhancing absorption rates while preventing secondary reactions. Their towers feature integrated mist eliminators that reduce solution carryover by up to 98% compared to conventional designs. The company has also pioneered corrosion-resistant materials selection, utilizing specialized alloys and polymer linings that extend equipment service life by 40-60% in highly acidic environments.
Strengths: Superior removal efficiency with proprietary packing materials; extensive operational experience in various petrochemical applications; advanced corrosion management systems. Weaknesses: Higher initial capital investment compared to conventional designs; requires specialized maintenance expertise; performance may degrade in applications with highly variable feed compositions.
Air Products & Chemicals, Inc.
Technical Solution: Air Products & Chemicals has developed sophisticated hydrosulfuric acid absorption tower technology utilizing advanced mass transfer principles. Their design incorporates proprietary high-efficiency structured packing that achieves 30-40% greater absorption capacity per unit volume compared to conventional random packing. The company's absorption towers feature precision liquid distribution systems that maintain uniform wetting across the entire cross-section, preventing channeling and ensuring maximum utilization of packing surface area. Air Products employs computational fluid dynamics (CFD) modeling to optimize gas flow patterns, reducing pressure drop by approximately 25% while maintaining high mass transfer coefficients. Their towers incorporate intelligent control systems that continuously adjust operating parameters based on real-time monitoring of inlet gas composition, flow rates, and temperature profiles. The company has also developed specialized materials of construction that resist corrosion in high-concentration H2S environments, including proprietary fluoropolymer linings and high-molybdenum stainless steel alloys that extend equipment life by up to 15 years.
Strengths: Industry-leading mass transfer efficiency; sophisticated modeling capabilities for custom designs; extensive experience across diverse industrial applications. Weaknesses: Premium pricing structure compared to standard absorption technologies; complex control systems require specialized training for operators; higher maintenance requirements for advanced components.
Critical Design Parameters and Engineering Principles
So 3 absorption tower
PatentWO2019081544A1
Innovation
- A single absorption tower is divided into two stages with a collecting tray in the middle, allowing for independent operation of cold and hot sulfuric acid streams, with temperature ranges of 60-90°C and 180-220°C respectively, enabling controlled acid flow and enhanced heat recovery without impairing medium-pressure steam production.
Sedimentation type film hydrogen sulfide absorbing tower for pyrolytic gas of waste tire configured for hydrogen sulfide treatment
PatentActiveTW201941815A
Innovation
- A sedimentation film hydrogen sulfide absorption tower is employed, utilizing a chemical reaction between an absorbent and hydrogen sulfide to remove it from waste tire pyrolysis oil, featuring a tower body, spraying device, sedimentation thin film device, and condensing device to enhance contact area and efficiency.
Environmental Compliance and Emission Standards
The regulatory landscape governing hydrosulfuric acid absorption towers has become increasingly stringent worldwide, with environmental protection agencies establishing comprehensive frameworks to control hydrogen sulfide emissions. In the United States, the Environmental Protection Agency (EPA) has set National Ambient Air Quality Standards (NAAQS) that limit H2S concentrations to 0.03 ppm averaged over a 1-hour period. The European Union, through its Industrial Emissions Directive (IED), mandates even stricter limits at 0.005 ppm for facilities near residential areas. These standards directly influence the design parameters of absorption towers, requiring minimum removal efficiencies of 99.9% in many jurisdictions.
Compliance monitoring requirements have evolved significantly, with continuous emission monitoring systems (CEMS) now mandatory for large-scale operations. These systems must demonstrate 95% uptime and undergo quarterly calibration to ensure accurate reporting. The data collected must be retained for a minimum of five years and be available for regulatory inspection upon request. Non-compliance penalties have increased substantially, with fines reaching up to $37,500 per violation per day in the United States and similar punitive measures in other developed nations.
Best Available Techniques (BAT) standards, particularly prominent in the EU regulatory framework, specify that absorption towers must incorporate multi-stage scrubbing processes with optimized liquid-to-gas ratios between 12:1 and 15:1. Additionally, modern designs must include mist eliminators capable of removing 99% of liquid droplets larger than 5 microns to prevent secondary pollution. These technical specifications are regularly updated, with the most recent revisions emphasizing energy efficiency alongside emission control.
Industry-specific regulations add another layer of complexity, with petroleum refineries facing Refinery Sector Rules that mandate maximum H2S emissions of 162 ppmv from sulfur recovery units. Similarly, natural gas processing facilities must comply with New Source Performance Standards (NSPS) that limit emissions to 230 mg/dscm. These sector-specific requirements often necessitate customized absorption tower designs with specialized packing materials and enhanced monitoring capabilities.
Emerging regulatory trends indicate a move toward lifecycle environmental impact assessment, with carbon footprint considerations becoming increasingly important in permitting processes. Several jurisdictions now require environmental impact statements that address not only direct emissions but also the energy consumption and chemical usage associated with absorption tower operations. This holistic approach is driving innovation in tower design, with emphasis on reducing chemical consumption and minimizing waste generation while maintaining high removal efficiencies.
Compliance monitoring requirements have evolved significantly, with continuous emission monitoring systems (CEMS) now mandatory for large-scale operations. These systems must demonstrate 95% uptime and undergo quarterly calibration to ensure accurate reporting. The data collected must be retained for a minimum of five years and be available for regulatory inspection upon request. Non-compliance penalties have increased substantially, with fines reaching up to $37,500 per violation per day in the United States and similar punitive measures in other developed nations.
Best Available Techniques (BAT) standards, particularly prominent in the EU regulatory framework, specify that absorption towers must incorporate multi-stage scrubbing processes with optimized liquid-to-gas ratios between 12:1 and 15:1. Additionally, modern designs must include mist eliminators capable of removing 99% of liquid droplets larger than 5 microns to prevent secondary pollution. These technical specifications are regularly updated, with the most recent revisions emphasizing energy efficiency alongside emission control.
Industry-specific regulations add another layer of complexity, with petroleum refineries facing Refinery Sector Rules that mandate maximum H2S emissions of 162 ppmv from sulfur recovery units. Similarly, natural gas processing facilities must comply with New Source Performance Standards (NSPS) that limit emissions to 230 mg/dscm. These sector-specific requirements often necessitate customized absorption tower designs with specialized packing materials and enhanced monitoring capabilities.
Emerging regulatory trends indicate a move toward lifecycle environmental impact assessment, with carbon footprint considerations becoming increasingly important in permitting processes. Several jurisdictions now require environmental impact statements that address not only direct emissions but also the energy consumption and chemical usage associated with absorption tower operations. This holistic approach is driving innovation in tower design, with emphasis on reducing chemical consumption and minimizing waste generation while maintaining high removal efficiencies.
Material Selection and Corrosion Management
Material selection for hydrosulfuric acid absorption towers represents a critical engineering challenge due to the highly corrosive nature of H2S and its aqueous solutions. Carbon steel, while economical, exhibits unacceptable corrosion rates in such environments, particularly at higher concentrations and temperatures. Stainless steel alloys, specifically 316L and 317L grades, offer significantly improved resistance but may still experience localized corrosion in high-chloride environments. For severe service conditions, higher-performance materials such as duplex stainless steels (2205, 2507) and nickel-based alloys (Hastelloy C-276, Inconel 625) demonstrate superior resistance to both general and localized corrosion.
Fiber-reinforced plastics (FRP), particularly those utilizing vinyl ester resins, present a cost-effective alternative for less demanding applications, offering excellent chemical resistance at lower temperatures. These non-metallic options eliminate electrochemical corrosion mechanisms entirely, though they impose operational temperature limitations typically below 120°C.
Corrosion management strategies must extend beyond material selection to include comprehensive monitoring systems. Online corrosion monitoring using electrical resistance probes, linear polarization resistance techniques, and ultrasonic thickness measurements enables real-time assessment of material degradation rates. These systems should be strategically positioned at critical points within the absorption tower, particularly in areas of high turbulence, temperature gradients, or phase transitions where corrosion rates typically accelerate.
Protective coatings and linings serve as additional defense mechanisms, with fluoropolymer linings (PTFE, PVDF) and specialized epoxy systems demonstrating excellent resistance to H2S environments. However, their application requires meticulous surface preparation and quality control to prevent premature failure through delamination or permeation.
Cathodic protection systems, while common in many chemical processing applications, require careful implementation in H2S service due to potential hydrogen embrittlement concerns in susceptible materials. Impressed current systems must be designed with appropriate current density limitations to prevent hydrogen evolution at the protected surface.
Regular inspection protocols utilizing advanced non-destructive testing methods such as phased array ultrasonics and guided wave testing should be established to detect early signs of material degradation before operational integrity is compromised. These inspections should be complemented by periodic material coupon testing to validate corrosion rate predictions under actual operating conditions.
The economic implications of material selection decisions must be evaluated through comprehensive life-cycle cost analysis rather than initial capital expenditure alone. Higher-grade alloys often demonstrate superior long-term value despite higher initial costs when considering maintenance requirements, operational reliability, and potential downtime costs associated with corrosion-related failures.
Fiber-reinforced plastics (FRP), particularly those utilizing vinyl ester resins, present a cost-effective alternative for less demanding applications, offering excellent chemical resistance at lower temperatures. These non-metallic options eliminate electrochemical corrosion mechanisms entirely, though they impose operational temperature limitations typically below 120°C.
Corrosion management strategies must extend beyond material selection to include comprehensive monitoring systems. Online corrosion monitoring using electrical resistance probes, linear polarization resistance techniques, and ultrasonic thickness measurements enables real-time assessment of material degradation rates. These systems should be strategically positioned at critical points within the absorption tower, particularly in areas of high turbulence, temperature gradients, or phase transitions where corrosion rates typically accelerate.
Protective coatings and linings serve as additional defense mechanisms, with fluoropolymer linings (PTFE, PVDF) and specialized epoxy systems demonstrating excellent resistance to H2S environments. However, their application requires meticulous surface preparation and quality control to prevent premature failure through delamination or permeation.
Cathodic protection systems, while common in many chemical processing applications, require careful implementation in H2S service due to potential hydrogen embrittlement concerns in susceptible materials. Impressed current systems must be designed with appropriate current density limitations to prevent hydrogen evolution at the protected surface.
Regular inspection protocols utilizing advanced non-destructive testing methods such as phased array ultrasonics and guided wave testing should be established to detect early signs of material degradation before operational integrity is compromised. These inspections should be complemented by periodic material coupon testing to validate corrosion rate predictions under actual operating conditions.
The economic implications of material selection decisions must be evaluated through comprehensive life-cycle cost analysis rather than initial capital expenditure alone. Higher-grade alloys often demonstrate superior long-term value despite higher initial costs when considering maintenance requirements, operational reliability, and potential downtime costs associated with corrosion-related failures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!