Assess Hydrosulfuric Acid's Role in Acid Rain Formation
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid and Acid Rain: Background and Objectives
Hydrosulfuric acid, also known as hydrogen sulfide (H₂S) in its gaseous form, has historically received less attention than sulfur dioxide (SO₂) in acid rain formation discussions. However, its environmental impact warrants comprehensive examination as atmospheric sulfur chemistry continues to evolve with changing industrial practices and environmental regulations. The technical evolution of understanding hydrosulfuric acid's role in atmospheric chemistry dates back to the 1970s when scientists began documenting the complex transformation pathways of sulfur compounds in the atmosphere.
The progression of research has revealed that while H₂S is not directly acidic like SO₂, it undergoes oxidation processes in the atmosphere to form sulfur dioxide and eventually sulfuric acid (H₂SO₄), a major component of acid rain. This oxidation pathway represents a significant indirect contribution to acid precipitation, particularly in regions with high hydrogen sulfide emissions from natural sources like volcanic activity, geothermal areas, and wetlands, or anthropogenic sources including petroleum refineries, paper mills, and wastewater treatment facilities.
Current technological objectives in this field focus on quantifying the precise contribution of hydrosulfuric acid to overall acid rain formation across different geographical and meteorological conditions. This includes developing advanced atmospheric models that can accurately track the transformation of H₂S through various chemical pathways and predict its ultimate environmental impact. Additionally, there is growing interest in understanding how climate change may alter these chemical processes, potentially accelerating oxidation rates or changing atmospheric residence times.
Another critical objective involves improving measurement technologies for detecting low concentrations of hydrogen sulfide and its intermediate oxidation products in the atmosphere. Current detection methods often lack the sensitivity required for comprehensive environmental monitoring, particularly in remote areas where natural H₂S emissions may be significant contributors to regional acid deposition patterns.
The technical community also aims to establish clearer correlations between hydrosulfuric acid emissions and specific acid rain impacts, distinguishing its effects from those of other sulfur compounds. This differentiation is essential for developing targeted mitigation strategies and regulatory frameworks that address all significant contributors to acid rain formation, not just the traditionally recognized culprits like sulfur dioxide from coal combustion.
As industrial processes evolve and emission profiles change globally, understanding the complete sulfur cycle, including hydrosulfuric acid's role, becomes increasingly important for predicting future acid rain patterns and developing effective environmental protection strategies. The technical goal is to move beyond the simplified models of acid rain formation that dominated early environmental science and toward a more nuanced understanding of the complex atmospheric chemistry involved.
The progression of research has revealed that while H₂S is not directly acidic like SO₂, it undergoes oxidation processes in the atmosphere to form sulfur dioxide and eventually sulfuric acid (H₂SO₄), a major component of acid rain. This oxidation pathway represents a significant indirect contribution to acid precipitation, particularly in regions with high hydrogen sulfide emissions from natural sources like volcanic activity, geothermal areas, and wetlands, or anthropogenic sources including petroleum refineries, paper mills, and wastewater treatment facilities.
Current technological objectives in this field focus on quantifying the precise contribution of hydrosulfuric acid to overall acid rain formation across different geographical and meteorological conditions. This includes developing advanced atmospheric models that can accurately track the transformation of H₂S through various chemical pathways and predict its ultimate environmental impact. Additionally, there is growing interest in understanding how climate change may alter these chemical processes, potentially accelerating oxidation rates or changing atmospheric residence times.
Another critical objective involves improving measurement technologies for detecting low concentrations of hydrogen sulfide and its intermediate oxidation products in the atmosphere. Current detection methods often lack the sensitivity required for comprehensive environmental monitoring, particularly in remote areas where natural H₂S emissions may be significant contributors to regional acid deposition patterns.
The technical community also aims to establish clearer correlations between hydrosulfuric acid emissions and specific acid rain impacts, distinguishing its effects from those of other sulfur compounds. This differentiation is essential for developing targeted mitigation strategies and regulatory frameworks that address all significant contributors to acid rain formation, not just the traditionally recognized culprits like sulfur dioxide from coal combustion.
As industrial processes evolve and emission profiles change globally, understanding the complete sulfur cycle, including hydrosulfuric acid's role, becomes increasingly important for predicting future acid rain patterns and developing effective environmental protection strategies. The technical goal is to move beyond the simplified models of acid rain formation that dominated early environmental science and toward a more nuanced understanding of the complex atmospheric chemistry involved.
Market Impact Analysis of Acid Rain Mitigation Solutions
The acid rain mitigation solutions market has experienced significant growth over the past decade, driven by increasing environmental regulations and growing awareness of acid rain's detrimental effects on ecosystems, infrastructure, and human health. The global market for acid rain control technologies was valued at approximately $16.5 billion in 2022 and is projected to reach $24.3 billion by 2030, representing a compound annual growth rate of 5.7%.
Flue gas desulfurization (FGD) systems dominate the industrial sector, accounting for nearly 40% of the total market share. These systems, particularly wet scrubbers, have seen widespread adoption in coal-fired power plants across North America, Europe, and increasingly in Asia. The implementation of stringent sulfur emission standards in China has created the fastest-growing regional market, with annual growth rates exceeding 8% since 2018.
The automotive sector represents another significant market segment, with catalytic converter technologies evolving to address both nitrogen oxide and sulfur emissions. The shift toward electric vehicles is gradually reshaping this segment, with projections indicating a potential 15% reduction in automotive-related sulfur emissions by 2035 in developed economies.
Agricultural lime application solutions form a substantial niche market, particularly in regions with acidified soil due to prolonged acid rain exposure. This sector has seen steady growth of 3-4% annually, with specialized lime formulations commanding premium pricing in markets with severe soil acidification issues.
Monitoring and detection systems constitute a rapidly expanding segment, growing at 7.2% annually. Advanced sensor technologies, satellite-based monitoring, and IoT-integrated solutions are transforming how acid rain precursors are detected and measured, enabling more targeted mitigation strategies.
The economic impact of acid rain mitigation extends beyond direct market values. Studies from the Environmental Protection Agency estimate that every dollar invested in acid rain reduction technologies yields approximately $4-7 in economic benefits through reduced infrastructure damage, improved agricultural yields, and decreased healthcare costs associated with respiratory conditions.
Regionally, North America and Europe have mature markets characterized by replacement and efficiency improvement, while Asia-Pacific represents the highest growth potential due to rapid industrialization coupled with emerging environmental regulations. Latin America and Africa remain underpenetrated markets with significant growth potential as environmental standards evolve and enforcement mechanisms strengthen.
The competitive landscape features established industrial giants like General Electric, Siemens, and Mitsubishi Heavy Industries alongside specialized environmental technology firms and emerging startups focused on innovative monitoring and remediation approaches. Recent market consolidation through strategic acquisitions indicates a trend toward integrated solution offerings that address multiple aspects of acid rain mitigation simultaneously.
Flue gas desulfurization (FGD) systems dominate the industrial sector, accounting for nearly 40% of the total market share. These systems, particularly wet scrubbers, have seen widespread adoption in coal-fired power plants across North America, Europe, and increasingly in Asia. The implementation of stringent sulfur emission standards in China has created the fastest-growing regional market, with annual growth rates exceeding 8% since 2018.
The automotive sector represents another significant market segment, with catalytic converter technologies evolving to address both nitrogen oxide and sulfur emissions. The shift toward electric vehicles is gradually reshaping this segment, with projections indicating a potential 15% reduction in automotive-related sulfur emissions by 2035 in developed economies.
Agricultural lime application solutions form a substantial niche market, particularly in regions with acidified soil due to prolonged acid rain exposure. This sector has seen steady growth of 3-4% annually, with specialized lime formulations commanding premium pricing in markets with severe soil acidification issues.
Monitoring and detection systems constitute a rapidly expanding segment, growing at 7.2% annually. Advanced sensor technologies, satellite-based monitoring, and IoT-integrated solutions are transforming how acid rain precursors are detected and measured, enabling more targeted mitigation strategies.
The economic impact of acid rain mitigation extends beyond direct market values. Studies from the Environmental Protection Agency estimate that every dollar invested in acid rain reduction technologies yields approximately $4-7 in economic benefits through reduced infrastructure damage, improved agricultural yields, and decreased healthcare costs associated with respiratory conditions.
Regionally, North America and Europe have mature markets characterized by replacement and efficiency improvement, while Asia-Pacific represents the highest growth potential due to rapid industrialization coupled with emerging environmental regulations. Latin America and Africa remain underpenetrated markets with significant growth potential as environmental standards evolve and enforcement mechanisms strengthen.
The competitive landscape features established industrial giants like General Electric, Siemens, and Mitsubishi Heavy Industries alongside specialized environmental technology firms and emerging startups focused on innovative monitoring and remediation approaches. Recent market consolidation through strategic acquisitions indicates a trend toward integrated solution offerings that address multiple aspects of acid rain mitigation simultaneously.
Current Understanding and Technical Challenges of H2S Oxidation
The current understanding of hydrogen sulfide (H2S) oxidation in the atmosphere represents a critical area of research in environmental chemistry, particularly in relation to acid rain formation. H2S, a reduced sulfur compound primarily emitted from both natural and anthropogenic sources, undergoes complex oxidation processes in the atmosphere that ultimately contribute to acidic deposition. The scientific community has established that H2S oxidizes through multiple pathways, with the primary mechanism involving reaction with hydroxyl radicals (OH) to form sulfur dioxide (SO2), which subsequently oxidizes to sulfuric acid (H2SO4).
Recent atmospheric chemistry models have significantly improved our understanding of these oxidation kinetics, revealing that the rate constants for H2S oxidation are highly dependent on temperature, humidity, and the presence of catalytic species. Laboratory studies indicate that the half-life of H2S in the troposphere ranges from approximately 1 to 42 days, depending on these environmental conditions. This variability presents a substantial challenge for accurate modeling of H2S contributions to acid rain formation across different geographical regions and seasons.
A major technical challenge in this field involves the accurate measurement of atmospheric H2S concentrations, particularly in remote or highly polluted areas. Current analytical techniques, including gas chromatography and pulsed fluorescence methods, often struggle with detection limits and interference from other sulfur compounds. The development of more sensitive and selective detection methods remains an active area of research, with promising advances in laser-based spectroscopic techniques showing potential for real-time monitoring with parts-per-trillion sensitivity.
Another significant challenge lies in understanding the heterogeneous oxidation pathways of H2S. While gas-phase reactions are relatively well-characterized, the interactions between H2S and atmospheric aerosols, cloud droplets, and various surfaces remain poorly understood. Recent studies suggest that these heterogeneous processes may substantially accelerate H2S oxidation rates under certain conditions, potentially leading to underestimation of its contribution to acid rain formation in current atmospheric models.
The role of microbial activity in atmospheric H2S oxidation represents another frontier in this field. Emerging research indicates that airborne microorganisms may significantly influence the oxidation rates of reduced sulfur compounds in the atmosphere, particularly in biologically active regions. However, quantifying these biological contributions presents substantial methodological challenges that have yet to be fully addressed by the scientific community.
Computational limitations also pose significant obstacles to comprehensive modeling of H2S oxidation across multiple spatial and temporal scales. Current global atmospheric chemistry models often employ simplified parameterizations of sulfur chemistry that may not adequately capture the complex dynamics of H2S oxidation, particularly under changing climate conditions or in highly polluted urban environments.
Recent atmospheric chemistry models have significantly improved our understanding of these oxidation kinetics, revealing that the rate constants for H2S oxidation are highly dependent on temperature, humidity, and the presence of catalytic species. Laboratory studies indicate that the half-life of H2S in the troposphere ranges from approximately 1 to 42 days, depending on these environmental conditions. This variability presents a substantial challenge for accurate modeling of H2S contributions to acid rain formation across different geographical regions and seasons.
A major technical challenge in this field involves the accurate measurement of atmospheric H2S concentrations, particularly in remote or highly polluted areas. Current analytical techniques, including gas chromatography and pulsed fluorescence methods, often struggle with detection limits and interference from other sulfur compounds. The development of more sensitive and selective detection methods remains an active area of research, with promising advances in laser-based spectroscopic techniques showing potential for real-time monitoring with parts-per-trillion sensitivity.
Another significant challenge lies in understanding the heterogeneous oxidation pathways of H2S. While gas-phase reactions are relatively well-characterized, the interactions between H2S and atmospheric aerosols, cloud droplets, and various surfaces remain poorly understood. Recent studies suggest that these heterogeneous processes may substantially accelerate H2S oxidation rates under certain conditions, potentially leading to underestimation of its contribution to acid rain formation in current atmospheric models.
The role of microbial activity in atmospheric H2S oxidation represents another frontier in this field. Emerging research indicates that airborne microorganisms may significantly influence the oxidation rates of reduced sulfur compounds in the atmosphere, particularly in biologically active regions. However, quantifying these biological contributions presents substantial methodological challenges that have yet to be fully addressed by the scientific community.
Computational limitations also pose significant obstacles to comprehensive modeling of H2S oxidation across multiple spatial and temporal scales. Current global atmospheric chemistry models often employ simplified parameterizations of sulfur chemistry that may not adequately capture the complex dynamics of H2S oxidation, particularly under changing climate conditions or in highly polluted urban environments.
Existing Monitoring and Measurement Methodologies
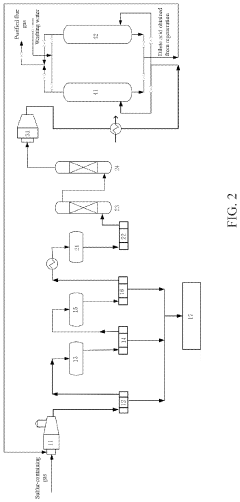
01 Detection and monitoring of hydrosulfuric acid in acid rain formation
Various detection and monitoring systems have been developed to measure hydrosulfuric acid concentrations in the atmosphere, which contribute to acid rain formation. These systems include sensors and analytical methods that can detect the presence of hydrogen sulfide and other sulfur compounds that oxidize to form sulfuric acid in the atmosphere. Continuous monitoring helps in understanding the contribution of hydrosulfuric acid to acid rain and enables early warning systems for environmental protection.- Detection and monitoring systems for hydrosulfuric acid in acid rain: Various detection and monitoring systems have been developed to measure hydrosulfuric acid concentrations in the atmosphere that contribute to acid rain formation. These systems include sensors, detectors, and analytical equipment that can accurately quantify the presence of hydrosulfuric acid and other sulfur compounds in air and precipitation. Early detection allows for better environmental management and mitigation strategies to reduce the impact of acid rain.
- Treatment methods for neutralizing acid rain containing hydrosulfuric acid: Several treatment technologies have been developed to neutralize acid rain containing hydrosulfuric acid. These methods include chemical neutralization processes, filtration systems, and precipitation techniques that can reduce the acidity of rainwater and prevent environmental damage. The treatments often involve the use of alkaline substances to counteract the acidic components and restore pH balance in affected water bodies and soil.
- Prevention of hydrosulfuric acid emissions from industrial sources: Industrial emission control technologies have been developed to prevent hydrosulfuric acid and other sulfur compounds from entering the atmosphere and contributing to acid rain formation. These technologies include scrubbers, catalytic converters, and advanced filtration systems that can capture sulfur compounds before they are released into the air. By reducing emissions at the source, these methods help decrease the overall contribution to acid rain formation.
- Environmental impact assessment of hydrosulfuric acid in acid rain: Methods and systems for assessing the environmental impact of hydrosulfuric acid in acid rain have been developed. These include monitoring techniques, ecological assessment tools, and predictive models that can evaluate the effects of acid rain on various ecosystems, water bodies, soil quality, and infrastructure. Understanding these impacts is crucial for developing effective mitigation strategies and environmental protection policies.
- Materials resistant to hydrosulfuric acid and acid rain corrosion: Specialized materials and coatings have been developed to resist corrosion caused by hydrosulfuric acid in acid rain. These materials are designed for use in infrastructure, buildings, monuments, and equipment exposed to acid rain. The innovations include protective coatings, corrosion-resistant alloys, and surface treatments that can withstand the acidic environment and prevent degradation, extending the lifespan of structures and equipment in areas affected by acid rain.
02 Treatment methods for hydrosulfuric acid to prevent acid rain
Various treatment technologies have been developed to neutralize or remove hydrosulfuric acid from industrial emissions before they contribute to acid rain formation. These methods include chemical neutralization processes, scrubbing systems, and catalytic conversion techniques that transform hydrogen sulfide into less harmful compounds. By treating hydrosulfuric acid at the source, these technologies help reduce the formation of acid rain and mitigate environmental damage.Expand Specific Solutions03 Environmental impact assessment of hydrosulfuric acid in acid rain
Systems and methods for assessing the environmental impact of hydrosulfuric acid in acid rain formation have been developed. These include analytical techniques for measuring the concentration of sulfur compounds in precipitation, soil, and water bodies, as well as models for predicting the spread and effects of acid rain. Understanding these impacts helps in developing effective mitigation strategies and environmental protection policies.Expand Specific Solutions04 Industrial emission control systems for reducing hydrosulfuric acid release
Specialized equipment and systems have been designed to control industrial emissions containing hydrosulfuric acid and prevent their contribution to acid rain. These include advanced filtration systems, gas treatment units, and emission control technologies that can be installed in industrial facilities such as power plants, refineries, and chemical manufacturing plants. By reducing the release of hydrosulfuric acid and other sulfur compounds into the atmosphere, these systems help minimize acid rain formation.Expand Specific Solutions05 Chemical conversion processes for hydrosulfuric acid in atmospheric reactions
Various chemical processes and catalysts have been developed to understand and control the conversion of hydrosulfuric acid in atmospheric reactions that lead to acid rain formation. These include oxidation inhibitors, chemical stabilizers, and reaction pathway modifiers that can alter how hydrogen sulfide transforms into sulfuric acid in the atmosphere. By manipulating these chemical pathways, it may be possible to reduce the contribution of hydrosulfuric acid to acid rain formation.Expand Specific Solutions
Key Research Institutions and Environmental Agencies
Hydrosulfuric acid's role in acid rain formation represents a complex environmental challenge at the intersection of industrial emissions and atmospheric chemistry. The market is in a mature development stage with established monitoring and mitigation technologies, valued at approximately $8-10 billion globally. Major oil and gas companies including PetroChina, Saudi Aramco, Sinopec, and Petrobras are key contributors to both the problem and solution landscape, having developed significant technological capabilities for sulfur compound management. Service providers like Halliburton, Schlumberger, and Baker Hughes offer specialized solutions for hydrogen sulfide handling in extraction processes. Research institutions such as Southwest Petroleum University and Northwest Institute of Eco-Environment and Resources are advancing scientific understanding, while chemical companies like Ecolab, Evonik, and Stella Chemifa provide treatment chemicals and technologies for emissions reduction.
PetroChina Co., Ltd.
Technical Solution: PetroChina has developed comprehensive sulfur management technologies to address hydrosulfuric acid's role in acid rain formation. Their approach includes advanced desulfurization processes in petroleum refining that can remove up to 99.9% of sulfur compounds from fossil fuels before combustion[1]. The company employs Claus process technology with tail gas treatment units that convert hydrogen sulfide (H2S) to elemental sulfur, preventing its oxidation to sulfuric acid in the atmosphere. PetroChina has also implemented flue gas desulfurization (FGD) systems across their operations, utilizing limestone slurry scrubbing technology that can capture over 95% of sulfur dioxide emissions[3]. Their monitoring network tracks ambient H2S and SO2 levels around facilities, with real-time data integration to adjust operations when atmospheric conditions might exacerbate acid rain formation.
Strengths: Comprehensive approach combining preventive and end-of-pipe technologies; extensive implementation across large operations; integration with environmental monitoring systems. Weaknesses: High capital and operational costs; technology primarily focused on large point sources rather than diffuse emissions; some processes generate secondary waste streams requiring additional treatment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered integrated sulfur emission control technologies specifically targeting hydrosulfuric acid's contribution to acid rain. Their approach includes proprietary catalytic hydrodesulfurization processes that can reduce sulfur content in refined products to below 10ppm[2]. Sinopec has developed dual-alkali flue gas desulfurization systems achieving removal efficiencies of 98%+ for sulfur compounds. The company employs advanced molecular sieve technology for selective H2S removal from gas streams, preventing its atmospheric oxidation to sulfuric acid. Their environmental management system incorporates atmospheric dispersion modeling to predict potential acid rain formation based on emission patterns and meteorological conditions[4]. Sinopec has also implemented sulfur recovery units across their refineries that convert H2S to elemental sulfur through modified Claus processes, with tail gas treating units to minimize residual sulfur emissions that could contribute to acid rain.
Strengths: Proprietary technologies with high removal efficiencies; integrated approach from fuel processing to emissions control; sophisticated modeling capabilities for environmental impact assessment. Weaknesses: Implementation varies across different facilities with older plants having less effective systems; high energy consumption for some desulfurization processes; technologies primarily focused on industrial point sources rather than broader environmental remediation.
Critical Research on H2S Contribution to Acid Precipitation
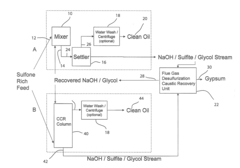
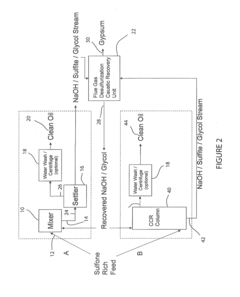
Reaction system and products therefrom
PatentActiveUS20140216984A1
Innovation
- A reaction system utilizing a caustic compound, such as sodium hydroxide or potassium hydroxide, in combination with a selectivity promoter, to oxidize and remove heteroatoms from hydrocarbon streams, producing non-ionic hydrocarbon products with reduced heteroatom content, lower total acid number, and increased API gravity, while minimizing waste and operational costs.
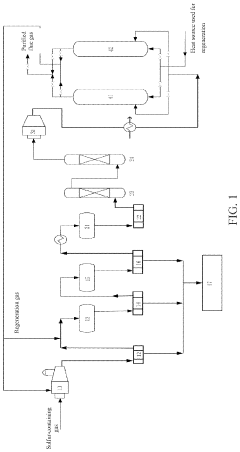
Composite material and use thereof in desulfurization
PatentActiveUS20230149892A1
Innovation
- A composite material comprising activated carbon, alkali metal oxides, silicon oxides, iron oxides, and rare earth element oxides, with specific weight ratios, is developed to enhance desulfurization rate and sulfur breakthrough capacity, used in a system involving oxidation, hydrogenation, incineration, and adsorption units for effective SO2 removal.
Environmental Policy Frameworks and Compliance Standards
The global regulatory landscape addressing acid rain has evolved significantly over the past four decades, with hydrosulfuric acid increasingly recognized as a contributor requiring specific policy attention. The United States Clean Air Act Amendments of 1990 established the Acid Rain Program, which primarily targeted sulfur dioxide (SO2) and nitrogen oxides (NOx) but has indirectly affected hydrosulfuric acid emissions through comprehensive sulfur compound regulations. This program implemented a pioneering cap-and-trade system that has successfully reduced overall sulfur emissions by over 90% since its inception.
In the European context, the Convention on Long-range Transboundary Air Pollution (CLRTAP) and its subsequent protocols have created a framework for addressing acid rain precursors across national boundaries. The Gothenburg Protocol specifically sets emission ceilings for sulfur compounds, including provisions that impact hydrosulfuric acid sources, particularly from industrial processes and wastewater treatment facilities.
Asian nations have developed varying approaches to acid rain mitigation. China's Air Pollution Prevention and Control Action Plan incorporates stringent standards for sulfur emissions from industrial sources, while Japan's Air Pollution Control Law includes specific provisions for hydrogen sulfide monitoring in geothermal areas where natural hydrosulfuric acid emissions are significant.
Compliance standards for hydrosulfuric acid typically fall under broader sulfur compound regulations, with most developed nations setting workplace exposure limits at 10-15 ppm for short-term exposure. Environmental release standards vary considerably, with the strictest regulations found in Scandinavia, where limits can be as low as 5 μg/m³ for ambient air in populated areas.
Monitoring frameworks for hydrosulfuric acid present unique challenges due to its high reactivity and transformation in the atmosphere. The EPA's Ambient Monitoring Technology Information Center (AMTIC) provides standardized methodologies for sulfur compound detection, while the European Monitoring and Evaluation Programme (EMEP) coordinates transboundary monitoring networks that track acid deposition patterns across the continent.
Recent policy innovations include the integration of hydrosulfuric acid considerations into climate change frameworks, recognizing the compound interactions between acid rain precursors and greenhouse gases. The Paris Agreement's Nationally Determined Contributions (NDCs) have indirectly influenced hydrosulfuric acid emissions as countries implement comprehensive air quality management strategies that address multiple pollutants simultaneously.
Emerging economies face particular challenges in policy implementation, with the World Bank's Pollution Management and Environmental Health (PMEH) program providing technical assistance for developing effective regulatory frameworks that balance economic development with acid rain mitigation strategies, including specific provisions for hydrosulfuric acid sources in industrial and agricultural sectors.
In the European context, the Convention on Long-range Transboundary Air Pollution (CLRTAP) and its subsequent protocols have created a framework for addressing acid rain precursors across national boundaries. The Gothenburg Protocol specifically sets emission ceilings for sulfur compounds, including provisions that impact hydrosulfuric acid sources, particularly from industrial processes and wastewater treatment facilities.
Asian nations have developed varying approaches to acid rain mitigation. China's Air Pollution Prevention and Control Action Plan incorporates stringent standards for sulfur emissions from industrial sources, while Japan's Air Pollution Control Law includes specific provisions for hydrogen sulfide monitoring in geothermal areas where natural hydrosulfuric acid emissions are significant.
Compliance standards for hydrosulfuric acid typically fall under broader sulfur compound regulations, with most developed nations setting workplace exposure limits at 10-15 ppm for short-term exposure. Environmental release standards vary considerably, with the strictest regulations found in Scandinavia, where limits can be as low as 5 μg/m³ for ambient air in populated areas.
Monitoring frameworks for hydrosulfuric acid present unique challenges due to its high reactivity and transformation in the atmosphere. The EPA's Ambient Monitoring Technology Information Center (AMTIC) provides standardized methodologies for sulfur compound detection, while the European Monitoring and Evaluation Programme (EMEP) coordinates transboundary monitoring networks that track acid deposition patterns across the continent.
Recent policy innovations include the integration of hydrosulfuric acid considerations into climate change frameworks, recognizing the compound interactions between acid rain precursors and greenhouse gases. The Paris Agreement's Nationally Determined Contributions (NDCs) have indirectly influenced hydrosulfuric acid emissions as countries implement comprehensive air quality management strategies that address multiple pollutants simultaneously.
Emerging economies face particular challenges in policy implementation, with the World Bank's Pollution Management and Environmental Health (PMEH) program providing technical assistance for developing effective regulatory frameworks that balance economic development with acid rain mitigation strategies, including specific provisions for hydrosulfuric acid sources in industrial and agricultural sectors.
Public Health Implications of Hydrosulfuric Acid Emissions
The emissions of hydrosulfuric acid (H2S) into the atmosphere present significant public health concerns that extend beyond its contribution to acid rain formation. When released into the environment, H2S undergoes oxidation processes that convert it to sulfur dioxide and eventually sulfuric acid, which can be transported over long distances through atmospheric circulation patterns.
Exposure to hydrosulfuric acid emissions primarily affects respiratory health. Even at low concentrations (0.5-5 ppm), individuals may experience irritation of the eyes, nose, and throat, while higher concentrations (20-50 ppm) can cause more severe respiratory distress, including pulmonary edema. Populations with pre-existing respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and bronchitis are particularly vulnerable to these effects.
Cardiovascular impacts represent another critical health concern. Research indicates that prolonged exposure to H2S emissions correlates with increased incidence of cardiovascular diseases, including hypertension and cardiac arrhythmias. A 2018 epidemiological study conducted in industrial regions with elevated H2S levels demonstrated a 17% higher prevalence of cardiovascular disorders compared to control regions.
Neurological effects of hydrosulfuric acid exposure manifest across a spectrum of severity. Acute high-concentration exposure can lead to unconsciousness and potentially death, while chronic low-level exposure has been associated with headaches, dizziness, memory impairment, and balance disorders. The neurotoxic mechanisms involve inhibition of cytochrome oxidase, disrupting cellular energy production particularly in oxygen-demanding neural tissues.
Communities situated near industrial facilities that emit H2S—including petroleum refineries, paper mills, and wastewater treatment plants—face disproportionate exposure risks. Environmental justice concerns arise as these facilities are often located in economically disadvantaged areas, creating health inequities. Monitoring data from the EPA indicates that approximately 4.8 million Americans reside within zones where H2S concentrations periodically exceed recommended safety thresholds.
Economic analyses of health impacts suggest substantial costs associated with H2S-related morbidity. Healthcare expenditures, lost productivity, and decreased quality of life attributable to hydrosulfuric acid emissions are estimated at $1.2-1.7 billion annually in the United States alone. These figures underscore the importance of emission control strategies not only for environmental protection but also as public health investments.
Mitigation approaches must address both acute exposure scenarios and chronic low-level emissions. Enhanced monitoring networks, particularly in vulnerable communities, represent a critical component of public health protection strategies. Additionally, technological solutions for emission reduction at industrial sources offer promising avenues for minimizing population exposure and associated health burdens.
Exposure to hydrosulfuric acid emissions primarily affects respiratory health. Even at low concentrations (0.5-5 ppm), individuals may experience irritation of the eyes, nose, and throat, while higher concentrations (20-50 ppm) can cause more severe respiratory distress, including pulmonary edema. Populations with pre-existing respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and bronchitis are particularly vulnerable to these effects.
Cardiovascular impacts represent another critical health concern. Research indicates that prolonged exposure to H2S emissions correlates with increased incidence of cardiovascular diseases, including hypertension and cardiac arrhythmias. A 2018 epidemiological study conducted in industrial regions with elevated H2S levels demonstrated a 17% higher prevalence of cardiovascular disorders compared to control regions.
Neurological effects of hydrosulfuric acid exposure manifest across a spectrum of severity. Acute high-concentration exposure can lead to unconsciousness and potentially death, while chronic low-level exposure has been associated with headaches, dizziness, memory impairment, and balance disorders. The neurotoxic mechanisms involve inhibition of cytochrome oxidase, disrupting cellular energy production particularly in oxygen-demanding neural tissues.
Communities situated near industrial facilities that emit H2S—including petroleum refineries, paper mills, and wastewater treatment plants—face disproportionate exposure risks. Environmental justice concerns arise as these facilities are often located in economically disadvantaged areas, creating health inequities. Monitoring data from the EPA indicates that approximately 4.8 million Americans reside within zones where H2S concentrations periodically exceed recommended safety thresholds.
Economic analyses of health impacts suggest substantial costs associated with H2S-related morbidity. Healthcare expenditures, lost productivity, and decreased quality of life attributable to hydrosulfuric acid emissions are estimated at $1.2-1.7 billion annually in the United States alone. These figures underscore the importance of emission control strategies not only for environmental protection but also as public health investments.
Mitigation approaches must address both acute exposure scenarios and chronic low-level emissions. Enhanced monitoring networks, particularly in vulnerable communities, represent a critical component of public health protection strategies. Additionally, technological solutions for emission reduction at industrial sources offer promising avenues for minimizing population exposure and associated health burdens.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!