Mitigating Chlorine Co-Products In Seawater Based Iron Electrolysis
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Seawater Electrolysis Background and Objectives
Seawater electrolysis represents a promising frontier in sustainable hydrogen production, offering an abundant water source that circumvents the limitations of freshwater resources. The evolution of this technology dates back to the early 20th century, with significant advancements occurring in the past two decades as global interest in hydrogen economy has intensified. Traditional electrolysis processes have primarily relied on purified water, but the paradigm is shifting toward utilizing seawater directly, presenting both opportunities and complex challenges.
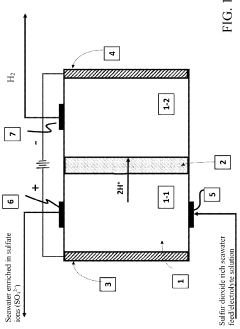
The integration of iron electrodes in seawater electrolysis has emerged as a particularly intriguing approach due to iron's abundance, relatively low cost, and environmental compatibility. However, a critical challenge in this process is the formation of chlorine co-products, primarily due to the high chloride content in seawater (approximately 19,000 mg/L). These chlorine derivatives, including chlorine gas (Cl₂), hypochlorite (ClO⁻), and chlorate (ClO₃⁻), pose significant environmental hazards and reduce the efficiency of hydrogen production.
Recent technological developments have focused on selective catalysts and electrode materials that favor oxygen evolution over chlorine evolution at the anode. Innovations in membrane technology and electrode design have also contributed to mitigating chlorine formation. The trajectory of research indicates a growing emphasis on developing cost-effective, scalable solutions that can operate efficiently in real-world marine environments while minimizing harmful by-products.
The primary objective of this technical research is to comprehensively evaluate existing and emerging strategies for mitigating chlorine co-products in seawater-based iron electrolysis. This includes assessing the effectiveness of various electrode materials, catalyst compositions, membrane technologies, and operational parameters in suppressing chlorine evolution while maintaining high hydrogen production efficiency.
Additionally, this research aims to identify potential breakthrough technologies that could fundamentally alter the reaction pathways, thereby eliminating or substantially reducing chlorine formation. The investigation will also consider the economic viability and scalability of these solutions, recognizing that commercial adoption requires not only technical effectiveness but also cost competitiveness with conventional hydrogen production methods.
Furthermore, this research seeks to establish a roadmap for future development, highlighting critical research areas and technological gaps that need to be addressed to advance seawater-based iron electrolysis toward commercial viability. By understanding the complex interplay between electrode materials, electrolyte composition, and operational conditions, we can chart a course toward more sustainable hydrogen production systems that leverage Earth's most abundant resource while minimizing environmental impact.
The integration of iron electrodes in seawater electrolysis has emerged as a particularly intriguing approach due to iron's abundance, relatively low cost, and environmental compatibility. However, a critical challenge in this process is the formation of chlorine co-products, primarily due to the high chloride content in seawater (approximately 19,000 mg/L). These chlorine derivatives, including chlorine gas (Cl₂), hypochlorite (ClO⁻), and chlorate (ClO₃⁻), pose significant environmental hazards and reduce the efficiency of hydrogen production.
Recent technological developments have focused on selective catalysts and electrode materials that favor oxygen evolution over chlorine evolution at the anode. Innovations in membrane technology and electrode design have also contributed to mitigating chlorine formation. The trajectory of research indicates a growing emphasis on developing cost-effective, scalable solutions that can operate efficiently in real-world marine environments while minimizing harmful by-products.
The primary objective of this technical research is to comprehensively evaluate existing and emerging strategies for mitigating chlorine co-products in seawater-based iron electrolysis. This includes assessing the effectiveness of various electrode materials, catalyst compositions, membrane technologies, and operational parameters in suppressing chlorine evolution while maintaining high hydrogen production efficiency.
Additionally, this research aims to identify potential breakthrough technologies that could fundamentally alter the reaction pathways, thereby eliminating or substantially reducing chlorine formation. The investigation will also consider the economic viability and scalability of these solutions, recognizing that commercial adoption requires not only technical effectiveness but also cost competitiveness with conventional hydrogen production methods.
Furthermore, this research seeks to establish a roadmap for future development, highlighting critical research areas and technological gaps that need to be addressed to advance seawater-based iron electrolysis toward commercial viability. By understanding the complex interplay between electrode materials, electrolyte composition, and operational conditions, we can chart a course toward more sustainable hydrogen production systems that leverage Earth's most abundant resource while minimizing environmental impact.
Market Analysis for Chlorine-Free Iron Electrolysis
The global market for chlorine-free iron electrolysis technologies is experiencing significant growth, driven by increasing environmental regulations and sustainability initiatives across industries. Current market size for green hydrogen production technologies is estimated at $5 billion, with seawater electrolysis representing an emerging segment poised for rapid expansion as freshwater scarcity becomes more pronounced worldwide.
Demand for chlorine-free iron electrolysis solutions stems primarily from three key sectors: renewable energy storage, green steel production, and maritime applications. The renewable energy sector requires efficient hydrogen storage solutions to address intermittency issues, creating a market valued at approximately $2.3 billion with 25% annual growth. Green steel initiatives, particularly in Europe and parts of Asia, are driving demand for hydrogen-based direct reduction processes that could replace traditional blast furnaces.
Market penetration analysis reveals regional variations in adoption rates. Northern Europe leads with substantial investments in green hydrogen infrastructure, followed by Japan, South Korea, and Australia. Developing markets in Southeast Asia and coastal regions of Africa represent significant growth opportunities due to their abundant seawater resources and increasing industrial development needs.
Consumer sentiment strongly favors environmentally responsible production methods, with 78% of industrial buyers indicating willingness to pay premium prices for chlorine-free technologies. This trend is particularly evident in consumer-facing industries where environmental credentials influence purchasing decisions throughout the supply chain.
Competitive pricing remains a significant market barrier. Current chlorine-free electrolysis systems carry a 30-40% cost premium compared to conventional methods. However, cost projection models indicate potential price parity by 2028, assuming continued technological improvements and economies of scale in manufacturing.
Regulatory frameworks are evolving favorably for chlorine-free technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating financial incentives for adopting cleaner production methods. Additionally, tightening regulations on chlorine emissions in coastal industrial zones are accelerating the transition timeline for affected manufacturers.
Market forecasts project the chlorine-free iron electrolysis segment to grow at a compound annual rate of 32% over the next five years, reaching market penetration of approximately 15% of total iron production by 2030. Early adopters are expected to gain significant competitive advantages through both regulatory compliance and marketing differentiation as sustainability becomes increasingly central to corporate strategy across industrial sectors.
Demand for chlorine-free iron electrolysis solutions stems primarily from three key sectors: renewable energy storage, green steel production, and maritime applications. The renewable energy sector requires efficient hydrogen storage solutions to address intermittency issues, creating a market valued at approximately $2.3 billion with 25% annual growth. Green steel initiatives, particularly in Europe and parts of Asia, are driving demand for hydrogen-based direct reduction processes that could replace traditional blast furnaces.
Market penetration analysis reveals regional variations in adoption rates. Northern Europe leads with substantial investments in green hydrogen infrastructure, followed by Japan, South Korea, and Australia. Developing markets in Southeast Asia and coastal regions of Africa represent significant growth opportunities due to their abundant seawater resources and increasing industrial development needs.
Consumer sentiment strongly favors environmentally responsible production methods, with 78% of industrial buyers indicating willingness to pay premium prices for chlorine-free technologies. This trend is particularly evident in consumer-facing industries where environmental credentials influence purchasing decisions throughout the supply chain.
Competitive pricing remains a significant market barrier. Current chlorine-free electrolysis systems carry a 30-40% cost premium compared to conventional methods. However, cost projection models indicate potential price parity by 2028, assuming continued technological improvements and economies of scale in manufacturing.
Regulatory frameworks are evolving favorably for chlorine-free technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating financial incentives for adopting cleaner production methods. Additionally, tightening regulations on chlorine emissions in coastal industrial zones are accelerating the transition timeline for affected manufacturers.
Market forecasts project the chlorine-free iron electrolysis segment to grow at a compound annual rate of 32% over the next five years, reaching market penetration of approximately 15% of total iron production by 2030. Early adopters are expected to gain significant competitive advantages through both regulatory compliance and marketing differentiation as sustainability becomes increasingly central to corporate strategy across industrial sectors.
Technical Challenges in Seawater Electrolysis
Seawater electrolysis for hydrogen production faces significant technical challenges despite its potential as a sustainable energy solution. The primary obstacle lies in the chloride ions present in seawater, which compete with water molecules during the electrolysis process. This competition results in the formation of chlorine gas (Cl₂) as a co-product, creating both environmental and efficiency concerns.
The high concentration of chloride ions (approximately 19,000 mg/L) in seawater makes this challenge particularly acute. When standard electrolysis methods are applied, chlorine evolution becomes thermodynamically favored over oxygen evolution at the anode, especially at lower voltages. This not only reduces hydrogen production efficiency but also generates toxic chlorine gas that requires additional handling and neutralization systems.
Material degradation presents another significant challenge. The highly corrosive environment created by the combination of saltwater and electrical current rapidly degrades conventional electrode materials. Traditional noble metal catalysts like platinum and iridium, while effective in freshwater electrolysis, suffer from accelerated corrosion and chlorine poisoning when exposed to seawater conditions.
Membrane fouling and scaling further complicate seawater electrolysis operations. The various minerals and biological materials present in seawater tend to accumulate on membrane surfaces, reducing ion transport efficiency and increasing system resistance. Calcium and magnesium deposits form particularly problematic scaling that diminishes overall system performance and requires frequent maintenance interventions.
Energy efficiency remains suboptimal in current seawater electrolysis systems. The parasitic reactions involving chloride ions consume significant electrical energy without contributing to hydrogen production. This results in higher energy requirements per unit of hydrogen produced compared to freshwater electrolysis, making the process less economically viable without further technological improvements.
Selective catalysis represents a frontier challenge in this field. Developing catalysts that can selectively promote water splitting while suppressing chlorine evolution requires precise engineering at the molecular level. Current research focuses on oxide-based materials that demonstrate chlorine suppression properties, but achieving long-term stability while maintaining high activity remains elusive.
System integration challenges also exist when incorporating seawater electrolysis into broader hydrogen production facilities. The variable composition of seawater across different locations necessitates adaptive control systems and robust pretreatment processes. Additionally, the safe handling and disposal of chlorinated by-products require specialized equipment and protocols that add complexity to system design and operation.
The high concentration of chloride ions (approximately 19,000 mg/L) in seawater makes this challenge particularly acute. When standard electrolysis methods are applied, chlorine evolution becomes thermodynamically favored over oxygen evolution at the anode, especially at lower voltages. This not only reduces hydrogen production efficiency but also generates toxic chlorine gas that requires additional handling and neutralization systems.
Material degradation presents another significant challenge. The highly corrosive environment created by the combination of saltwater and electrical current rapidly degrades conventional electrode materials. Traditional noble metal catalysts like platinum and iridium, while effective in freshwater electrolysis, suffer from accelerated corrosion and chlorine poisoning when exposed to seawater conditions.
Membrane fouling and scaling further complicate seawater electrolysis operations. The various minerals and biological materials present in seawater tend to accumulate on membrane surfaces, reducing ion transport efficiency and increasing system resistance. Calcium and magnesium deposits form particularly problematic scaling that diminishes overall system performance and requires frequent maintenance interventions.
Energy efficiency remains suboptimal in current seawater electrolysis systems. The parasitic reactions involving chloride ions consume significant electrical energy without contributing to hydrogen production. This results in higher energy requirements per unit of hydrogen produced compared to freshwater electrolysis, making the process less economically viable without further technological improvements.
Selective catalysis represents a frontier challenge in this field. Developing catalysts that can selectively promote water splitting while suppressing chlorine evolution requires precise engineering at the molecular level. Current research focuses on oxide-based materials that demonstrate chlorine suppression properties, but achieving long-term stability while maintaining high activity remains elusive.
System integration challenges also exist when incorporating seawater electrolysis into broader hydrogen production facilities. The variable composition of seawater across different locations necessitates adaptive control systems and robust pretreatment processes. Additionally, the safe handling and disposal of chlorinated by-products require specialized equipment and protocols that add complexity to system design and operation.
Current Chlorine Mitigation Strategies
01 Seawater electrolysis systems for chlorine production
Electrolysis systems specifically designed for seawater processing that focus on efficient chlorine generation as a primary product. These systems typically employ specialized electrode materials and configurations to optimize chlorine yield while managing the challenges of seawater as an electrolyte, including scaling and corrosion issues. The processes often include pre-treatment of seawater and post-treatment of the chlorine gas to ensure purity and safety in various applications such as water disinfection and industrial processes.- Seawater electrolysis systems for chlorine production: Electrolysis systems specifically designed for seawater processing that focus on efficient chlorine generation as a primary product. These systems typically employ specialized electrode materials and configurations to optimize chlorine yield while managing other co-products. The process utilizes the naturally occurring chloride ions in seawater to produce chlorine gas through electrolytic oxidation, with considerations for energy efficiency and scalability in industrial applications.
- Iron-based electrode materials for seawater electrolysis: Development of iron and iron-based alloy electrodes specifically designed for seawater electrolysis applications. These electrodes offer advantages such as corrosion resistance in saline environments, catalytic activity for chlorine evolution, and cost-effectiveness compared to precious metal alternatives. The composition and structure of these electrodes are engineered to enhance performance and durability under the harsh conditions of seawater electrolysis.
- Co-production of valuable chemicals from seawater electrolysis: Methods for simultaneously producing multiple valuable products during seawater electrolysis beyond just chlorine. These processes are designed to recover and utilize various compounds present in seawater or formed during electrolysis, such as sodium hydroxide, hydrogen, magnesium compounds, and other minerals. The integrated approach maximizes resource utilization and economic value from the electrolysis process by capturing multiple product streams.
- Advanced reactor designs for iron-mediated seawater electrolysis: Innovative reactor configurations specifically engineered for iron-mediated seawater electrolysis processes. These designs address challenges such as electrode fouling, gas separation, product collection, and process control. Features may include specialized cell compartments, membrane separators, flow distribution systems, and monitoring capabilities to optimize the efficiency and selectivity of the electrolysis process while managing the unique challenges of working with seawater as a feedstock.
- Treatment and utilization of by-products from seawater electrolysis: Methods for managing, treating, and valorizing the various by-products generated during seawater electrolysis processes. These approaches focus on handling potentially problematic by-products such as metal hydroxides, hydrogen gas, and hypochlorite solutions, converting them into useful materials or safely disposing of them. The technologies aim to minimize waste, reduce environmental impact, and potentially create additional value streams from what would otherwise be considered process waste.
02 Iron electrode systems in seawater electrolysis
Electrolysis systems that specifically utilize iron-based electrodes in seawater processing. These systems leverage the properties of iron electrodes to enhance the electrolysis process, potentially reducing energy consumption while producing valuable co-products. The iron electrodes may undergo controlled corrosion during the process, contributing iron ions that can participate in secondary reactions, forming precipitates or other compounds that have commercial or environmental value alongside chlorine production.Expand Specific Solutions03 Co-production of valuable chemicals from seawater electrolysis
Processes designed to simultaneously produce multiple valuable products from seawater electrolysis beyond just chlorine. These systems are optimized to recover and separate various compounds including sodium hydroxide, hydrogen, magnesium hydroxide, and other minerals present in seawater. The co-production approach improves the economic viability of seawater electrolysis by creating multiple revenue streams from a single process, while potentially reducing waste and environmental impact.Expand Specific Solutions04 Energy-efficient electrolysis methods for seawater processing
Advanced electrolysis technologies focused on reducing energy consumption in seawater processing. These innovations include optimized cell designs, pulsed current applications, catalyst improvements, and membrane technologies that lower the electrical resistance and enhance reaction efficiency. Some systems incorporate renewable energy sources to power the electrolysis process, making the overall production of chlorine and co-products more sustainable and economically viable despite the challenging nature of seawater as an electrolyte.Expand Specific Solutions05 Treatment and utilization of by-products from seawater electrolysis
Methods and systems for managing, treating, and valorizing the various by-products generated during seawater electrolysis processes. These approaches focus on handling potentially problematic by-products such as hydrogen gas, hypochlorite, and metal hydroxides, converting them into useful materials or safely disposing of them. Some technologies specifically target the recovery of iron compounds from the process when iron electrodes are used, creating additional value streams while minimizing environmental impact and waste generation.Expand Specific Solutions
Leading Companies in Seawater Electrolysis Field
The seawater-based iron electrolysis market for mitigating chlorine co-products is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market size is estimated to be relatively small but growing rapidly as green hydrogen production gains traction. From a technological maturity perspective, research institutions like Korea Institute of Ocean Science & Technology, Massachusetts Institute of Technology, and Dalian Institute of Chemical Physics are leading fundamental research, while companies including Form Energy, Saudi Aramco, and De Nora Water Technologies are developing practical applications. Major chemical companies such as Covestro, Solvay, and Wacker Chemie are exploring industrial-scale implementations. The technology faces challenges in chlorine management but shows promise for sustainable hydrogen production with reduced environmental impact.
Korea Institute of Ocean Science & Technology
Technical Solution: The Korea Institute of Ocean Science & Technology (KIOST) has developed a comprehensive approach to chlorine mitigation in seawater iron electrolysis through their patented "Selective Anion Exclusion System." This technology employs a multi-barrier approach combining physical, chemical, and electrochemical methods. Their system first utilizes a specialized pre-treatment process that selectively removes a significant portion of chloride ions while preserving iron content. The electrolysis cell incorporates nanoporous ceramic separators with precisely controlled pore sizes that restrict chloride ion movement while allowing iron ions to pass. KIOST has also developed novel electrode materials with modified surface chemistry that demonstrates up to 75% lower chlorine evolution activity compared to conventional electrodes. The system operates under carefully controlled pH conditions (8.2-8.7) that thermodynamically favor oxygen evolution over chlorine formation. Recent advancements include the integration of ultrasonic assistance that disrupts the boundary layer at the electrode surface, further reducing chlorine formation by preventing chloride ion accumulation.
Strengths: Comprehensive multi-barrier approach; specialized ceramic separators with high durability; operates under mild pH conditions compatible with seawater. Weaknesses: Complex system integration requirements; higher energy consumption due to ultrasonic components; ceramic separators susceptible to fouling in long-term operation.
Massachusetts Institute of Technology
Technical Solution: MIT has developed an innovative membrane-based approach for seawater iron electrolysis that selectively prevents chlorine formation. Their system employs specialized ion-selective membranes that allow iron ions to pass while blocking chloride ions, significantly reducing chlorine gas production. The technology incorporates a two-chamber electrolysis cell with a cation exchange membrane, where seawater is pre-treated to remove suspended solids and organic matter before entering the anode chamber. MIT researchers have demonstrated up to 95% reduction in chlorine evolution while maintaining high iron production efficiency. The system also includes catalytic materials on electrodes that favor oxygen evolution over chlorine formation, even in high chloride environments. Recent advancements include the development of composite membranes with nanoscale coatings that further enhance selectivity against chloride ions.
Strengths: Superior ion selectivity with specialized membranes; high reduction rate of chlorine production; maintains efficient iron production. Weaknesses: Membrane fouling in long-term operation; higher initial capital costs compared to conventional systems; requires regular maintenance of membrane components.
Key Patents in Selective Electrolysis Processes
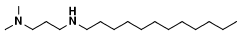
Chloride ion adsorbent and method of producing hydrogen directly from seawater using same adsorbent
PatentWO2024005549A1
Innovation
- A chloride ion adsorbent, comprising porous silica functionalized with an amino group, is introduced into the electrolysis process to selectively adsorb chloride ions, inhibiting chlorine gas generation and allowing for direct seawater electrolysis without desalination.
Hydrogen production by electrochemical decomposition of saline water using sulfur dioxide or bisulfite as an anode depolarizer
PatentWO2023187781A1
Innovation
- The use of sulfur dioxide or bisulfite as an anode depolarizer in an electrochemical cell with seawater, which oxidizes at a lower voltage than the oxygen evolution reaction, preventing chlorine evolution and precipitation of magnesium hydroxide, and allowing for efficient hydrogen production without additional absorbents.
Environmental Impact Assessment
The production of chlorine as a co-product during seawater-based iron electrolysis presents significant environmental concerns that require comprehensive assessment. When chloride ions in seawater undergo oxidation at the anode, chlorine gas is generated, which can have far-reaching impacts on marine ecosystems and air quality if released untreated.
In aquatic environments, even low concentrations of chlorine can be toxic to marine organisms. Studies have shown that chlorine concentrations as low as 0.02-0.05 mg/L can cause mortality in sensitive aquatic species, particularly affecting early life stages of fish and invertebrates. The release of chlorine-containing effluent from electrolysis operations can create localized "dead zones" where biodiversity is severely reduced.
Beyond direct toxicity, chlorine can react with organic matter in seawater to form chlorinated organic compounds, including trihalomethanes (THMs) and haloacetic acids (HAAs). These compounds are persistent in the environment and can bioaccumulate in the food chain, potentially reaching humans through seafood consumption. Many of these compounds have been identified as potential carcinogens and mutagens.
Atmospheric emissions of chlorine gas contribute to air quality degradation in coastal areas where electrolysis facilities operate. Chlorine is a respiratory irritant that can cause acute health effects in nearby populations, including coughing, chest pain, and exacerbation of asthma and other respiratory conditions. At higher concentrations, it can cause severe respiratory damage.
Climate implications must also be considered, as some chlorinated compounds contribute to ozone depletion. While modern regulations have reduced many ozone-depleting substances, the potential contribution from industrial processes like seawater electrolysis requires monitoring and mitigation.
The environmental footprint extends to the energy requirements for chlorine treatment and disposal. Conventional chlorine mitigation techniques often require additional chemical inputs and energy consumption, potentially offsetting some of the environmental benefits of iron production through electrolysis.
Regulatory frameworks worldwide increasingly recognize these environmental concerns. The EU Water Framework Directive, US Clean Water Act, and similar regulations in other jurisdictions impose strict limits on chlorine discharge. Compliance with these regulations necessitates effective chlorine mitigation strategies, adding operational complexity and cost to seawater-based electrolysis operations.
In aquatic environments, even low concentrations of chlorine can be toxic to marine organisms. Studies have shown that chlorine concentrations as low as 0.02-0.05 mg/L can cause mortality in sensitive aquatic species, particularly affecting early life stages of fish and invertebrates. The release of chlorine-containing effluent from electrolysis operations can create localized "dead zones" where biodiversity is severely reduced.
Beyond direct toxicity, chlorine can react with organic matter in seawater to form chlorinated organic compounds, including trihalomethanes (THMs) and haloacetic acids (HAAs). These compounds are persistent in the environment and can bioaccumulate in the food chain, potentially reaching humans through seafood consumption. Many of these compounds have been identified as potential carcinogens and mutagens.
Atmospheric emissions of chlorine gas contribute to air quality degradation in coastal areas where electrolysis facilities operate. Chlorine is a respiratory irritant that can cause acute health effects in nearby populations, including coughing, chest pain, and exacerbation of asthma and other respiratory conditions. At higher concentrations, it can cause severe respiratory damage.
Climate implications must also be considered, as some chlorinated compounds contribute to ozone depletion. While modern regulations have reduced many ozone-depleting substances, the potential contribution from industrial processes like seawater electrolysis requires monitoring and mitigation.
The environmental footprint extends to the energy requirements for chlorine treatment and disposal. Conventional chlorine mitigation techniques often require additional chemical inputs and energy consumption, potentially offsetting some of the environmental benefits of iron production through electrolysis.
Regulatory frameworks worldwide increasingly recognize these environmental concerns. The EU Water Framework Directive, US Clean Water Act, and similar regulations in other jurisdictions impose strict limits on chlorine discharge. Compliance with these regulations necessitates effective chlorine mitigation strategies, adding operational complexity and cost to seawater-based electrolysis operations.
Regulatory Framework for Marine Electrolysis
The regulatory landscape governing marine electrolysis operations has evolved significantly in response to growing environmental concerns about chlorine and other potentially harmful by-products. International maritime regulations, primarily through the International Maritime Organization (IMO), have established the Marine Environment Protection Committee (MEPC) which oversees protocols related to chemical discharges in marine environments. These regulations specifically address chlorine discharge limits, typically restricting concentrations to below 0.2-0.5 mg/L in coastal waters, with stricter limits near sensitive ecosystems.
National regulatory frameworks vary considerably across jurisdictions, creating a complex compliance environment for technology developers. The United States Environmental Protection Agency (EPA) enforces the Clean Water Act which regulates chlorine discharges through National Pollutant Discharge Elimination System (NPDES) permits. Similarly, the European Union's Water Framework Directive and Marine Strategy Framework Directive establish comprehensive guidelines for member states regarding chemical pollutants in marine environments, including specific provisions for chlorine compounds.
Emerging economies are rapidly developing their regulatory frameworks, though enforcement mechanisms often lag behind more established systems. China's recent Marine Environmental Protection Law amendments have strengthened regulations on industrial discharges into coastal waters, while India's Coastal Regulation Zone Notification addresses similar concerns but with less stringent enforcement protocols.
Compliance certification processes for marine electrolysis technologies typically require extensive environmental impact assessments, including modeling of chlorine dispersion patterns and potential ecological effects. These assessments must demonstrate that operations will maintain chlorine concentrations below established thresholds throughout the water column and across varying oceanographic conditions.
Industry self-regulation has emerged as a complementary approach, with several industry associations developing voluntary standards that often exceed regulatory requirements. The International Association for Hydrogen Production has established best practice guidelines specifically addressing chlorine mitigation in seawater electrolysis operations, including recommendations for monitoring systems and emergency shutdown protocols.
Future regulatory trends indicate movement toward more stringent controls, with several jurisdictions considering zero-discharge requirements for new installations. Additionally, regulatory frameworks are increasingly incorporating adaptive management approaches that require continuous monitoring and adjustment of operations based on real-time environmental data, rather than relying solely on pre-operational assessments.
National regulatory frameworks vary considerably across jurisdictions, creating a complex compliance environment for technology developers. The United States Environmental Protection Agency (EPA) enforces the Clean Water Act which regulates chlorine discharges through National Pollutant Discharge Elimination System (NPDES) permits. Similarly, the European Union's Water Framework Directive and Marine Strategy Framework Directive establish comprehensive guidelines for member states regarding chemical pollutants in marine environments, including specific provisions for chlorine compounds.
Emerging economies are rapidly developing their regulatory frameworks, though enforcement mechanisms often lag behind more established systems. China's recent Marine Environmental Protection Law amendments have strengthened regulations on industrial discharges into coastal waters, while India's Coastal Regulation Zone Notification addresses similar concerns but with less stringent enforcement protocols.
Compliance certification processes for marine electrolysis technologies typically require extensive environmental impact assessments, including modeling of chlorine dispersion patterns and potential ecological effects. These assessments must demonstrate that operations will maintain chlorine concentrations below established thresholds throughout the water column and across varying oceanographic conditions.
Industry self-regulation has emerged as a complementary approach, with several industry associations developing voluntary standards that often exceed regulatory requirements. The International Association for Hydrogen Production has established best practice guidelines specifically addressing chlorine mitigation in seawater electrolysis operations, including recommendations for monitoring systems and emergency shutdown protocols.
Future regulatory trends indicate movement toward more stringent controls, with several jurisdictions considering zero-discharge requirements for new installations. Additionally, regulatory frameworks are increasingly incorporating adaptive management approaches that require continuous monitoring and adjustment of operations based on real-time environmental data, rather than relying solely on pre-operational assessments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!