Assessing Hydrosulfuric Acid Impact on Natural Water Bodies
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Contamination Background and Objectives
Hydrosulfuric acid, also known as hydrogen sulfide (H₂S) in aqueous solution, has emerged as a significant environmental contaminant affecting natural water bodies globally. The historical trajectory of this contaminant traces back to the industrial revolution when increased manufacturing activities, mining operations, and petroleum refining began releasing substantial amounts of sulfur compounds into the environment. Over the past century, the concentration of hydrosulfuric acid in water bodies has shown a concerning upward trend, particularly in regions with intensive industrial development.
The evolution of hydrosulfuric acid contamination has been closely linked to technological advancements in various industries. Initially, limited understanding of environmental impacts resulted in unregulated discharge practices. The mid-20th century saw growing awareness of water pollution issues, leading to preliminary monitoring efforts. Recent decades have witnessed significant improvements in detection technologies and environmental regulations, yet hydrosulfuric acid contamination persists as a complex challenge requiring multifaceted solutions.
Current research indicates that hydrosulfuric acid enters water systems through multiple pathways, including industrial wastewater discharge, agricultural runoff, natural geological processes, and decomposition of organic matter in oxygen-depleted environments. The compound's high water solubility facilitates its rapid dispersion throughout aquatic ecosystems, creating widespread contamination patterns that can be difficult to trace and remediate.
The technical objectives of this assessment are multifold. First, we aim to establish comprehensive baseline data on hydrosulfuric acid concentrations across diverse water bodies, including rivers, lakes, groundwater systems, and coastal environments. Second, we seek to quantify the relationship between contamination levels and specific industrial activities to identify primary contributors. Third, we intend to evaluate the effectiveness of current detection methodologies and develop improved monitoring protocols for early identification of contamination events.
Additionally, this assessment will investigate the chemical behavior of hydrosulfuric acid in various aquatic environments, particularly focusing on its transformation processes, persistence factors, and interaction with other water contaminants. Understanding these dynamics is crucial for developing effective remediation strategies and predicting long-term environmental impacts.
The ultimate goal of this technical investigation is to establish a scientific foundation for developing innovative treatment technologies, informing regulatory frameworks, and implementing preventive measures to mitigate hydrosulfuric acid contamination in natural water bodies. This work aligns with broader environmental protection initiatives and sustainable development goals focused on preserving water quality for ecological and human health.
The evolution of hydrosulfuric acid contamination has been closely linked to technological advancements in various industries. Initially, limited understanding of environmental impacts resulted in unregulated discharge practices. The mid-20th century saw growing awareness of water pollution issues, leading to preliminary monitoring efforts. Recent decades have witnessed significant improvements in detection technologies and environmental regulations, yet hydrosulfuric acid contamination persists as a complex challenge requiring multifaceted solutions.
Current research indicates that hydrosulfuric acid enters water systems through multiple pathways, including industrial wastewater discharge, agricultural runoff, natural geological processes, and decomposition of organic matter in oxygen-depleted environments. The compound's high water solubility facilitates its rapid dispersion throughout aquatic ecosystems, creating widespread contamination patterns that can be difficult to trace and remediate.
The technical objectives of this assessment are multifold. First, we aim to establish comprehensive baseline data on hydrosulfuric acid concentrations across diverse water bodies, including rivers, lakes, groundwater systems, and coastal environments. Second, we seek to quantify the relationship between contamination levels and specific industrial activities to identify primary contributors. Third, we intend to evaluate the effectiveness of current detection methodologies and develop improved monitoring protocols for early identification of contamination events.
Additionally, this assessment will investigate the chemical behavior of hydrosulfuric acid in various aquatic environments, particularly focusing on its transformation processes, persistence factors, and interaction with other water contaminants. Understanding these dynamics is crucial for developing effective remediation strategies and predicting long-term environmental impacts.
The ultimate goal of this technical investigation is to establish a scientific foundation for developing innovative treatment technologies, informing regulatory frameworks, and implementing preventive measures to mitigate hydrosulfuric acid contamination in natural water bodies. This work aligns with broader environmental protection initiatives and sustainable development goals focused on preserving water quality for ecological and human health.
Market Analysis of Water Quality Monitoring Solutions
The global water quality monitoring solutions market has experienced significant growth in recent years, driven by increasing concerns about water pollution and its environmental impacts. Currently valued at approximately 4.5 billion USD, this market is projected to reach 6.7 billion USD by 2027, growing at a CAGR of 7.3% during the forecast period. This growth trajectory is particularly relevant when considering solutions for monitoring hydrosulfuric acid contamination in natural water bodies.
The market segmentation reveals distinct categories based on monitoring approaches: continuous monitoring systems, laboratory-based analysis equipment, and portable testing kits. Continuous monitoring solutions, which enable real-time detection of hydrosulfuric acid and other contaminants, represent the fastest-growing segment with a 9.2% annual growth rate. This acceleration reflects the increasing demand for immediate data collection and analysis capabilities in environmental protection efforts.
Geographically, North America dominates the water quality monitoring market with a 35% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is experiencing the most rapid expansion due to industrialization, population growth, and strengthening environmental regulations in countries like China and India. Latin America and Africa represent emerging markets with substantial growth potential as these regions address increasing water quality challenges.
By end-user segmentation, government environmental agencies remain the largest consumer of water quality monitoring solutions, accounting for 42% of the market. Industrial sectors, particularly mining, oil and gas, and chemical manufacturing—all potential sources of hydrosulfuric acid contamination—constitute 31% of the market. Research institutions and academic facilities represent 18% of market demand, while private environmental consulting firms account for the remaining 9%.
Customer needs analysis indicates evolving requirements for more sensitive detection methods specifically for hydrosulfuric acid, which can be toxic to aquatic life even at concentrations as low as 0.5 ppm. End-users increasingly demand integrated systems that can simultaneously monitor multiple parameters beyond just hydrosulfuric acid, including pH, dissolved oxygen, and other sulfur compounds that may indicate potential contamination sources.
Price sensitivity varies significantly across market segments. While government agencies and large industrial operations typically invest in comprehensive monitoring systems ranging from 50,000 to 250,000 USD, smaller entities and developing regions seek more affordable solutions under 10,000 USD. This price disparity has created market opportunities for tiered solution offerings that address various budget constraints while maintaining essential monitoring capabilities for hydrosulfuric acid detection.
The market segmentation reveals distinct categories based on monitoring approaches: continuous monitoring systems, laboratory-based analysis equipment, and portable testing kits. Continuous monitoring solutions, which enable real-time detection of hydrosulfuric acid and other contaminants, represent the fastest-growing segment with a 9.2% annual growth rate. This acceleration reflects the increasing demand for immediate data collection and analysis capabilities in environmental protection efforts.
Geographically, North America dominates the water quality monitoring market with a 35% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is experiencing the most rapid expansion due to industrialization, population growth, and strengthening environmental regulations in countries like China and India. Latin America and Africa represent emerging markets with substantial growth potential as these regions address increasing water quality challenges.
By end-user segmentation, government environmental agencies remain the largest consumer of water quality monitoring solutions, accounting for 42% of the market. Industrial sectors, particularly mining, oil and gas, and chemical manufacturing—all potential sources of hydrosulfuric acid contamination—constitute 31% of the market. Research institutions and academic facilities represent 18% of market demand, while private environmental consulting firms account for the remaining 9%.
Customer needs analysis indicates evolving requirements for more sensitive detection methods specifically for hydrosulfuric acid, which can be toxic to aquatic life even at concentrations as low as 0.5 ppm. End-users increasingly demand integrated systems that can simultaneously monitor multiple parameters beyond just hydrosulfuric acid, including pH, dissolved oxygen, and other sulfur compounds that may indicate potential contamination sources.
Price sensitivity varies significantly across market segments. While government agencies and large industrial operations typically invest in comprehensive monitoring systems ranging from 50,000 to 250,000 USD, smaller entities and developing regions seek more affordable solutions under 10,000 USD. This price disparity has created market opportunities for tiered solution offerings that address various budget constraints while maintaining essential monitoring capabilities for hydrosulfuric acid detection.
Current Technical Challenges in H2S Detection in Aquatic Environments
The detection of hydrogen sulfide (H2S) in aquatic environments presents significant technical challenges that impede accurate assessment and monitoring efforts. Current detection methods face limitations in sensitivity, specificity, and real-time capabilities, particularly at the low concentrations relevant to ecological impact assessment. Conventional analytical techniques such as methylene blue method and iodometric titration require sample collection and laboratory processing, introducing delays and potential sample degradation due to H2S's volatile nature.
Field-deployable sensors struggle with interference from other sulfur compounds and dissolved organic matter commonly present in natural water bodies. This cross-sensitivity significantly reduces measurement accuracy in complex aquatic matrices. Additionally, existing electrochemical sensors suffer from electrode fouling when deployed in biologically active waters, requiring frequent maintenance and recalibration that limits their practical application in remote monitoring scenarios.
The spatial and temporal variability of H2S concentrations in natural waters presents another substantial challenge. H2S levels can fluctuate dramatically based on microbial activity, temperature changes, and oxygen availability. Current monitoring systems lack the capability to capture these dynamic fluctuations effectively, often missing critical concentration peaks that may have acute ecological impacts.
Miniaturization of detection systems for underwater deployment faces technical barriers related to power requirements, data transmission, and material durability in corrosive environments. While laboratory instruments can achieve detection limits in the low μg/L range, field-deployable systems typically have detection limits orders of magnitude higher, creating a significant gap between research capabilities and practical monitoring applications.
Calibration and standardization across different water matrices remain problematic. The performance of H2S detection methods varies significantly depending on salinity, pH, temperature, and organic content of the water body. This variability makes it difficult to establish universal calibration protocols and complicates data comparison across different aquatic environments.
Recent advances in optical sensing technologies, particularly those utilizing fluorescent and colorimetric indicators, show promise but still face challenges in long-term stability when exposed to natural waters. Similarly, emerging technologies like miniaturized mass spectrometry and spectroscopic methods offer improved sensitivity but remain prohibitively expensive and complex for widespread deployment.
The integration of detection systems with data analytics platforms represents another frontier challenge. Current systems generally lack the capability to automatically process and interpret measurement data in real-time, limiting their utility for early warning applications and immediate intervention in contamination events.
Field-deployable sensors struggle with interference from other sulfur compounds and dissolved organic matter commonly present in natural water bodies. This cross-sensitivity significantly reduces measurement accuracy in complex aquatic matrices. Additionally, existing electrochemical sensors suffer from electrode fouling when deployed in biologically active waters, requiring frequent maintenance and recalibration that limits their practical application in remote monitoring scenarios.
The spatial and temporal variability of H2S concentrations in natural waters presents another substantial challenge. H2S levels can fluctuate dramatically based on microbial activity, temperature changes, and oxygen availability. Current monitoring systems lack the capability to capture these dynamic fluctuations effectively, often missing critical concentration peaks that may have acute ecological impacts.
Miniaturization of detection systems for underwater deployment faces technical barriers related to power requirements, data transmission, and material durability in corrosive environments. While laboratory instruments can achieve detection limits in the low μg/L range, field-deployable systems typically have detection limits orders of magnitude higher, creating a significant gap between research capabilities and practical monitoring applications.
Calibration and standardization across different water matrices remain problematic. The performance of H2S detection methods varies significantly depending on salinity, pH, temperature, and organic content of the water body. This variability makes it difficult to establish universal calibration protocols and complicates data comparison across different aquatic environments.
Recent advances in optical sensing technologies, particularly those utilizing fluorescent and colorimetric indicators, show promise but still face challenges in long-term stability when exposed to natural waters. Similarly, emerging technologies like miniaturized mass spectrometry and spectroscopic methods offer improved sensitivity but remain prohibitively expensive and complex for widespread deployment.
The integration of detection systems with data analytics platforms represents another frontier challenge. Current systems generally lack the capability to automatically process and interpret measurement data in real-time, limiting their utility for early warning applications and immediate intervention in contamination events.
Existing Methodologies for Hydrosulfuric Acid Impact Assessment
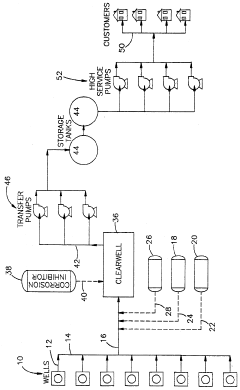
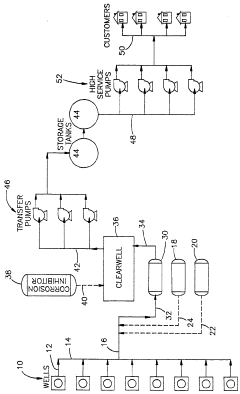
01 Corrosion prevention in industrial systems
Hydrosulfuric acid (H2S) causes significant corrosion in industrial equipment, particularly in oil and gas processing systems. Various compounds and methods have been developed to mitigate this corrosion, including specialized coatings, inhibitors, and treatment processes that can neutralize or remove H2S from industrial systems, thereby extending equipment life and preventing failures.- Corrosion prevention in industrial systems: Hydrosulfuric acid (H2S) causes significant corrosion in industrial systems, particularly in oil and gas pipelines and equipment. Various corrosion inhibitors and protective coatings have been developed to mitigate this impact. These solutions include polymer-based coatings, chemical inhibitors, and specialized alloys that resist sulfide-induced degradation, extending the operational life of industrial equipment exposed to hydrosulfuric acid environments.
- Environmental remediation technologies: Technologies for treating hydrosulfuric acid contamination in environmental settings focus on neutralization and removal processes. These include biological treatment systems using specialized bacteria, chemical oxidation methods, and absorption techniques. Advanced filtration systems and scrubbers have been developed to capture and convert hydrosulfuric acid from industrial emissions, reducing its environmental impact and protecting ecosystems from its harmful effects.
- Material modification for acid resistance: Hydrosulfuric acid resistance can be enhanced in materials through specific modification techniques. Polymer composites with sulfur-resistant additives, surface treatment methods, and specialized curing processes have been developed to improve material durability when exposed to hydrosulfuric acid. These modifications create barriers that prevent acid penetration and chemical degradation, maintaining structural integrity in harsh environments.
- Detection and monitoring systems: Accurate detection and monitoring of hydrosulfuric acid is crucial for safety in industrial and environmental settings. Sensing technologies include electrochemical sensors, optical detection systems, and real-time monitoring devices that can detect even low concentrations of hydrosulfuric acid. These systems provide early warning of dangerous levels, allowing for prompt intervention to prevent equipment damage, environmental contamination, or health hazards to workers.
- Waste treatment and neutralization processes: Specialized processes have been developed for treating waste streams containing hydrosulfuric acid. These include chemical neutralization methods using alkaline reagents, oxidation processes that convert sulfides to less harmful compounds, and precipitation techniques that remove sulfur compounds from solution. Advanced treatment systems incorporate multiple stages to ensure complete removal of hydrosulfuric acid from industrial effluents before discharge, protecting water resources and complying with environmental regulations.
02 Environmental impact and remediation
Hydrosulfuric acid emissions pose significant environmental hazards, including air pollution and water contamination. Technologies have been developed for capturing, treating, and neutralizing H2S from industrial processes and natural sources. These remediation methods include biological treatments, chemical oxidation processes, and specialized filtration systems designed to reduce the environmental impact of H2S releases.Expand Specific Solutions03 Polymer modification and protection
Hydrosulfuric acid can significantly degrade polymer materials through chemical attack. Research has focused on developing polymer formulations with enhanced resistance to H2S exposure. These include modified polymer compositions, protective additives, and cross-linking agents that improve the durability and longevity of polymeric materials in H2S-rich environments, particularly important for applications in chemical processing and oil extraction.Expand Specific Solutions04 Detection and monitoring systems
Given the toxicity and corrosivity of hydrosulfuric acid, accurate detection and monitoring systems are crucial for safety. Advanced sensor technologies have been developed to detect H2S at low concentrations, providing early warning of dangerous levels. These systems incorporate various detection principles including electrochemical, optical, and semiconductor-based sensors, often integrated with automated alarm systems for industrial and environmental monitoring applications.Expand Specific Solutions05 Chemical processing applications
Despite its hazardous nature, hydrosulfuric acid serves important functions in various chemical processes. It is utilized in the synthesis of organic compounds, metal sulfide production, and as a reagent in analytical chemistry. Innovations focus on safer handling methods, controlled reaction environments, and process optimizations that maximize the utility of H2S while minimizing its risks in manufacturing and chemical production settings.Expand Specific Solutions
Leading Organizations in Water Quality Assessment Industry
The hydrosulfuric acid impact assessment market is currently in a growth phase, with increasing environmental regulations driving demand for water quality monitoring solutions. The global market size for water treatment technologies addressing sulfide contamination is estimated at $8-10 billion, expanding at 5-7% annually. From a technological maturity perspective, the field shows varied development levels. Evoqua Water Technologies and Veolia Water Solutions lead with advanced treatment systems, while Schlumberger companies contribute specialized monitoring technologies from the energy sector. Academic institutions like Xiamen University and Louisiana State University are advancing fundamental research, while Eni SpA and UOP LLC focus on industrial applications. Emerging players like Fuhuan Qingyun Technology are developing innovative carbon capture technologies that integrate with sulfide management, indicating a trend toward comprehensive environmental solutions.
Evoqua Water Technologies LLC
Technical Solution: Evoqua Water Technologies has developed comprehensive solutions for hydrosulfuric acid (H2S) impact assessment and remediation in natural water bodies. Their approach combines real-time monitoring systems with advanced treatment technologies. The company's monitoring platform utilizes electrochemical sensors with selective membranes that can detect H2S concentrations as low as 0.1 ppm in various water conditions. This is coupled with their proprietary OdorLoggerTM system that provides continuous data collection and remote monitoring capabilities. For treatment, Evoqua employs a multi-barrier approach including their BIOXIDE® solution, which uses calcium nitrate to biochemically prevent H2S formation, and advanced oxidation processes using their Barrier® M chlorine dioxide technology that effectively oxidizes dissolved sulfides without producing harmful byproducts. Their integrated assessment methodology incorporates ecological impact evaluation tools that measure effects on aquatic organisms at different trophic levels.
Strengths: Comprehensive end-to-end solution from detection to remediation; proprietary technologies with proven effectiveness in various water conditions; extensive experience across industrial and municipal applications. Weaknesses: Higher implementation costs compared to simpler solutions; requires specialized technical expertise for optimal system configuration; some treatment approaches may require ongoing chemical inputs.
Schlumberger Technology BV
Technical Solution: Schlumberger Technology has pioneered advanced solutions for hydrosulfuric acid assessment in natural water bodies through their environmental services division. Their approach integrates sophisticated monitoring technologies with predictive modeling capabilities. The company's H2S-WaterTrack™ system employs specialized ion-selective electrodes and optical sensors that can continuously monitor H2S concentrations at multiple depths throughout water columns. This data is processed through their proprietary DELFI™ cognitive environmental platform, which applies machine learning algorithms to predict dispersion patterns and potential ecological impacts. Schlumberger's assessment methodology includes comprehensive water chemistry analysis that examines how H2S interacts with other compounds in specific water bodies, particularly in high-salinity environments where they have significant expertise from their oil field operations. Their technology can distinguish between geogenic (naturally occurring) and anthropogenic sources of sulfides through isotopic fingerprinting techniques, allowing for more targeted remediation strategies. The company has successfully deployed these systems in environmentally sensitive areas near industrial operations and in natural water bodies affected by agricultural runoff.
Strengths: Exceptional data analytics capabilities that provide predictive insights beyond simple monitoring; extensive experience with complex water chemistry in diverse environments; integrated approach that connects assessment to actionable remediation strategies. Weaknesses: Solutions are often designed for industrial-scale applications and may be cost-prohibitive for smaller environmental projects; heavy reliance on proprietary technology creates potential vendor lock-in; systems require significant technical expertise to operate effectively.
Critical Patents and Research on H2S Detection Technologies
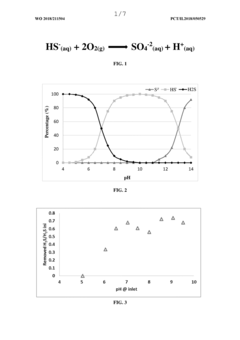
Method for removing hydrogen sulfide from untreated water
PatentInactiveUS5269944A
Innovation
- Adjusting the pH of untreated water to convert bicarbonate ions to carbon dioxide and then treating it with an oxidizing agent like chlorine to convert hydrogen sulfide to sulfate ions, avoiding the formation of elemental sulfur by maintaining a pH below 6.0 and using neutralization to raise the pH for potable water.
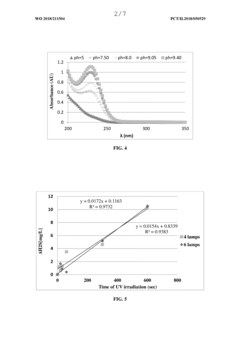
Method for decreasing the concentration of hydrogen sulfide
PatentWO2018211504A1
Innovation
- A method involving maintaining the pH of the medium at 5.2 or above, contacting it with an oxidant like oxygen or ozone, and applying ultraviolet (UV) irradiation in the range of 200 to 270 nm to decrease H2S concentration, with the UV irradiation intensity sufficient to achieve a rate of at least 8% per minute.
Environmental Regulatory Framework for Sulfide Compounds
The regulatory landscape governing sulfide compounds in water bodies has evolved significantly over the past decades, reflecting growing awareness of environmental impacts. At the international level, the World Health Organization (WHO) has established guidelines for hydrogen sulfide concentrations in water, recommending levels below 0.05 mg/L to prevent taste and odor issues, though no health-based guideline value has been established due to the compound's rapid oxidation in water.
In the United States, the Environmental Protection Agency (EPA) regulates sulfide compounds under multiple frameworks. The Clean Water Act (CWA) establishes water quality criteria for aquatic life protection, with acute exposure limits for hydrogen sulfide set at 2.0 μg/L and chronic exposure limits at 0.2 μg/L in freshwater systems. Additionally, the Safe Drinking Water Act (SDWA) addresses secondary standards related to taste and odor, indirectly affecting sulfide regulation.
The European Union's Water Framework Directive (2000/60/EC) takes a comprehensive approach to water protection, requiring member states to achieve "good ecological status" for all water bodies. While not specifically targeting hydrogen sulfide, the directive's ecological quality standards encompass parameters affected by sulfide contamination, including dissolved oxygen levels and biological indicators.
Emerging economies have developed varying regulatory approaches. China's Environmental Quality Standards for Surface Water (GB 3838-2002) classifies water bodies into five categories based on function and sets corresponding sulfide limits ranging from 0.05 mg/L to 1.0 mg/L. India's Water (Prevention and Control of Pollution) Act establishes standards for industrial effluents, limiting sulfide discharge to 2.0 mg/L.
Industry-specific regulations provide another layer of control, particularly for sectors known to generate sulfide-containing waste streams. Mining operations face stringent requirements under frameworks like the International Cyanide Management Code, which includes provisions for managing sulfide-bearing tailings. Similarly, the oil and gas industry must comply with regulations governing the treatment and disposal of hydrogen sulfide-containing produced water.
Recent regulatory trends show movement toward ecosystem-based approaches that consider cumulative impacts rather than single-parameter compliance. This shift acknowledges the complex interactions between sulfide compounds and other water quality parameters, particularly in sensitive ecosystems like wetlands and estuaries where natural sulfur cycling plays a crucial ecological role.
In the United States, the Environmental Protection Agency (EPA) regulates sulfide compounds under multiple frameworks. The Clean Water Act (CWA) establishes water quality criteria for aquatic life protection, with acute exposure limits for hydrogen sulfide set at 2.0 μg/L and chronic exposure limits at 0.2 μg/L in freshwater systems. Additionally, the Safe Drinking Water Act (SDWA) addresses secondary standards related to taste and odor, indirectly affecting sulfide regulation.
The European Union's Water Framework Directive (2000/60/EC) takes a comprehensive approach to water protection, requiring member states to achieve "good ecological status" for all water bodies. While not specifically targeting hydrogen sulfide, the directive's ecological quality standards encompass parameters affected by sulfide contamination, including dissolved oxygen levels and biological indicators.
Emerging economies have developed varying regulatory approaches. China's Environmental Quality Standards for Surface Water (GB 3838-2002) classifies water bodies into five categories based on function and sets corresponding sulfide limits ranging from 0.05 mg/L to 1.0 mg/L. India's Water (Prevention and Control of Pollution) Act establishes standards for industrial effluents, limiting sulfide discharge to 2.0 mg/L.
Industry-specific regulations provide another layer of control, particularly for sectors known to generate sulfide-containing waste streams. Mining operations face stringent requirements under frameworks like the International Cyanide Management Code, which includes provisions for managing sulfide-bearing tailings. Similarly, the oil and gas industry must comply with regulations governing the treatment and disposal of hydrogen sulfide-containing produced water.
Recent regulatory trends show movement toward ecosystem-based approaches that consider cumulative impacts rather than single-parameter compliance. This shift acknowledges the complex interactions between sulfide compounds and other water quality parameters, particularly in sensitive ecosystems like wetlands and estuaries where natural sulfur cycling plays a crucial ecological role.
Ecological Risk Assessment Methodologies for Sulfide Contamination
Ecological risk assessment for sulfide contamination in natural water bodies requires systematic methodologies to evaluate potential environmental impacts. These methodologies typically follow a tiered approach, beginning with screening-level assessments and progressing to more detailed analyses as warranted by initial findings.
The first tier involves hazard identification, where potential sources of hydrosulfuric acid are mapped and characterized. This includes both anthropogenic sources such as industrial discharges, mining operations, and agricultural runoff, as well as natural sources like volcanic activity and biological decomposition processes. Historical data on sulfide concentrations and pH levels provide valuable baseline information for comparison.
Exposure assessment constitutes the second tier, focusing on the fate and transport of sulfides in aquatic environments. This involves measuring or modeling sulfide concentrations across different environmental compartments, considering factors such as water flow rates, temperature, dissolved oxygen levels, and sediment characteristics. Advanced sampling techniques including passive samplers and automated monitoring systems enable continuous data collection under varying conditions.
The third tier encompasses dose-response assessment, which establishes relationships between sulfide concentrations and adverse effects on aquatic organisms. Standardized toxicity tests using indicator species from multiple trophic levels (e.g., algae, invertebrates, fish) help determine acute and chronic toxicity thresholds. These tests typically measure endpoints such as mortality, growth inhibition, reproductive impairment, and behavioral changes.
Risk characterization integrates exposure and effects data to quantify ecological risks. This often employs hazard quotients (HQ) or risk quotients (RQ), calculated by dividing measured environmental concentrations by toxicity reference values. Probabilistic risk assessment techniques, including Monte Carlo simulations, help account for variability and uncertainty in both exposure and effects data.
Biomonitoring serves as a crucial component of comprehensive assessment programs, involving the collection and analysis of biological samples to evaluate actual ecosystem impacts. Bioindicator species, community structure analyses, and biomarker responses provide evidence of exposure and effects under real-world conditions. These biological metrics often detect subtle ecosystem changes before conventional chemical monitoring reveals problems.
Weight-of-evidence approaches integrate multiple lines of evidence from chemical, toxicological, and ecological investigations to strengthen risk conclusions. This holistic evaluation helps address the inherent complexity of sulfide impacts, which can vary dramatically based on environmental conditions and biological communities present in different water bodies.
The first tier involves hazard identification, where potential sources of hydrosulfuric acid are mapped and characterized. This includes both anthropogenic sources such as industrial discharges, mining operations, and agricultural runoff, as well as natural sources like volcanic activity and biological decomposition processes. Historical data on sulfide concentrations and pH levels provide valuable baseline information for comparison.
Exposure assessment constitutes the second tier, focusing on the fate and transport of sulfides in aquatic environments. This involves measuring or modeling sulfide concentrations across different environmental compartments, considering factors such as water flow rates, temperature, dissolved oxygen levels, and sediment characteristics. Advanced sampling techniques including passive samplers and automated monitoring systems enable continuous data collection under varying conditions.
The third tier encompasses dose-response assessment, which establishes relationships between sulfide concentrations and adverse effects on aquatic organisms. Standardized toxicity tests using indicator species from multiple trophic levels (e.g., algae, invertebrates, fish) help determine acute and chronic toxicity thresholds. These tests typically measure endpoints such as mortality, growth inhibition, reproductive impairment, and behavioral changes.
Risk characterization integrates exposure and effects data to quantify ecological risks. This often employs hazard quotients (HQ) or risk quotients (RQ), calculated by dividing measured environmental concentrations by toxicity reference values. Probabilistic risk assessment techniques, including Monte Carlo simulations, help account for variability and uncertainty in both exposure and effects data.
Biomonitoring serves as a crucial component of comprehensive assessment programs, involving the collection and analysis of biological samples to evaluate actual ecosystem impacts. Bioindicator species, community structure analyses, and biomarker responses provide evidence of exposure and effects under real-world conditions. These biological metrics often detect subtle ecosystem changes before conventional chemical monitoring reveals problems.
Weight-of-evidence approaches integrate multiple lines of evidence from chemical, toxicological, and ecological investigations to strengthen risk conclusions. This holistic evaluation helps address the inherent complexity of sulfide impacts, which can vary dramatically based on environmental conditions and biological communities present in different water bodies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!