Anode Coating Technologies To Reduce Oxygen Evolution Penalties
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anode Coating Evolution and Objectives
Anode coating technologies have undergone significant evolution over the past decades, transforming from simple protective layers to sophisticated multifunctional interfaces designed to address specific electrochemical challenges. The development trajectory began in the 1980s with basic carbon coatings primarily focused on preventing electrolyte decomposition. By the early 2000s, research shifted toward metal oxide coatings that offered improved stability and conductivity properties.
The oxygen evolution reaction (OER) represents one of the most significant efficiency barriers in numerous electrochemical systems, including water electrolysis, metal-air batteries, and certain fuel cells. This parasitic reaction consumes energy, generates unwanted byproducts, and accelerates electrode degradation. The penalties associated with oxygen evolution include reduced coulombic efficiency, accelerated capacity fading, and safety concerns related to gas generation and pressure buildup within cells.
Recent technological advancements have focused on developing specialized anode coating materials that can selectively suppress oxygen evolution while maintaining desired electrochemical performance. These include fluorinated compounds, phosphate-based materials, and advanced polymer composites that create physical and chemical barriers against oxygen generation pathways.
The primary objective of current research in anode coating technologies is to develop materials that can effectively mitigate oxygen evolution penalties while simultaneously enhancing overall system performance. This includes designing coatings with precise thickness control (typically 5-50 nm), optimized ionic conductivity, and mechanical stability under operational conditions.
Secondary objectives include developing scalable and cost-effective coating deposition methods suitable for industrial implementation. Current techniques range from atomic layer deposition (ALD) offering nanometer precision but limited throughput, to more scalable approaches like solution-based methods that balance manufacturing practicality with coating quality.
Looking forward, the field aims to achieve multifunctional coatings capable of addressing multiple challenges simultaneously. These next-generation materials will likely incorporate self-healing properties, adaptive interfaces that respond to local electrochemical conditions, and hierarchical structures optimized at multiple length scales.
The ultimate goal remains developing coating technologies that can effectively eliminate oxygen evolution penalties without compromising other performance metrics, thereby enabling higher energy density, longer cycle life, and improved safety across various electrochemical energy storage and conversion systems.
The oxygen evolution reaction (OER) represents one of the most significant efficiency barriers in numerous electrochemical systems, including water electrolysis, metal-air batteries, and certain fuel cells. This parasitic reaction consumes energy, generates unwanted byproducts, and accelerates electrode degradation. The penalties associated with oxygen evolution include reduced coulombic efficiency, accelerated capacity fading, and safety concerns related to gas generation and pressure buildup within cells.
Recent technological advancements have focused on developing specialized anode coating materials that can selectively suppress oxygen evolution while maintaining desired electrochemical performance. These include fluorinated compounds, phosphate-based materials, and advanced polymer composites that create physical and chemical barriers against oxygen generation pathways.
The primary objective of current research in anode coating technologies is to develop materials that can effectively mitigate oxygen evolution penalties while simultaneously enhancing overall system performance. This includes designing coatings with precise thickness control (typically 5-50 nm), optimized ionic conductivity, and mechanical stability under operational conditions.
Secondary objectives include developing scalable and cost-effective coating deposition methods suitable for industrial implementation. Current techniques range from atomic layer deposition (ALD) offering nanometer precision but limited throughput, to more scalable approaches like solution-based methods that balance manufacturing practicality with coating quality.
Looking forward, the field aims to achieve multifunctional coatings capable of addressing multiple challenges simultaneously. These next-generation materials will likely incorporate self-healing properties, adaptive interfaces that respond to local electrochemical conditions, and hierarchical structures optimized at multiple length scales.
The ultimate goal remains developing coating technologies that can effectively eliminate oxygen evolution penalties without compromising other performance metrics, thereby enabling higher energy density, longer cycle life, and improved safety across various electrochemical energy storage and conversion systems.
Market Analysis for Advanced Anode Materials
The global market for advanced anode materials is experiencing significant growth, driven primarily by the expanding lithium-ion battery sector. Current market valuations place the advanced anode materials market at approximately 3.2 billion USD in 2023, with projections indicating a compound annual growth rate of 16.8% through 2030. This growth trajectory is largely attributed to increasing demand for high-performance batteries in electric vehicles, consumer electronics, and grid-scale energy storage systems.
Anode coating technologies specifically designed to reduce oxygen evolution penalties represent a critical segment within this market. These technologies address one of the most persistent challenges in battery performance: the parasitic oxygen evolution reaction that occurs at the anode-electrolyte interface, leading to capacity fade and reduced battery lifespan. The market demand for such solutions has intensified as manufacturers seek to extend battery life cycles and improve safety profiles.
Regional analysis reveals that Asia-Pacific dominates the advanced anode materials market, accounting for over 65% of global production capacity. This concentration is primarily due to the established battery manufacturing ecosystems in China, Japan, and South Korea. North America and Europe are rapidly expanding their market shares, driven by strategic investments in domestic battery supply chains and supportive regulatory frameworks promoting clean energy technologies.
Consumer electronics currently represent the largest application segment for advanced anode materials, but electric vehicles are projected to become the dominant market driver by 2025. This shift reflects the accelerating global transition toward electrified transportation and the corresponding demand for batteries with higher energy density, faster charging capabilities, and longer operational lifespans.
Market segmentation by coating technology type shows silicon-based coatings leading with approximately 38% market share, followed by carbon-based coatings at 27% and metal oxide coatings at 21%. Emerging technologies, including composite coatings and atomic layer deposition methods, are gaining traction due to their superior performance in mitigating oxygen evolution penalties.
Key market drivers include stringent environmental regulations promoting clean energy adoption, declining battery costs enabling broader market penetration, and increasing consumer awareness regarding sustainable energy solutions. Technological advancements in coating methodologies, particularly those addressing interface stability and ion transport efficiency, are creating new market opportunities and competitive advantages for early adopters.
Market challenges persist in the form of raw material supply constraints, particularly for critical minerals used in advanced coating formulations. Additionally, the complex manufacturing processes associated with precision coating technologies present scalability challenges that impact production economics and market accessibility for smaller manufacturers.
Anode coating technologies specifically designed to reduce oxygen evolution penalties represent a critical segment within this market. These technologies address one of the most persistent challenges in battery performance: the parasitic oxygen evolution reaction that occurs at the anode-electrolyte interface, leading to capacity fade and reduced battery lifespan. The market demand for such solutions has intensified as manufacturers seek to extend battery life cycles and improve safety profiles.
Regional analysis reveals that Asia-Pacific dominates the advanced anode materials market, accounting for over 65% of global production capacity. This concentration is primarily due to the established battery manufacturing ecosystems in China, Japan, and South Korea. North America and Europe are rapidly expanding their market shares, driven by strategic investments in domestic battery supply chains and supportive regulatory frameworks promoting clean energy technologies.
Consumer electronics currently represent the largest application segment for advanced anode materials, but electric vehicles are projected to become the dominant market driver by 2025. This shift reflects the accelerating global transition toward electrified transportation and the corresponding demand for batteries with higher energy density, faster charging capabilities, and longer operational lifespans.
Market segmentation by coating technology type shows silicon-based coatings leading with approximately 38% market share, followed by carbon-based coatings at 27% and metal oxide coatings at 21%. Emerging technologies, including composite coatings and atomic layer deposition methods, are gaining traction due to their superior performance in mitigating oxygen evolution penalties.
Key market drivers include stringent environmental regulations promoting clean energy adoption, declining battery costs enabling broader market penetration, and increasing consumer awareness regarding sustainable energy solutions. Technological advancements in coating methodologies, particularly those addressing interface stability and ion transport efficiency, are creating new market opportunities and competitive advantages for early adopters.
Market challenges persist in the form of raw material supply constraints, particularly for critical minerals used in advanced coating formulations. Additionally, the complex manufacturing processes associated with precision coating technologies present scalability challenges that impact production economics and market accessibility for smaller manufacturers.
Current Challenges in Oxygen Evolution Reaction
The oxygen evolution reaction (OER) represents a significant bottleneck in various electrochemical systems, particularly in lithium-air batteries, water electrolysis, and metal-air batteries. Despite extensive research efforts, several fundamental challenges continue to impede the widespread implementation of efficient anode coating technologies designed to mitigate oxygen evolution penalties.
A primary challenge lies in the inherent thermodynamic limitations of the OER process. The four-electron transfer mechanism requires substantial energy input, resulting in high overpotentials that significantly reduce overall system efficiency. Even state-of-the-art catalysts typically require overpotentials exceeding 300-400 mV to achieve industrially relevant current densities, which translates directly to energy losses in practical applications.
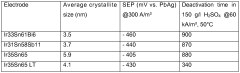
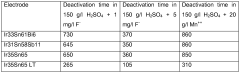
Catalyst stability presents another critical hurdle. Under the highly oxidative conditions necessary for oxygen evolution, many materials undergo degradation through mechanisms such as dissolution, surface reconstruction, or phase transformation. Noble metal-based catalysts like IrO₂ and RuO₂ demonstrate superior activity but suffer from high costs and limited abundance, while more earth-abundant alternatives often lack sufficient stability in acidic environments or adequate conductivity for efficient electron transfer.
The interface between the catalyst coating and the underlying anode substrate introduces additional complications. Poor adhesion can lead to delamination during operation, while inadequate electronic connectivity increases internal resistance. Furthermore, the three-phase boundary where solid catalyst, liquid electrolyte, and gaseous oxygen meet creates mass transport limitations that can significantly hinder reaction kinetics at high current densities.
Scalability and manufacturing challenges further complicate industrial implementation. Many high-performance coating technologies developed in laboratory settings rely on complex synthesis procedures, expensive precursors, or specialized equipment that prove difficult to scale up economically. Techniques such as atomic layer deposition offer precise control over coating properties but face throughput limitations for large-scale production.
The electrolyte environment introduces additional constraints, as pH fluctuations near the electrode surface can dramatically alter catalyst performance. In lithium-air batteries specifically, the formation of insulating lithium peroxide during discharge creates passivation layers that impede electron transfer and oxygen diffusion, while carbonate-based electrolytes suffer from decomposition due to nucleophilic attack by superoxide intermediates.
Recent research has highlighted the importance of understanding reaction mechanisms at the molecular level, as the rate-determining step and reaction pathway can vary significantly depending on catalyst composition, structure, and operating conditions. This mechanistic complexity makes rational catalyst design particularly challenging, often necessitating empirical approaches to optimization.
A primary challenge lies in the inherent thermodynamic limitations of the OER process. The four-electron transfer mechanism requires substantial energy input, resulting in high overpotentials that significantly reduce overall system efficiency. Even state-of-the-art catalysts typically require overpotentials exceeding 300-400 mV to achieve industrially relevant current densities, which translates directly to energy losses in practical applications.
Catalyst stability presents another critical hurdle. Under the highly oxidative conditions necessary for oxygen evolution, many materials undergo degradation through mechanisms such as dissolution, surface reconstruction, or phase transformation. Noble metal-based catalysts like IrO₂ and RuO₂ demonstrate superior activity but suffer from high costs and limited abundance, while more earth-abundant alternatives often lack sufficient stability in acidic environments or adequate conductivity for efficient electron transfer.
The interface between the catalyst coating and the underlying anode substrate introduces additional complications. Poor adhesion can lead to delamination during operation, while inadequate electronic connectivity increases internal resistance. Furthermore, the three-phase boundary where solid catalyst, liquid electrolyte, and gaseous oxygen meet creates mass transport limitations that can significantly hinder reaction kinetics at high current densities.
Scalability and manufacturing challenges further complicate industrial implementation. Many high-performance coating technologies developed in laboratory settings rely on complex synthesis procedures, expensive precursors, or specialized equipment that prove difficult to scale up economically. Techniques such as atomic layer deposition offer precise control over coating properties but face throughput limitations for large-scale production.
The electrolyte environment introduces additional constraints, as pH fluctuations near the electrode surface can dramatically alter catalyst performance. In lithium-air batteries specifically, the formation of insulating lithium peroxide during discharge creates passivation layers that impede electron transfer and oxygen diffusion, while carbonate-based electrolytes suffer from decomposition due to nucleophilic attack by superoxide intermediates.
Recent research has highlighted the importance of understanding reaction mechanisms at the molecular level, as the rate-determining step and reaction pathway can vary significantly depending on catalyst composition, structure, and operating conditions. This mechanistic complexity makes rational catalyst design particularly challenging, often necessitating empirical approaches to optimization.
Existing Coating Solutions for OER Efficiency
01 Metal oxide coatings for improved OER performance
Metal oxide coatings such as iridium oxide, ruthenium oxide, and mixed metal oxides can be applied to anode surfaces to reduce oxygen evolution penalties. These coatings enhance catalytic activity, improve electron transfer, and reduce overpotential during the oxygen evolution reaction. The optimized metal oxide layers provide better stability in harsh electrolyte conditions while minimizing energy losses associated with oxygen evolution.- Metal oxide coatings for improved OER performance: Metal oxide coatings such as iridium oxide, ruthenium oxide, and mixed metal oxides can be applied to anode surfaces to reduce oxygen evolution penalties. These coatings enhance catalytic activity, improve electron transfer, and reduce overpotential during oxygen evolution reactions. The optimized composition and structure of these metal oxide layers can significantly improve the efficiency of electrochemical processes while minimizing energy losses.
- Nanostructured catalyst coatings for anodes: Nanostructured materials applied as anode coatings provide increased surface area and active sites for oxygen evolution reactions. These nanoscale architectures, including nanoparticles, nanowires, and nanotubes, enhance catalytic performance while reducing the energy penalties associated with oxygen evolution. The controlled morphology at the nanoscale allows for optimized electron transport pathways and improved interaction with electrolytes, resulting in lower overpotentials.
- Composite and doped coating materials: Composite coatings combining multiple materials or doped coating systems can significantly reduce oxygen evolution penalties at anodes. These formulations often incorporate transition metals, rare earth elements, or carbon-based materials to enhance conductivity and catalytic properties. By carefully engineering the composition and dopant concentrations, these coatings can achieve synergistic effects that improve stability and reduce energy losses during oxygen evolution reactions.
- Surface modification techniques for anodes: Various surface modification techniques can be applied to anodes to minimize oxygen evolution penalties. These include plasma treatment, chemical etching, electrochemical activation, and surface functionalization. These processes create optimized surface structures with improved wettability, enhanced active site density, and reduced charge transfer resistance, leading to more efficient oxygen evolution reactions with lower energy requirements.
- Protective and anti-corrosion coatings: Protective coatings that enhance anode durability while maintaining efficient oxygen evolution are crucial for reducing long-term operational penalties. These coatings prevent degradation of the anode material in harsh electrochemical environments, extending service life while maintaining catalytic performance. Advanced anti-corrosion formulations incorporate barrier layers, self-healing components, or sacrificial materials that protect the underlying anode while allowing efficient oxygen evolution to continue.
02 Nanostructured anode coatings for enhanced surface area
Nanostructured coatings including nanoparticles, nanowires, and nanosheets can be applied to anode surfaces to significantly increase the active surface area. These nanostructured materials provide more catalytic sites for oxygen evolution, reducing the reaction penalties. The enhanced surface area improves electron transfer efficiency and reduces the overpotential required for oxygen evolution, resulting in overall improved electrochemical performance.Expand Specific Solutions03 Composite and doped coating materials
Composite coatings combining multiple materials or doped coatings with specific elements can enhance anode performance during oxygen evolution. These materials may include carbon-supported catalysts, perovskite structures, or transition metal compounds doped with elements like nitrogen or phosphorus. The synergistic effects between different components in these composite materials help to reduce oxygen evolution penalties by optimizing electron transfer pathways and catalytic activity.Expand Specific Solutions04 Protective layers to prevent anode degradation
Protective coating layers can be applied to anodes to prevent degradation during oxygen evolution reactions. These coatings shield the underlying anode material from corrosion, oxidation, and dissolution while still allowing efficient electron transfer. Materials such as conductive polymers, ceramic layers, or passivation films help extend anode lifetime and maintain consistent performance by reducing the penalties associated with anode degradation during oxygen evolution.Expand Specific Solutions05 Surface modification techniques for improved wettability
Surface modification techniques can be employed to improve the wettability and electrolyte accessibility of anode coatings. These techniques include plasma treatment, chemical etching, and functionalization with hydrophilic groups. Enhanced wettability ensures better contact between the electrolyte and the anode surface, facilitating more efficient oxygen evolution reactions with reduced penalties. These modifications also help prevent gas bubble accumulation on the anode surface, which can block active sites and increase resistance.Expand Specific Solutions
Leading Companies and Research Institutions in Anode Technology
The anode coating technologies market for reducing oxygen evolution penalties is currently in a growth phase, characterized by increasing demand for more efficient electrochemical processes across industrial applications. The market size is expanding as industries prioritize energy efficiency and sustainability in electrochemical operations. Technologically, the field shows varying maturity levels, with established players like Industrie De Nora and BASF leading with advanced coating solutions, while newer entrants such as Sunrui Marine Environment Engineering and Jiangyin Luojia Green Carbon Technology are developing innovative approaches. Johnson Matthey and Umicore Galvanotechnik bring significant expertise in precious metal catalysts and electroplating, while research institutions like California Institute of Technology and King Saud University contribute fundamental advancements. The competitive landscape reflects a blend of industrial chemical giants, specialized electrochemistry firms, and emerging technology developers focused on improving anode performance and reducing energy consumption.
Industrie De Nora SpA
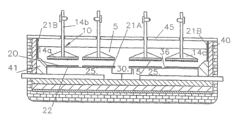
Technical Solution: De Nora has developed proprietary Dimensional Stable Anode (DSA) coating technologies specifically designed to minimize oxygen evolution reactions in electrochemical processes. Their approach involves applying mixed metal oxide coatings containing iridium, ruthenium, and tantalum on titanium substrates using thermal decomposition methods. These coatings create a catalytic surface that selectively promotes desired electrochemical reactions while suppressing parasitic oxygen evolution. De Nora's latest generation coatings incorporate nanostructured materials with optimized porosity and surface area, achieving up to 30% reduction in oxygen evolution compared to conventional anodes. The company has also pioneered gradient composition coatings where the elemental ratios vary from the substrate interface to the electrolyte-facing surface, creating tailored electrochemical properties throughout the coating thickness.
Strengths: Industry-leading expertise in electrochemical coatings with over 50 years of experience; proprietary manufacturing processes ensuring coating durability in harsh environments; extensive industrial implementation experience. Weaknesses: Higher initial cost compared to conventional anodes; requires specialized application equipment; performance can degrade in certain extreme pH environments.
BASF Corp.
Technical Solution: BASF has pioneered composite anode coating technologies that incorporate conductive polymers and ceramic materials to create barrier layers against oxygen evolution. Their approach utilizes a multi-layer coating strategy where an inner layer provides electrical conductivity while outer layers contain oxygen evolution inhibitors and selective catalysts. BASF's proprietary sol-gel deposition process enables precise control of coating thickness and composition at the nanoscale, creating tailored interfaces that minimize parasitic reactions. Their latest innovation involves incorporating fluorinated compounds and rare-earth elements that modify the electronic structure of the anode surface, shifting reaction potentials away from oxygen evolution pathways. Laboratory tests demonstrate these coatings can reduce oxygen evolution by up to 45% while maintaining primary reaction efficiency above 90%. BASF has also developed self-assembling coating systems where components reorganize during initial operation to form optimal catalytic structures.
Strengths: Extensive materials science expertise; comprehensive characterization capabilities; strong integration with other chemical processes. Weaknesses: Some formulations show decreased performance in high-temperature applications; coating adhesion can be challenging on certain substrate materials; requires precise process control during application.
Key Innovations in Oxygen Evolution Suppression
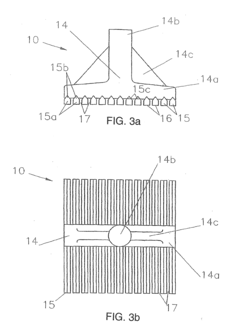
Anode for oxygen evolution
PatentWO2012175673A1
Innovation
- A valve metal substrate with a catalytic layer comprising a mixture of iridium, tin, and a doping element such as bismuth, antimony, or niobium, combined with a protective valve metal oxide layer, forming small crystallites for enhanced catalytic activity and corrosion resistance, achieved through thermal decomposition of precursor salts at reduced temperatures.
Metallic oxygen evolving anode operating at high current density for aluminium reduction cells
PatentInactiveUS20110192728A1
Innovation
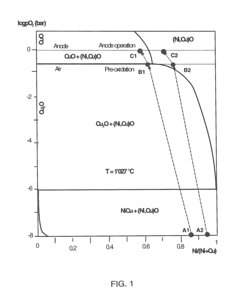
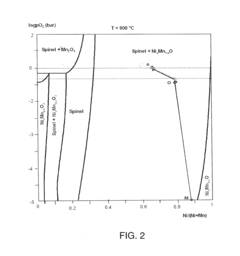
- A metallic oxygen evolving anode alloy composition of Nickel-Iron-Manganese-Copper-Silicon, with specific weight ratios and a pre-oxidized surface, forming a stable nickel manganese oxide or nickel ferrite layer that maintains p-semiconductor character even at high oxygen activity, preventing n-p semiconductor junctions and ensuring stable operation at high current densities.
Environmental Impact Assessment of Coating Materials
The environmental impact of anode coating materials represents a critical consideration in the development and deployment of advanced battery technologies aimed at reducing oxygen evolution penalties. Current coating technologies utilize a variety of materials including transition metal oxides, noble metals, and composite structures, each carrying distinct environmental footprints throughout their lifecycle.
Primary extraction processes for coating materials, particularly rare earth elements and noble metals like iridium and ruthenium, involve energy-intensive mining operations that contribute significantly to habitat destruction, soil erosion, and water contamination. These processes typically generate 8-12 tons of waste material per ton of extracted rare earth elements, creating substantial environmental liabilities in mining regions.
Manufacturing processes for anode coatings involve chemical treatments, high-temperature sintering, and precision deposition techniques that consume considerable energy and often utilize environmentally hazardous solvents and reagents. Notably, physical vapor deposition methods commonly employed for uniform coating application consume between 50-200 kWh per kilogram of coating material, contributing to the carbon footprint of battery production.
Toxicity profiles of coating materials vary significantly, with certain compounds presenting environmental persistence concerns. Cobalt-based coatings, while effective at suppressing oxygen evolution, pose documented ecotoxicological risks to aquatic ecosystems at concentrations as low as 5 μg/L. Similarly, fluoride-containing compounds used in some coating formulations present groundwater contamination risks if improperly managed during manufacturing or disposal phases.
End-of-life considerations reveal significant challenges in the recyclability of coated anodes. Current recycling technologies achieve recovery rates of only 35-60% for coating materials, with the remainder typically directed to landfills or incinerators. This inefficiency represents both resource loss and potential environmental contamination pathways.
Recent life cycle assessments indicate that environmentally optimized coating technologies could reduce the overall environmental impact by 30-45% compared to conventional approaches. Promising developments include water-based coating processes that eliminate organic solvent usage, bio-derived coating precursors, and design-for-recycling approaches that facilitate material recovery at end-of-life.
Regulatory frameworks increasingly influence coating material selection, with the European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations worldwide driving manufacturers toward less environmentally problematic alternatives. This regulatory landscape is expected to continue evolving, potentially accelerating the transition toward more sustainable coating technologies in the coming decade.
Primary extraction processes for coating materials, particularly rare earth elements and noble metals like iridium and ruthenium, involve energy-intensive mining operations that contribute significantly to habitat destruction, soil erosion, and water contamination. These processes typically generate 8-12 tons of waste material per ton of extracted rare earth elements, creating substantial environmental liabilities in mining regions.
Manufacturing processes for anode coatings involve chemical treatments, high-temperature sintering, and precision deposition techniques that consume considerable energy and often utilize environmentally hazardous solvents and reagents. Notably, physical vapor deposition methods commonly employed for uniform coating application consume between 50-200 kWh per kilogram of coating material, contributing to the carbon footprint of battery production.
Toxicity profiles of coating materials vary significantly, with certain compounds presenting environmental persistence concerns. Cobalt-based coatings, while effective at suppressing oxygen evolution, pose documented ecotoxicological risks to aquatic ecosystems at concentrations as low as 5 μg/L. Similarly, fluoride-containing compounds used in some coating formulations present groundwater contamination risks if improperly managed during manufacturing or disposal phases.
End-of-life considerations reveal significant challenges in the recyclability of coated anodes. Current recycling technologies achieve recovery rates of only 35-60% for coating materials, with the remainder typically directed to landfills or incinerators. This inefficiency represents both resource loss and potential environmental contamination pathways.
Recent life cycle assessments indicate that environmentally optimized coating technologies could reduce the overall environmental impact by 30-45% compared to conventional approaches. Promising developments include water-based coating processes that eliminate organic solvent usage, bio-derived coating precursors, and design-for-recycling approaches that facilitate material recovery at end-of-life.
Regulatory frameworks increasingly influence coating material selection, with the European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations worldwide driving manufacturers toward less environmentally problematic alternatives. This regulatory landscape is expected to continue evolving, potentially accelerating the transition toward more sustainable coating technologies in the coming decade.
Scalability and Manufacturing Considerations
The scalability of anode coating technologies represents a critical factor in their commercial viability for large-scale energy storage applications. Current laboratory-scale coating methods often face significant challenges when transitioning to industrial production. Physical vapor deposition (PVD) techniques, while offering excellent coating uniformity and thickness control, typically suffer from low throughput and high equipment costs when scaled up. These limitations make PVD less economically viable for mass production of coated anodes despite their technical advantages.
Solution-based coating methods such as dip coating, spin coating, and spray coating demonstrate better scalability potential. Among these, roll-to-roll processing stands out as particularly promising for continuous, high-volume manufacturing of coated anodes. This approach allows for coating deposition on flexible substrates at speeds of up to several meters per minute, significantly reducing production time compared to batch processes. However, maintaining coating quality and uniformity at high production speeds remains challenging, often requiring sophisticated tension control systems and precision application mechanisms.
Cost considerations play a decisive role in manufacturing scalability. Material costs for advanced protective coatings such as atomic layer deposition (ALD) of metal oxides can be prohibitively expensive for large-scale implementation. Recent research has focused on developing cost-effective alternatives using earth-abundant materials while maintaining comparable oxygen evolution suppression properties. Additionally, process simplification efforts aim to reduce the number of manufacturing steps, thereby lowering both capital and operational expenditures.
Environmental and safety factors also impact scalability considerations. Many coating processes involve volatile organic compounds or toxic precursors that require specialized handling and disposal procedures. The development of water-based or solvent-free coating technologies represents an important direction for environmentally sustainable manufacturing. These green chemistry approaches not only reduce environmental impact but also potentially lower regulatory compliance costs associated with hazardous material management.
Quality control and process monitoring present significant challenges when scaling up anode coating technologies. In-line measurement techniques such as optical monitoring, electrical resistance testing, and spectroscopic methods are being integrated into production lines to ensure coating consistency. Advanced manufacturing concepts from Industry 4.0, including real-time data analytics and machine learning algorithms, are increasingly being applied to optimize coating parameters and detect defects early in the production process.
The integration of anode coating processes into existing battery manufacturing lines represents another critical consideration. Retrofitting established production facilities with new coating technologies often requires substantial capital investment and production downtime. Modular coating systems that can be seamlessly incorporated into current manufacturing workflows offer a potential solution to this challenge, allowing for incremental implementation and validation before full-scale deployment.
Solution-based coating methods such as dip coating, spin coating, and spray coating demonstrate better scalability potential. Among these, roll-to-roll processing stands out as particularly promising for continuous, high-volume manufacturing of coated anodes. This approach allows for coating deposition on flexible substrates at speeds of up to several meters per minute, significantly reducing production time compared to batch processes. However, maintaining coating quality and uniformity at high production speeds remains challenging, often requiring sophisticated tension control systems and precision application mechanisms.
Cost considerations play a decisive role in manufacturing scalability. Material costs for advanced protective coatings such as atomic layer deposition (ALD) of metal oxides can be prohibitively expensive for large-scale implementation. Recent research has focused on developing cost-effective alternatives using earth-abundant materials while maintaining comparable oxygen evolution suppression properties. Additionally, process simplification efforts aim to reduce the number of manufacturing steps, thereby lowering both capital and operational expenditures.
Environmental and safety factors also impact scalability considerations. Many coating processes involve volatile organic compounds or toxic precursors that require specialized handling and disposal procedures. The development of water-based or solvent-free coating technologies represents an important direction for environmentally sustainable manufacturing. These green chemistry approaches not only reduce environmental impact but also potentially lower regulatory compliance costs associated with hazardous material management.
Quality control and process monitoring present significant challenges when scaling up anode coating technologies. In-line measurement techniques such as optical monitoring, electrical resistance testing, and spectroscopic methods are being integrated into production lines to ensure coating consistency. Advanced manufacturing concepts from Industry 4.0, including real-time data analytics and machine learning algorithms, are increasingly being applied to optimize coating parameters and detect defects early in the production process.
The integration of anode coating processes into existing battery manufacturing lines represents another critical consideration. Retrofitting established production facilities with new coating technologies often requires substantial capital investment and production downtime. Modular coating systems that can be seamlessly incorporated into current manufacturing workflows offer a potential solution to this challenge, allowing for incremental implementation and validation before full-scale deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!