Process Control And Real-Time Monitoring For Electrochemical Iron Cells
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Iron Cell Technology Evolution and Objectives
Electrochemical iron cell technology has evolved significantly over the past decades, transforming from rudimentary laboratory experiments to sophisticated industrial applications. The journey began in the early 1970s with basic electrochemical cells designed primarily for academic research, focusing on understanding the fundamental principles of iron electrodeposition and dissolution. By the 1980s, these cells had evolved to incorporate basic monitoring systems, primarily utilizing manual sampling and offline analysis methods.
The 1990s marked a significant turning point with the introduction of computerized control systems, enabling more precise regulation of electrochemical parameters such as current density, temperature, and electrolyte composition. This period also witnessed the first integration of real-time pH and conductivity sensors, though their reliability and accuracy remained limited in harsh electrochemical environments.
The early 2000s brought miniaturization and enhanced sensor technologies, allowing for more compact cell designs with improved efficiency. Concurrently, data acquisition systems became more sophisticated, enabling continuous monitoring of multiple parameters simultaneously. The development of corrosion-resistant materials during this period also significantly extended cell lifespans and reduced maintenance requirements.
Recent advancements since 2010 have focused on intelligent control systems incorporating machine learning algorithms for predictive maintenance and optimization. Modern electrochemical iron cells now feature integrated sensor networks capable of monitoring not only basic parameters but also electrolyte impurities, cell degradation indicators, and energy efficiency metrics in real-time.
The primary objective of current electrochemical iron cell technology development is to achieve comprehensive process control and real-time monitoring capabilities that maximize efficiency while minimizing energy consumption and environmental impact. Specific goals include developing robust sensors capable of withstanding harsh electrochemical environments, implementing advanced data analytics for process optimization, and creating adaptive control systems that can respond to changing conditions autonomously.
Additional objectives include reducing production costs through improved cell designs, extending operational lifespans through better materials and maintenance protocols, and enhancing safety features to protect both equipment and operators. The industry is also increasingly focused on sustainability goals, including reducing water usage, minimizing waste generation, and lowering the carbon footprint of electrochemical iron production processes.
The technology trajectory suggests future developments will likely incorporate more sophisticated artificial intelligence for autonomous operation, advanced materials for enhanced durability, and comprehensive digital twin modeling for virtual testing and optimization before physical implementation.
The 1990s marked a significant turning point with the introduction of computerized control systems, enabling more precise regulation of electrochemical parameters such as current density, temperature, and electrolyte composition. This period also witnessed the first integration of real-time pH and conductivity sensors, though their reliability and accuracy remained limited in harsh electrochemical environments.
The early 2000s brought miniaturization and enhanced sensor technologies, allowing for more compact cell designs with improved efficiency. Concurrently, data acquisition systems became more sophisticated, enabling continuous monitoring of multiple parameters simultaneously. The development of corrosion-resistant materials during this period also significantly extended cell lifespans and reduced maintenance requirements.
Recent advancements since 2010 have focused on intelligent control systems incorporating machine learning algorithms for predictive maintenance and optimization. Modern electrochemical iron cells now feature integrated sensor networks capable of monitoring not only basic parameters but also electrolyte impurities, cell degradation indicators, and energy efficiency metrics in real-time.
The primary objective of current electrochemical iron cell technology development is to achieve comprehensive process control and real-time monitoring capabilities that maximize efficiency while minimizing energy consumption and environmental impact. Specific goals include developing robust sensors capable of withstanding harsh electrochemical environments, implementing advanced data analytics for process optimization, and creating adaptive control systems that can respond to changing conditions autonomously.
Additional objectives include reducing production costs through improved cell designs, extending operational lifespans through better materials and maintenance protocols, and enhancing safety features to protect both equipment and operators. The industry is also increasingly focused on sustainability goals, including reducing water usage, minimizing waste generation, and lowering the carbon footprint of electrochemical iron production processes.
The technology trajectory suggests future developments will likely incorporate more sophisticated artificial intelligence for autonomous operation, advanced materials for enhanced durability, and comprehensive digital twin modeling for virtual testing and optimization before physical implementation.
Market Analysis for Advanced Electrochemical Process Control Systems
The global market for advanced electrochemical process control systems is experiencing robust growth, driven primarily by increasing industrial automation demands and the rising need for energy-efficient manufacturing processes. The electrochemical iron cell sector represents a significant segment within this market, with an estimated market value exceeding $3.2 billion in 2023 and projected to reach $5.7 billion by 2028, growing at a compound annual growth rate of 12.3%.
Key market drivers include stringent environmental regulations mandating reduced carbon emissions and improved energy efficiency in industrial processes. The electrochemical iron production industry, traditionally energy-intensive, faces mounting pressure to optimize operations while minimizing environmental impact. This regulatory landscape has created substantial demand for sophisticated process control and real-time monitoring solutions.
Regional analysis reveals that Asia-Pacific currently dominates the market, accounting for approximately 42% of global demand. This dominance stems from rapid industrialization in countries like China and India, where steel and iron production capacities continue to expand. North America and Europe follow with market shares of 28% and 23% respectively, with their demand primarily driven by modernization of existing facilities rather than new installations.
End-user segmentation indicates that large-scale iron and steel manufacturers constitute the primary customer base, representing 67% of market revenue. However, medium-sized enterprises are showing the fastest adoption rate growth as advanced control systems become more accessible and scalable.
The competitive landscape features both established industrial automation giants and specialized electrochemical process control providers. Major players include Siemens, ABB, Emerson Electric, and Honeywell in the general automation sector, while specialized firms like Hatch, Outotec, and SMS group offer tailored solutions specifically for electrochemical iron cells.
Customer demand trends highlight increasing preference for integrated solutions that combine process control with predictive maintenance capabilities. Real-time monitoring systems that enable immediate intervention to prevent quality issues or equipment failures are experiencing particularly strong demand growth. Additionally, solutions offering data analytics and artificial intelligence components for process optimization are commanding premium pricing in the market.
Market challenges include high initial implementation costs, technical complexity requiring specialized expertise, and integration difficulties with legacy systems. These factors have created entry barriers for smaller manufacturers, though cloud-based and modular solutions are gradually addressing these limitations.
Key market drivers include stringent environmental regulations mandating reduced carbon emissions and improved energy efficiency in industrial processes. The electrochemical iron production industry, traditionally energy-intensive, faces mounting pressure to optimize operations while minimizing environmental impact. This regulatory landscape has created substantial demand for sophisticated process control and real-time monitoring solutions.
Regional analysis reveals that Asia-Pacific currently dominates the market, accounting for approximately 42% of global demand. This dominance stems from rapid industrialization in countries like China and India, where steel and iron production capacities continue to expand. North America and Europe follow with market shares of 28% and 23% respectively, with their demand primarily driven by modernization of existing facilities rather than new installations.
End-user segmentation indicates that large-scale iron and steel manufacturers constitute the primary customer base, representing 67% of market revenue. However, medium-sized enterprises are showing the fastest adoption rate growth as advanced control systems become more accessible and scalable.
The competitive landscape features both established industrial automation giants and specialized electrochemical process control providers. Major players include Siemens, ABB, Emerson Electric, and Honeywell in the general automation sector, while specialized firms like Hatch, Outotec, and SMS group offer tailored solutions specifically for electrochemical iron cells.
Customer demand trends highlight increasing preference for integrated solutions that combine process control with predictive maintenance capabilities. Real-time monitoring systems that enable immediate intervention to prevent quality issues or equipment failures are experiencing particularly strong demand growth. Additionally, solutions offering data analytics and artificial intelligence components for process optimization are commanding premium pricing in the market.
Market challenges include high initial implementation costs, technical complexity requiring specialized expertise, and integration difficulties with legacy systems. These factors have created entry barriers for smaller manufacturers, though cloud-based and modular solutions are gradually addressing these limitations.
Current Challenges in Iron Cell Monitoring and Control
Electrochemical iron cells face significant monitoring and control challenges that impede their widespread industrial adoption and efficiency optimization. The harsh operating environment within these cells presents substantial obstacles for conventional sensing technologies. Extreme pH conditions, typically highly alkaline or acidic depending on the specific process, rapidly degrade standard sensors and measurement equipment, leading to frequent replacements and unreliable data collection.
Temperature variations across the cell create additional complications, as thermal gradients can reach up to 50°C between different regions. These variations not only affect reaction kinetics but also introduce measurement inconsistencies when attempting to implement uniform control strategies. The presence of hydrogen evolution as a side reaction further complicates monitoring efforts, as gas bubbles interfere with electrode surfaces and sensor readings.
Real-time monitoring of iron ion concentration remains particularly challenging. Current methods often rely on offline sampling and laboratory analysis, creating significant time delays between process changes and corrective actions. Spectroscopic techniques show promise but struggle with the opaque nature of the electrolyte solutions and interference from suspended particles and bubbles.
Electrode degradation presents another critical challenge, as the progressive fouling and passivation of electrodes alter cell performance over time. Without effective real-time monitoring of electrode surface conditions, control systems cannot adequately compensate for these changes, resulting in decreased efficiency and unpredictable performance.
Data integration represents a significant hurdle in developing comprehensive control systems. The diverse parameters requiring monitoring—including temperature, pH, conductivity, iron concentration, impurity levels, and gas evolution rates—generate heterogeneous data streams that must be synchronized and interpreted holistically. Current systems often operate in silos, with limited integration between different monitoring subsystems.
Energy efficiency optimization remains elusive without precise, real-time feedback on cell performance. The inability to accurately measure instantaneous energy consumption relative to iron production rates prevents the implementation of dynamic control strategies that could significantly reduce operational costs and environmental impact.
Scaling issues further complicate matters when transitioning from laboratory to industrial implementation. Monitoring solutions that function effectively in small-scale experimental setups often fail to perform reliably in full-scale industrial cells, where electrode spacing, electrolyte volumes, and current densities differ substantially. This scaling gap has hindered the translation of promising laboratory advances into commercial applications.
Temperature variations across the cell create additional complications, as thermal gradients can reach up to 50°C between different regions. These variations not only affect reaction kinetics but also introduce measurement inconsistencies when attempting to implement uniform control strategies. The presence of hydrogen evolution as a side reaction further complicates monitoring efforts, as gas bubbles interfere with electrode surfaces and sensor readings.
Real-time monitoring of iron ion concentration remains particularly challenging. Current methods often rely on offline sampling and laboratory analysis, creating significant time delays between process changes and corrective actions. Spectroscopic techniques show promise but struggle with the opaque nature of the electrolyte solutions and interference from suspended particles and bubbles.
Electrode degradation presents another critical challenge, as the progressive fouling and passivation of electrodes alter cell performance over time. Without effective real-time monitoring of electrode surface conditions, control systems cannot adequately compensate for these changes, resulting in decreased efficiency and unpredictable performance.
Data integration represents a significant hurdle in developing comprehensive control systems. The diverse parameters requiring monitoring—including temperature, pH, conductivity, iron concentration, impurity levels, and gas evolution rates—generate heterogeneous data streams that must be synchronized and interpreted holistically. Current systems often operate in silos, with limited integration between different monitoring subsystems.
Energy efficiency optimization remains elusive without precise, real-time feedback on cell performance. The inability to accurately measure instantaneous energy consumption relative to iron production rates prevents the implementation of dynamic control strategies that could significantly reduce operational costs and environmental impact.
Scaling issues further complicate matters when transitioning from laboratory to industrial implementation. Monitoring solutions that function effectively in small-scale experimental setups often fail to perform reliably in full-scale industrial cells, where electrode spacing, electrolyte volumes, and current densities differ substantially. This scaling gap has hindered the translation of promising laboratory advances into commercial applications.
State-of-the-Art Process Control Methodologies for Iron Cells
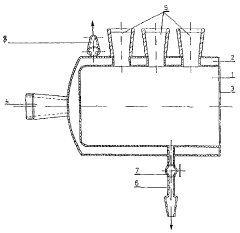
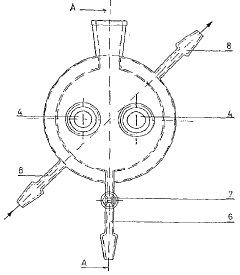
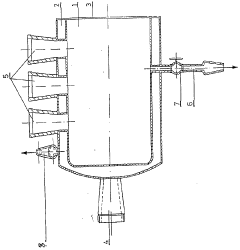
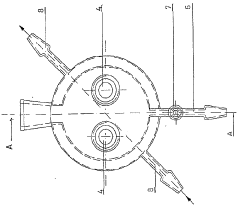
01 Real-time monitoring systems for electrochemical iron cells
Advanced monitoring systems are employed to track the performance of electrochemical iron cells in real-time. These systems utilize sensors and data acquisition technologies to continuously measure critical parameters such as current density, voltage, temperature, and electrolyte composition. Real-time monitoring enables immediate detection of operational anomalies and helps maintain optimal cell performance, thereby improving energy efficiency and product quality in iron production processes.- Real-time monitoring systems for electrochemical iron cells: Advanced monitoring systems are employed to track the performance of electrochemical iron cells in real-time. These systems utilize sensors and data acquisition technologies to continuously measure critical parameters such as current density, voltage, temperature, and electrolyte composition. Real-time monitoring enables immediate detection of operational anomalies and helps maintain optimal cell performance, thereby improving energy efficiency and product quality in electrochemical iron processing.
- Process control algorithms for electrochemical iron production: Sophisticated control algorithms are implemented to regulate electrochemical iron cell operations. These algorithms process data from monitoring systems to make automated adjustments to operating parameters, maintaining optimal conditions throughout the production cycle. Advanced control strategies include predictive modeling, adaptive control, and feedback mechanisms that respond to changes in cell conditions. These algorithms help optimize energy consumption, increase production efficiency, and ensure consistent product quality in electrochemical iron processing.
- Sensor integration and data acquisition for iron cell monitoring: Specialized sensor systems are integrated into electrochemical iron cells to collect comprehensive operational data. These sensors measure various parameters including pH levels, redox potential, iron concentration, and impurity levels in the electrolyte. The data acquisition systems collect, process, and transmit this information to control centers for analysis. Advanced sensor technologies include electrochemical sensors, spectroscopic analyzers, and microelectronic monitoring devices that withstand the harsh conditions of electrochemical cells while providing accurate, continuous measurements.
- Remote monitoring and control interfaces for iron cell operations: Remote monitoring and control interfaces enable operators to oversee and manage electrochemical iron cell operations from centralized control rooms or even off-site locations. These interfaces feature user-friendly dashboards that display real-time data, historical trends, and system alerts. Remote access capabilities allow technical experts to troubleshoot issues, optimize processes, and implement control adjustments without being physically present at the production facility, improving operational efficiency and reducing response time to process deviations.
- Predictive maintenance and fault detection systems: Predictive maintenance systems utilize data analytics and machine learning algorithms to forecast potential equipment failures in electrochemical iron cells before they occur. These systems analyze patterns in operational data to identify early warning signs of degradation or malfunction. Fault detection mechanisms continuously monitor for abnormal conditions and trigger alerts when parameters deviate from acceptable ranges. Implementation of these systems reduces unplanned downtime, extends equipment lifespan, and optimizes maintenance scheduling, resulting in improved overall operational reliability of electrochemical iron processing facilities.
02 Process control algorithms for electrochemical iron production
Sophisticated control algorithms are implemented to regulate electrochemical iron cell operations. These algorithms process data from monitoring systems and adjust operational parameters to maintain optimal conditions. They incorporate predictive modeling and adaptive control strategies to handle process variations and disturbances. Advanced process control systems can automatically adjust electrolyte flow rates, current density, and temperature to optimize energy consumption and ensure consistent product quality in iron electrodeposition processes.Expand Specific Solutions03 Sensor technologies for iron cell parameter measurement
Specialized sensor technologies are developed for accurate measurement of critical parameters in electrochemical iron cells. These include electrochemical sensors for monitoring electrolyte composition, optical sensors for detecting impurities, and advanced electrode sensors for measuring current distribution. The sensors are designed to withstand harsh operating conditions while providing high-precision measurements. Integration of multiple sensor types creates comprehensive monitoring networks that enable detailed analysis of cell performance and early detection of potential issues.Expand Specific Solutions04 Data analytics and visualization for iron cell operations
Data analytics platforms process the large volumes of information generated by electrochemical iron cell monitoring systems. These platforms employ statistical analysis, machine learning algorithms, and pattern recognition to identify trends, predict failures, and optimize operations. Visualization tools present complex operational data in intuitive formats, enabling operators to quickly assess cell status and make informed decisions. Historical data analysis helps identify long-term performance trends and opportunities for process improvement in iron electrodeposition systems.Expand Specific Solutions05 Integration of control systems with production management
Electrochemical iron cell control systems are integrated with broader production management platforms to optimize overall manufacturing efficiency. These integrated systems coordinate cell operations with upstream and downstream processes, manage material flows, and schedule maintenance activities. They incorporate quality control data to ensure product specifications are consistently met. The integration enables comprehensive production optimization, reducing energy consumption and operational costs while maintaining high product quality in iron electrochemical processes.Expand Specific Solutions
Leading Companies in Electrochemical Monitoring Solutions
The electrochemical iron cell process control and real-time monitoring market is in its growth phase, with increasing demand driven by renewable energy storage applications and industrial decarbonization efforts. The competitive landscape features established industrial automation leaders like Siemens AG, Robert Bosch GmbH, and National Instruments providing sophisticated monitoring solutions, alongside specialized battery technology companies such as BYD, Li-Tec Battery, and Saft Groupe. Technical maturity varies significantly, with companies like Tokyo Electron and KLA Corp bringing semiconductor-grade precision monitoring capabilities, while research institutions including Caltech and CNRS contribute cutting-edge innovations. State Grid Corporation of China and its subsidiaries are advancing grid-integration technologies, positioning this sector for substantial growth as electrochemical iron technologies become increasingly central to energy transition strategies.
BYD Co., Ltd.
Technical Solution: BYD has developed an integrated control and monitoring system for electrochemical iron cells that leverages their extensive experience in battery manufacturing. Their solution employs a hierarchical control architecture with three distinct layers: cell-level monitoring, module-level control, and system-level optimization. At the cell level, BYD utilizes proprietary sensors that continuously measure electrolyte conductivity, temperature distribution, and electrode potential with high precision. These sensors are designed to withstand the corrosive environment of iron cells while maintaining measurement accuracy. The module-level control system implements advanced algorithms that dynamically adjust current density and electrolyte flow rates to maintain optimal operating conditions across multiple cells. BYD's system incorporates real-time impedance spectroscopy techniques that can detect early signs of electrode degradation or electrolyte imbalance before they affect production quality. Their cloud-based analytics platform aggregates historical performance data to identify long-term trends and optimize process parameters, resulting in reported energy efficiency improvements of up to 15% compared to conventional control methods.
Strengths: BYD's solution benefits from their extensive practical experience with electrochemical systems at scale, resulting in robust hardware designed for industrial environments. Their integrated approach provides seamless coordination between monitoring and control functions. Weaknesses: The system is somewhat optimized for BYD's own manufacturing processes, potentially requiring significant adaptation for different electrochemical cell designs. Their proprietary sensor technology may create dependency on BYD for maintenance and replacement parts.
National Instruments Corp.
Technical Solution: National Instruments has developed a modular and highly customizable solution for electrochemical iron cell monitoring and control based on their CompactRIO platform. Their system features high-speed data acquisition hardware capable of sampling at rates up to 1 MHz, allowing for detailed analysis of electrochemical reactions in real-time. The solution incorporates specialized signal conditioning modules designed specifically for the harsh electrical environments of electrochemical cells, ensuring accurate measurements even in the presence of significant electromagnetic interference. National Instruments' LabVIEW-based software environment provides a flexible programming interface that enables complex control algorithms to be implemented and modified rapidly. Their system employs a distributed architecture where processing can occur both at the edge (near the cells) and in centralized servers, optimizing response times for critical control functions while enabling comprehensive data analysis. The platform includes advanced visualization tools that present real-time cell performance metrics through customizable dashboards, allowing operators to quickly identify trends and anomalies in the electrochemical process.
Strengths: National Instruments' solution offers exceptional flexibility and customization capabilities, allowing for rapid adaptation to changing process requirements. Their hardware is renowned for measurement accuracy and reliability in industrial environments. Weaknesses: The system requires significant programming expertise to fully utilize its capabilities, potentially necessitating specialized staff. Integration with legacy control systems may require additional engineering effort compared to more standardized industrial control platforms.
Critical Patents in Real-Time Electrochemical Monitoring
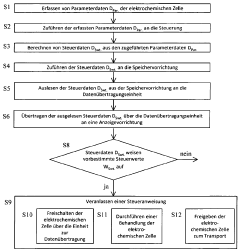
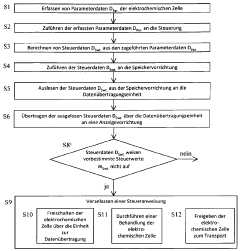
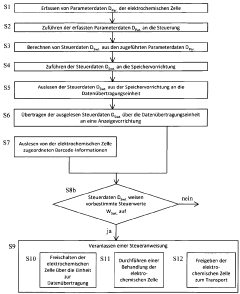
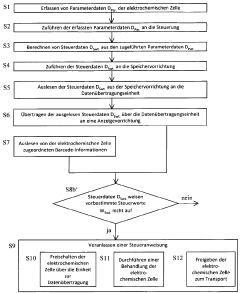
Method for the control and handling of electrochemical cells or batteries, electrochemical cell and battery
PatentWO2012130393A1
Innovation
- A method involving a controller with sensors, non-volatile memory, and data transmission units to acquire, calculate, and transmit parameter and control data wirelessly for display, allowing for real-time monitoring and decision-making during maintenance, including the option to activate or transport cells/batteries based on read data and barcode information.
Horizontal electrochemical cell for electro-optical analysis of electrochemical processes
PatentWO2002033379A1
Innovation
- A horizontal electrochemical cell with a double cylindrical glass container arrangement, featuring coaxial inner and outer bodies, perpendicular lid, and strategically placed tubular penetrations for electrodes, gas bubbler, sensors, and a stop valve, allowing for optical image acquisition and real-time process interpretation.
Energy Efficiency Considerations in Electrochemical Iron Production
Energy efficiency represents a critical factor in the economic and environmental viability of electrochemical iron production processes. Current electrochemical iron cells typically operate at energy efficiency levels between 40-60%, indicating substantial room for improvement. The primary energy consumption occurs during the electrolysis process, where electrical energy converts iron ions to metallic iron at the cathode.
Several factors influence energy efficiency in these systems. Cell voltage optimization stands as a fundamental consideration, with lower voltages generally corresponding to higher efficiency. However, maintaining adequate reaction rates requires balancing voltage reduction against production throughput. Temperature management also plays a crucial role, as operating temperatures affect both electrolyte conductivity and reaction kinetics.
Electrode design and materials significantly impact energy utilization. Advanced electrode materials with higher catalytic activity and conductivity can reduce overpotential requirements. Recent developments in nanostructured electrodes have demonstrated efficiency improvements of 15-20% compared to traditional flat electrodes by increasing active surface area and enhancing mass transfer.
Electrolyte composition represents another critical factor affecting energy consumption. Optimized electrolyte formulations can reduce internal resistance and improve ion mobility. Research indicates that additives such as specific organic compounds can decrease energy requirements by 5-10% through modification of the electrode-electrolyte interface properties.
Process control strategies directly influence energy efficiency. Pulsed current techniques have shown promise in reducing energy consumption by 8-12% compared to constant current operation by minimizing concentration polarization effects. Additionally, precise control of electrolyte flow rates ensures optimal mass transfer while minimizing pumping energy requirements.
Heat recovery systems present significant opportunities for efficiency improvement. Waste heat from electrochemical cells can be captured and repurposed for electrolyte preheating or facility heating, potentially recovering 15-25% of input energy. Integration with renewable energy sources further enhances sustainability, though intermittency challenges must be addressed through energy storage or hybrid systems.
Economic analyses indicate that energy typically represents 30-40% of operational costs in electrochemical iron production. Therefore, efficiency improvements directly translate to cost competitiveness. Furthermore, reduced energy consumption correlates with lower carbon emissions, enhancing environmental sustainability. Industry benchmarks suggest that next-generation electrochemical iron cells should target minimum efficiency levels of 75-80% to achieve economic parity with conventional production methods.
Several factors influence energy efficiency in these systems. Cell voltage optimization stands as a fundamental consideration, with lower voltages generally corresponding to higher efficiency. However, maintaining adequate reaction rates requires balancing voltage reduction against production throughput. Temperature management also plays a crucial role, as operating temperatures affect both electrolyte conductivity and reaction kinetics.
Electrode design and materials significantly impact energy utilization. Advanced electrode materials with higher catalytic activity and conductivity can reduce overpotential requirements. Recent developments in nanostructured electrodes have demonstrated efficiency improvements of 15-20% compared to traditional flat electrodes by increasing active surface area and enhancing mass transfer.
Electrolyte composition represents another critical factor affecting energy consumption. Optimized electrolyte formulations can reduce internal resistance and improve ion mobility. Research indicates that additives such as specific organic compounds can decrease energy requirements by 5-10% through modification of the electrode-electrolyte interface properties.
Process control strategies directly influence energy efficiency. Pulsed current techniques have shown promise in reducing energy consumption by 8-12% compared to constant current operation by minimizing concentration polarization effects. Additionally, precise control of electrolyte flow rates ensures optimal mass transfer while minimizing pumping energy requirements.
Heat recovery systems present significant opportunities for efficiency improvement. Waste heat from electrochemical cells can be captured and repurposed for electrolyte preheating or facility heating, potentially recovering 15-25% of input energy. Integration with renewable energy sources further enhances sustainability, though intermittency challenges must be addressed through energy storage or hybrid systems.
Economic analyses indicate that energy typically represents 30-40% of operational costs in electrochemical iron production. Therefore, efficiency improvements directly translate to cost competitiveness. Furthermore, reduced energy consumption correlates with lower carbon emissions, enhancing environmental sustainability. Industry benchmarks suggest that next-generation electrochemical iron cells should target minimum efficiency levels of 75-80% to achieve economic parity with conventional production methods.
Safety Standards and Compliance for Industrial Electrochemical Systems
Electrochemical iron cell operations must adhere to stringent safety standards and compliance frameworks to ensure worker safety, environmental protection, and operational integrity. The International Electrotechnical Commission (IEC) has established specific guidelines for electrochemical systems, including IEC 61508 for functional safety and IEC 60079 for equipment used in explosive atmospheres, which are particularly relevant for hydrogen generation during iron electrodeposition.
OSHA regulations in the United States mandate comprehensive hazard assessments and implementation of engineering controls for electrochemical processes. These include requirements for proper ventilation systems to manage potentially hazardous gas emissions, emergency shutdown protocols, and regular equipment inspections. The European Union's ATEX Directive similarly governs equipment and protective systems intended for use in potentially explosive atmospheres, directly applicable to electrochemical iron cell operations.
Chemical safety standards such as NFPA 400 (Hazardous Materials Code) provide guidelines for handling the corrosive electrolytes commonly used in iron electrochemical cells. These standards specify containment requirements, spill management procedures, and personal protective equipment specifications for operators. Additionally, ISO 45001 outlines occupational health and safety management systems that facilities must implement to identify hazards and minimize risks associated with electrochemical processes.
Real-time monitoring systems for electrochemical iron cells must comply with IEC 61511 for safety instrumented systems in the process industry. This standard requires that monitoring equipment maintain specific safety integrity levels (SIL) appropriate to the risk profile of the operation. Monitoring systems must undergo rigorous validation and verification processes to ensure reliability in detecting unsafe conditions such as electrolyte leakage, abnormal gas concentrations, or thermal runaway scenarios.
Environmental compliance frameworks, including the EPA's Resource Conservation and Recovery Act (RCRA) in the US and the EU's Industrial Emissions Directive, govern waste management and emissions from electrochemical processes. These regulations establish permissible limits for effluents and require implementation of best available techniques (BAT) for pollution prevention and control in industrial electrochemical operations.
Certification processes for electrochemical iron cell facilities typically involve third-party assessment by organizations such as UL, TÜV, or DNV GL. These assessments verify compliance with applicable standards and often include performance testing under various operational conditions. Regular compliance audits are mandatory in most jurisdictions, with documentation requirements for safety procedures, incident reporting, and corrective actions taken in response to safety events.
OSHA regulations in the United States mandate comprehensive hazard assessments and implementation of engineering controls for electrochemical processes. These include requirements for proper ventilation systems to manage potentially hazardous gas emissions, emergency shutdown protocols, and regular equipment inspections. The European Union's ATEX Directive similarly governs equipment and protective systems intended for use in potentially explosive atmospheres, directly applicable to electrochemical iron cell operations.
Chemical safety standards such as NFPA 400 (Hazardous Materials Code) provide guidelines for handling the corrosive electrolytes commonly used in iron electrochemical cells. These standards specify containment requirements, spill management procedures, and personal protective equipment specifications for operators. Additionally, ISO 45001 outlines occupational health and safety management systems that facilities must implement to identify hazards and minimize risks associated with electrochemical processes.
Real-time monitoring systems for electrochemical iron cells must comply with IEC 61511 for safety instrumented systems in the process industry. This standard requires that monitoring equipment maintain specific safety integrity levels (SIL) appropriate to the risk profile of the operation. Monitoring systems must undergo rigorous validation and verification processes to ensure reliability in detecting unsafe conditions such as electrolyte leakage, abnormal gas concentrations, or thermal runaway scenarios.
Environmental compliance frameworks, including the EPA's Resource Conservation and Recovery Act (RCRA) in the US and the EU's Industrial Emissions Directive, govern waste management and emissions from electrochemical processes. These regulations establish permissible limits for effluents and require implementation of best available techniques (BAT) for pollution prevention and control in industrial electrochemical operations.
Certification processes for electrochemical iron cell facilities typically involve third-party assessment by organizations such as UL, TÜV, or DNV GL. These assessments verify compliance with applicable standards and often include performance testing under various operational conditions. Regular compliance audits are mandatory in most jurisdictions, with documentation requirements for safety procedures, incident reporting, and corrective actions taken in response to safety events.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!