Reactor Configurations For Continuous Iron Electrowinning
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron Electrowinning Technology Background and Objectives
Iron electrowinning represents a significant advancement in metallurgical extraction processes, evolving from traditional pyrometallurgical methods to more environmentally sustainable electrochemical approaches. The technology has roots dating back to the early 20th century but has seen substantial development in recent decades due to increasing environmental regulations and energy efficiency requirements. Iron electrowinning specifically involves the electrochemical reduction of iron ions from solution to produce metallic iron at the cathode, offering a direct route from solution to metal without the intermediate steps required in conventional blast furnace operations.
The evolution of iron electrowinning technology has been marked by several key developments, including improvements in electrode materials, cell design, and electrolyte composition. Early systems suffered from low current efficiencies, electrode deterioration, and product contamination issues. Modern approaches have increasingly focused on continuous processing capabilities, which represent a significant departure from batch processing methods that dominated earlier implementations.
The primary technical objectives in iron electrowinning development center on achieving higher current efficiencies, reducing energy consumption, improving product purity, and establishing stable continuous operation. These objectives align with broader industry goals of reducing carbon emissions and operational costs while maintaining or enhancing product quality. Continuous electrowinning processes offer particular advantages in terms of production consistency, automation potential, and reduced labor requirements.
Reactor configuration represents a critical aspect of iron electrowinning technology advancement. Various designs have emerged, including parallel plate cells, fluidized bed electrodes, and more recently, membrane-separated compartments that allow for better control of electrolyte conditions. The configuration directly impacts mass transfer efficiency, current distribution, and ultimately the morphology and purity of the deposited iron.
Current research and development efforts are increasingly focused on overcoming the challenges associated with hydrogen evolution, which competes with iron deposition and reduces current efficiency. Additionally, significant attention is being directed toward scaling up laboratory successes to industrial implementation, particularly in continuous operation modes that can integrate with existing production systems.
The technology trajectory suggests a convergence toward hybrid systems that combine the advantages of electrowinning with complementary processes, potentially offering more comprehensive solutions for iron production and recycling. As global steel production continues to face pressure to reduce its environmental footprint, electrowinning technologies present a promising alternative pathway that aligns with sustainability objectives while maintaining economic viability.
The evolution of iron electrowinning technology has been marked by several key developments, including improvements in electrode materials, cell design, and electrolyte composition. Early systems suffered from low current efficiencies, electrode deterioration, and product contamination issues. Modern approaches have increasingly focused on continuous processing capabilities, which represent a significant departure from batch processing methods that dominated earlier implementations.
The primary technical objectives in iron electrowinning development center on achieving higher current efficiencies, reducing energy consumption, improving product purity, and establishing stable continuous operation. These objectives align with broader industry goals of reducing carbon emissions and operational costs while maintaining or enhancing product quality. Continuous electrowinning processes offer particular advantages in terms of production consistency, automation potential, and reduced labor requirements.
Reactor configuration represents a critical aspect of iron electrowinning technology advancement. Various designs have emerged, including parallel plate cells, fluidized bed electrodes, and more recently, membrane-separated compartments that allow for better control of electrolyte conditions. The configuration directly impacts mass transfer efficiency, current distribution, and ultimately the morphology and purity of the deposited iron.
Current research and development efforts are increasingly focused on overcoming the challenges associated with hydrogen evolution, which competes with iron deposition and reduces current efficiency. Additionally, significant attention is being directed toward scaling up laboratory successes to industrial implementation, particularly in continuous operation modes that can integrate with existing production systems.
The technology trajectory suggests a convergence toward hybrid systems that combine the advantages of electrowinning with complementary processes, potentially offering more comprehensive solutions for iron production and recycling. As global steel production continues to face pressure to reduce its environmental footprint, electrowinning technologies present a promising alternative pathway that aligns with sustainability objectives while maintaining economic viability.
Market Analysis for Continuous Iron Electrowinning
The global market for continuous iron electrowinning technology is experiencing significant growth, driven primarily by increasing demand for high-purity iron products across various industrial applications. The market size for iron electrowinning equipment and services was valued at approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate of 6.8% through 2030.
Key market segments for continuous iron electrowinning include the steel manufacturing industry, where electrowinning processes offer advantages in producing specialized high-purity iron products with reduced environmental impact compared to traditional blast furnace methods. The electronics industry represents another substantial market segment, utilizing high-purity iron in various components and specialized applications.
Geographically, Asia-Pacific dominates the market landscape, accounting for over 45% of global demand, with China being the primary contributor due to its massive steel production capacity and government initiatives promoting cleaner production technologies. North America and Europe follow, with growing adoption rates driven by stringent environmental regulations and the push toward greener manufacturing processes.
Market dynamics are significantly influenced by environmental factors, as continuous electrowinning processes typically generate fewer emissions compared to conventional pyrometallurgical methods. This environmental advantage is becoming increasingly valuable as regulatory frameworks worldwide impose stricter emissions standards on industrial operations.
Economic factors also play a crucial role in market development. While initial capital investment for continuous electrowinning systems remains relatively high, operational costs can be lower over time due to energy efficiency improvements and reduced waste management requirements. The return on investment timeline typically ranges from 3-5 years, depending on scale and implementation specifics.
Customer demand patterns indicate growing interest in modular and scalable reactor configurations that can be adapted to varying production requirements. This flexibility is particularly valued by mid-sized manufacturers seeking to optimize capital expenditure while maintaining production versatility.
Market barriers include technological complexity, high initial investment costs, and competition from established conventional processes. However, these barriers are gradually diminishing as technological advancements improve efficiency and reduce implementation costs.
Future market growth is expected to be driven by technological innovations in reactor design, electrode materials, and process control systems that enhance energy efficiency and production rates while minimizing operational disruptions.
Key market segments for continuous iron electrowinning include the steel manufacturing industry, where electrowinning processes offer advantages in producing specialized high-purity iron products with reduced environmental impact compared to traditional blast furnace methods. The electronics industry represents another substantial market segment, utilizing high-purity iron in various components and specialized applications.
Geographically, Asia-Pacific dominates the market landscape, accounting for over 45% of global demand, with China being the primary contributor due to its massive steel production capacity and government initiatives promoting cleaner production technologies. North America and Europe follow, with growing adoption rates driven by stringent environmental regulations and the push toward greener manufacturing processes.
Market dynamics are significantly influenced by environmental factors, as continuous electrowinning processes typically generate fewer emissions compared to conventional pyrometallurgical methods. This environmental advantage is becoming increasingly valuable as regulatory frameworks worldwide impose stricter emissions standards on industrial operations.
Economic factors also play a crucial role in market development. While initial capital investment for continuous electrowinning systems remains relatively high, operational costs can be lower over time due to energy efficiency improvements and reduced waste management requirements. The return on investment timeline typically ranges from 3-5 years, depending on scale and implementation specifics.
Customer demand patterns indicate growing interest in modular and scalable reactor configurations that can be adapted to varying production requirements. This flexibility is particularly valued by mid-sized manufacturers seeking to optimize capital expenditure while maintaining production versatility.
Market barriers include technological complexity, high initial investment costs, and competition from established conventional processes. However, these barriers are gradually diminishing as technological advancements improve efficiency and reduce implementation costs.
Future market growth is expected to be driven by technological innovations in reactor design, electrode materials, and process control systems that enhance energy efficiency and production rates while minimizing operational disruptions.
Current Reactor Technologies and Technical Barriers
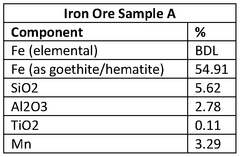
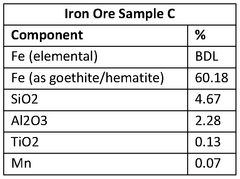
The current landscape of iron electrowinning reactor technologies presents a diverse array of configurations, each with distinct advantages and limitations. Conventional cell designs predominantly utilize parallel-plate electrodes arranged in monopolar or bipolar configurations. Monopolar systems offer operational simplicity and maintenance accessibility but suffer from lower space-time yields. Bipolar arrangements achieve higher production densities through more efficient space utilization, though they introduce challenges in current distribution and electrode connection complexity.
Filter-press reactors represent a significant advancement, featuring compressed electrode assemblies with minimal inter-electrode gaps. These systems demonstrate enhanced mass transfer characteristics and reduced ohmic losses, resulting in energy efficiency improvements of 15-25% compared to traditional designs. However, they face challenges in scaling beyond certain capacities due to pressure drop considerations and flow distribution complexities across larger electrode surfaces.
Fluidized bed electrochemical reactors have emerged as promising alternatives, particularly for processing iron-containing solutions with suspended solids. These systems utilize particulate electrodes in a fluidized state, offering exceptional mass transfer rates and tolerance to solution impurities. Despite these advantages, they struggle with current efficiency limitations and electrode particle management during continuous operation.
A critical technical barrier across all configurations remains the management of hydrogen evolution as a competing cathodic reaction. This parasitic process not only reduces current efficiency but also creates safety hazards and affects deposit morphology. Current mitigation strategies include specialized membrane separators and electrolyte additives, though none have achieved comprehensive resolution of this challenge.
Membrane technology integration presents another significant hurdle. Ion-exchange membranes can effectively separate anolyte and catholyte compartments, preventing cross-contamination and oxidation of ferrous to ferric iron. However, these membranes suffer from fouling in industrial environments and contribute substantially to overall cell resistance, increasing energy consumption by 20-30% in many implementations.
Scale formation and electrode passivation represent persistent operational challenges. Iron hydroxide precipitation on cathode surfaces progressively reduces active area and increases cell voltage. While periodic reverse current pulses and chemical cleaning protocols offer temporary remediation, they interrupt continuous operation and introduce system complexity.
Heat management emerges as a critical consideration in high-current-density operations. Excessive temperature increases accelerate side reactions and membrane degradation while potentially compromising deposit quality. Current cooling strategies rely primarily on electrolyte recirculation through external heat exchangers, introducing additional pumping requirements and system complexity.
Filter-press reactors represent a significant advancement, featuring compressed electrode assemblies with minimal inter-electrode gaps. These systems demonstrate enhanced mass transfer characteristics and reduced ohmic losses, resulting in energy efficiency improvements of 15-25% compared to traditional designs. However, they face challenges in scaling beyond certain capacities due to pressure drop considerations and flow distribution complexities across larger electrode surfaces.
Fluidized bed electrochemical reactors have emerged as promising alternatives, particularly for processing iron-containing solutions with suspended solids. These systems utilize particulate electrodes in a fluidized state, offering exceptional mass transfer rates and tolerance to solution impurities. Despite these advantages, they struggle with current efficiency limitations and electrode particle management during continuous operation.
A critical technical barrier across all configurations remains the management of hydrogen evolution as a competing cathodic reaction. This parasitic process not only reduces current efficiency but also creates safety hazards and affects deposit morphology. Current mitigation strategies include specialized membrane separators and electrolyte additives, though none have achieved comprehensive resolution of this challenge.
Membrane technology integration presents another significant hurdle. Ion-exchange membranes can effectively separate anolyte and catholyte compartments, preventing cross-contamination and oxidation of ferrous to ferric iron. However, these membranes suffer from fouling in industrial environments and contribute substantially to overall cell resistance, increasing energy consumption by 20-30% in many implementations.
Scale formation and electrode passivation represent persistent operational challenges. Iron hydroxide precipitation on cathode surfaces progressively reduces active area and increases cell voltage. While periodic reverse current pulses and chemical cleaning protocols offer temporary remediation, they interrupt continuous operation and introduce system complexity.
Heat management emerges as a critical consideration in high-current-density operations. Excessive temperature increases accelerate side reactions and membrane degradation while potentially compromising deposit quality. Current cooling strategies rely primarily on electrolyte recirculation through external heat exchangers, introducing additional pumping requirements and system complexity.
Existing Continuous Reactor Configurations and Performance
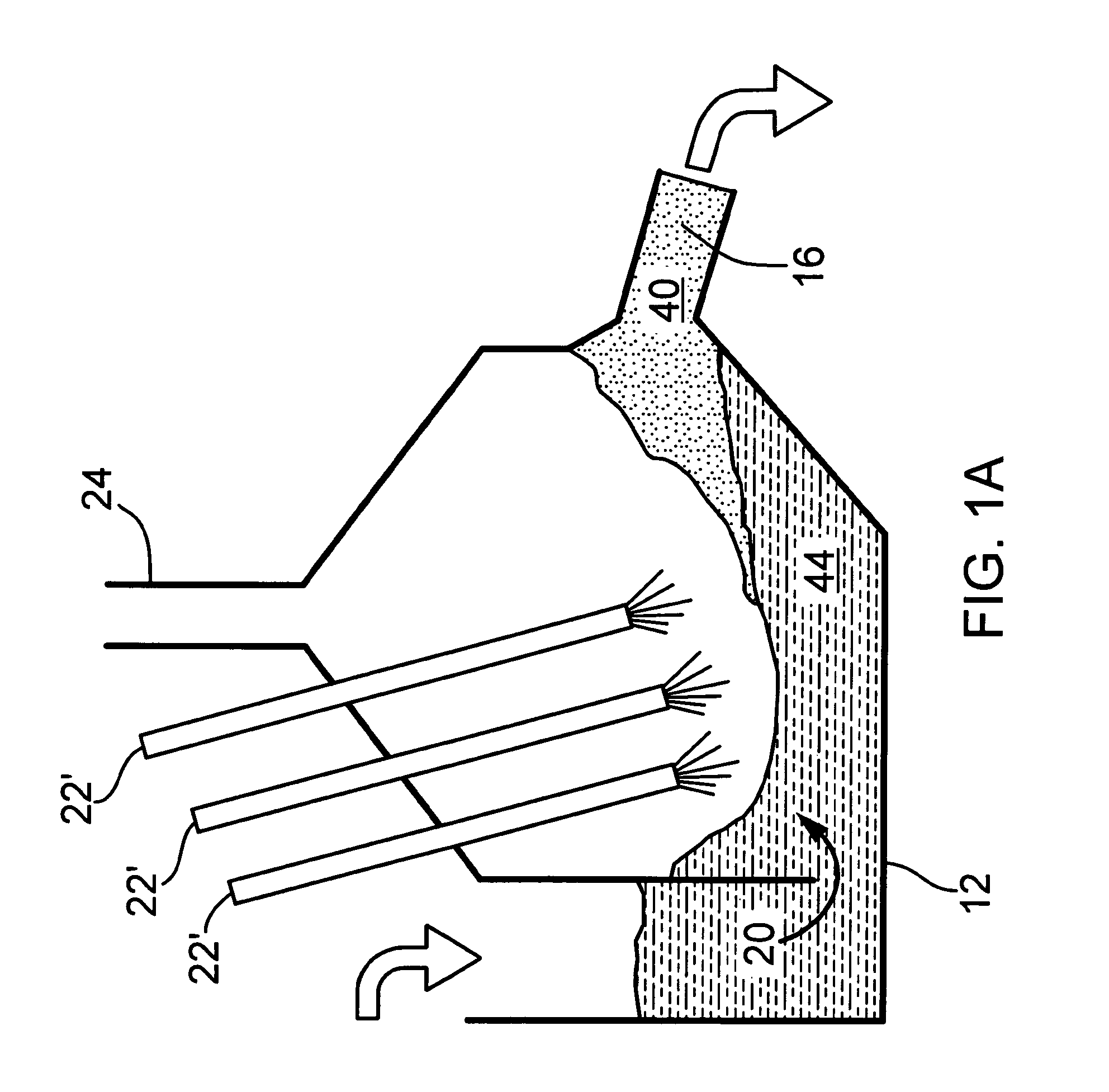
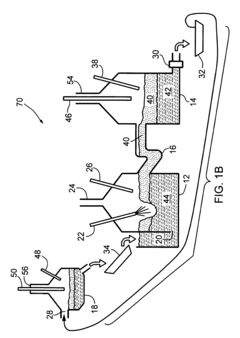
01 Vertical cell configurations for iron electrowinning
Vertical cell configurations are used in iron electrowinning reactors to optimize space utilization and improve efficiency. These designs typically feature vertically aligned electrodes with specific spacing to enhance mass transfer and current distribution. The vertical arrangement allows for better management of gas evolution during the electrowinning process and can facilitate the removal of deposited iron from the cathodes. These configurations often incorporate systems for circulation of electrolyte to maintain uniform concentration and temperature throughout the cell.- Vertical cell configurations for iron electrowinning: Vertical cell configurations are used in iron electrowinning reactors to optimize space and improve efficiency. These designs feature vertically aligned electrodes with specific spacing to enhance mass transfer and current distribution. The vertical arrangement allows for better management of gas evolution during the electrowinning process and can facilitate easier maintenance operations. These configurations often incorporate specialized flow patterns to ensure uniform electrolyte distribution across the electrode surfaces.
- Horizontal cell designs for iron electrowinning: Horizontal cell designs for iron electrowinning reactors feature horizontally arranged electrodes that maximize surface area contact with the electrolyte. These configurations often allow for easier electrode removal and replacement, reducing maintenance downtime. Horizontal designs can be particularly advantageous for managing sludge formation and preventing short circuits between electrodes. Some designs incorporate innovative flow distribution systems to ensure uniform current density across the electrode surfaces.
- Advanced membrane and diaphragm systems: Advanced membrane and diaphragm systems are incorporated into iron electrowinning reactors to separate anodic and cathodic compartments, preventing cross-contamination of solutions and improving current efficiency. These systems utilize ion-selective membranes or porous diaphragms that allow specific ion transfer while blocking unwanted species. The membrane configurations can significantly reduce power consumption and increase metal recovery rates by preventing side reactions. Some designs feature reinforced membrane structures to withstand the harsh chemical environment of electrowinning processes.
- Flow-through electrode configurations: Flow-through electrode configurations in iron electrowinning reactors feature porous or perforated electrodes that allow electrolyte to flow directly through the electrode structure. This design enhances mass transfer rates and reduces concentration polarization at the electrode surface. The improved electrolyte circulation helps maintain consistent ion concentrations throughout the cell and can significantly increase current efficiency. These configurations often incorporate specialized flow distributors to ensure uniform electrolyte flow across the entire electrode area.
- Modular and scalable reactor designs: Modular and scalable reactor designs for iron electrowinning allow for flexible capacity adjustment and easier maintenance. These configurations feature standardized cell units that can be added or removed to adjust production capacity according to demand. The modular approach facilitates easier replacement of individual components without disrupting the entire system. Some designs incorporate innovative connection systems between modules to ensure proper electrical contact and fluid sealing. These configurations often include centralized control systems that can manage multiple cell modules simultaneously.
02 Fluidized bed reactor designs for iron electrowinning
Fluidized bed reactor designs utilize suspended particles as cathodes in the electrowinning process. The fluidized bed approach provides enhanced mass transfer rates and increased surface area for iron deposition. These reactors typically operate with continuous circulation of electrolyte through a bed of conductive particles, allowing for improved current efficiency and metal recovery rates. The fluidized nature of the cathode material helps prevent dendrite formation and enables continuous harvesting of the deposited iron.Expand Specific Solutions03 Membrane-separated electrowinning cells
Membrane-separated electrowinning cells employ ion-selective membranes to divide the anode and cathode compartments. This configuration prevents oxidation of ferrous ions at the anode and reduces contamination between the compartments. The membrane technology allows for different electrolyte compositions in each compartment, optimizing conditions for both the anodic and cathodic reactions. These designs can achieve higher current efficiencies and produce purer iron deposits by minimizing side reactions and preventing the migration of unwanted species between compartments.Expand Specific Solutions04 Modular and scalable reactor configurations
Modular and scalable reactor configurations allow for flexible capacity adjustment in iron electrowinning operations. These designs feature standardized cell units that can be connected in series or parallel to achieve desired production rates. The modular approach facilitates maintenance by allowing individual units to be taken offline without disrupting the entire operation. These configurations often incorporate advanced monitoring and control systems to maintain optimal operating conditions across multiple cells, ensuring consistent product quality regardless of the scale of operation.Expand Specific Solutions05 Advanced electrode materials and configurations
Advanced electrode materials and configurations enhance the efficiency and durability of iron electrowinning reactors. These innovations include specialized cathode designs that facilitate easy removal of deposited iron and anode materials resistant to corrosion in aggressive electrolytes. Some configurations utilize dimensionally stable anodes with catalytic coatings to reduce oxygen evolution overpotential. Electrode spacing and geometry are optimized to improve current distribution and minimize energy consumption. These advanced designs often incorporate features to manage gas evolution and maintain uniform electrolyte flow across the electrode surfaces.Expand Specific Solutions
Leading Companies and Research Institutions in Iron Electrowinning
The continuous iron electrowinning technology market is currently in its early growth phase, characterized by significant research activity but limited commercial deployment. The market size remains relatively modest but shows promising expansion potential as industrial decarbonization efforts intensify. From a technical maturity perspective, the field is still evolving with various reactor configurations being explored. Key players include established industrial giants like Siemens AG and FANUC Corp. focusing on automation aspects, while specialized entities such as Paul Wurth SA and Johnson Matthey Davy Technologies contribute metallurgical expertise. Research institutions including CNRS, CSIC, and universities (Shanghai University, University of Florida) are advancing fundamental science, while energy companies like ExxonMobil and Shell are exploring applications within their sustainability initiatives.
Paul Wurth SA
Technical Solution: Paul Wurth has developed sophisticated reactor configurations for continuous iron electrowinning that integrate with their broader ironmaking expertise. Their system employs a horizontal flow-through cell design with specialized high-surface-area electrodes that maximize reaction efficiency. The technology incorporates advanced electrolyte management systems that continuously filter and regenerate the solution, maintaining optimal iron ion concentrations and pH levels (typically 1.8-2.2). Their reactors feature proprietary membrane technology that effectively separates anodic and cathodic reactions while minimizing resistance. Paul Wurth's approach includes precise thermal management systems that maintain optimal operating temperatures (50-70°C) throughout the process, enhancing reaction kinetics and energy efficiency. The design incorporates automated harvesting mechanisms that allow for continuous metal collection without process interruption, achieving production rates up to 30-40% higher than batch processes.
Strengths: Seamless integration with existing ironmaking infrastructure; excellent production continuity with minimal downtime; superior product consistency due to stable operating parameters. Weaknesses: Higher complexity in system design increases initial capital costs; requires specialized expertise for operation; more sensitive to feed material variations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative reactor configurations for continuous iron electrowinning that integrate with their broader chemical processing expertise. Their system employs a horizontal flow reactor design with specialized composite electrodes that demonstrate enhanced durability in aggressive electrolyte environments. The technology incorporates advanced electrolyte management systems that continuously monitor and adjust solution chemistry, maintaining optimal iron concentration (typically 40-60 g/L) and pH (1.5-2.0). Sinopec's approach includes proprietary additive formulations that significantly improve current efficiency while reducing energy consumption by approximately 18-22% compared to conventional methods. Their reactors feature sophisticated heat recovery systems that capture and reuse thermal energy from the electrowinning process, further enhancing overall energy efficiency. The design incorporates automated metal harvesting mechanisms that allow for continuous operation with minimal human intervention, achieving production capacities of 5,000-8,000 tons annually per reactor unit.
Strengths: Excellent energy efficiency through integrated heat recovery systems; high production capacity suitable for large-scale operations; robust design with minimal maintenance requirements. Weaknesses: Less flexible for small-scale applications; requires consistent feed quality; higher complexity in control systems increases initial technical expertise requirements.
Key Patents and Technical Innovations in Electrowinning Reactors
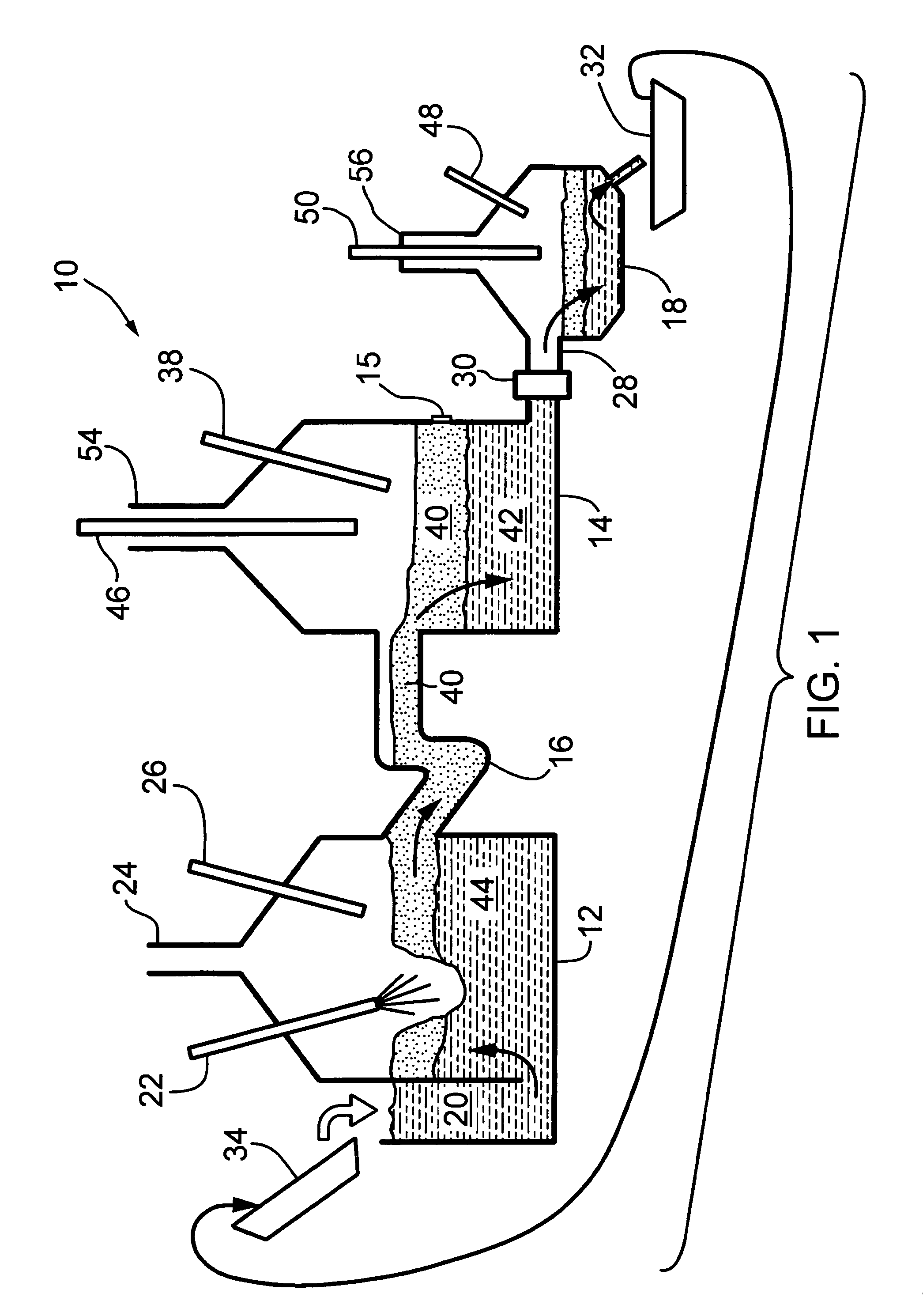
Process and system for electrolytically producing an iron-bearing product from iron ore particles
PatentWO2024250053A1
Innovation
- A continuous electrolytic process using an electrochemical flow reactor with a cathodic compartment and an anodic compartment, applying an electrical potential greater than 1.4V to reduce iron ore particles in an alkaline solution, allowing for the separation of solid iron-bearing products without disassembling the reactor, and recycling the catholyte for continuous processing.
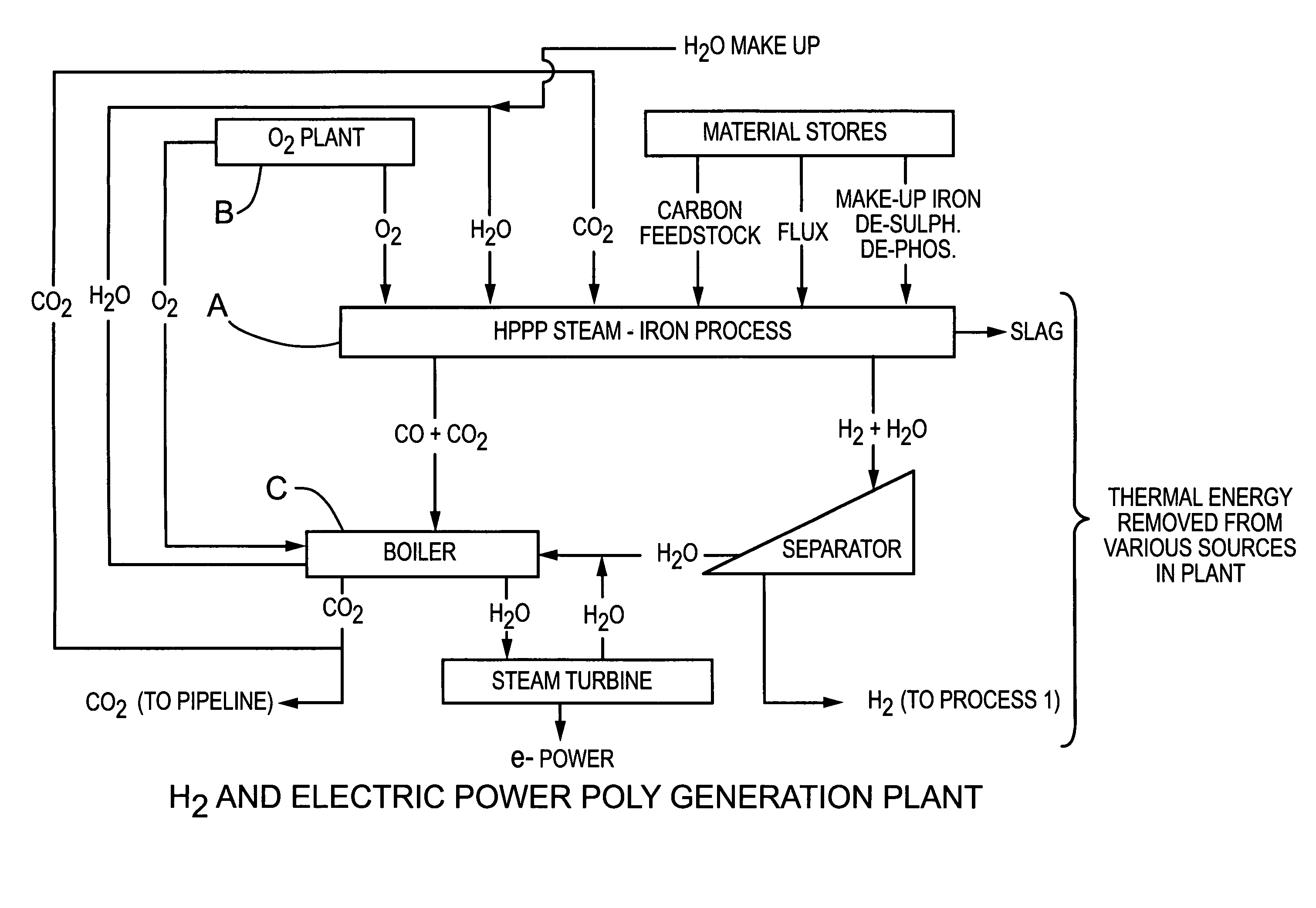
Reactor and process for the continuous production of hydrogen based on steam oxidation of molten iron
PatentInactiveUS7914765B2
Innovation
- A method and apparatus utilizing a molten iron reactor with three distinct reaction zones for steam oxidation, carbon reduction, and decarbonization, allowing for continuous hydrogen production by circulating molten iron through these zones, with optional separate decarbonization, to produce a pure hydrogen stream and CO2 suitable for capture, and incorporating a backup iron supply for process reliability.
Energy Efficiency and Sustainability Considerations
Energy efficiency represents a critical dimension in the development and implementation of continuous iron electrowinning reactor configurations. Current electrowinning processes typically consume between 2.5-3.5 kWh per kilogram of iron produced, highlighting significant opportunities for optimization. Advanced reactor designs incorporating improved electrode materials, such as titanium substrates with specialized coatings, have demonstrated potential energy savings of 15-20% compared to conventional systems.
Membrane technology integration in reactor configurations offers another avenue for enhancing energy efficiency. Selective ion exchange membranes can reduce parasitic reactions and minimize energy losses, potentially decreasing overall energy consumption by 8-12%. Additionally, optimized electrolyte circulation systems that maintain uniform concentration gradients near electrode surfaces have shown improvements in current efficiency exceeding 10%.
Temperature management within continuous electrowinning reactors presents another critical sustainability consideration. Research indicates that maintaining optimal operating temperatures between 40-60°C can significantly reduce energy requirements while extending electrode lifespan. Heat recovery systems integrated into reactor designs can recapture up to 30% of thermal energy, further enhancing overall process efficiency.
From a sustainability perspective, continuous iron electrowinning offers substantial advantages over traditional pyrometallurgical processes. Life cycle assessments indicate potential reductions in carbon emissions by 40-60% when compared to conventional blast furnace operations. This reduction stems primarily from the elimination of carbon-based reducing agents and lower operating temperatures.
Water management represents another key sustainability consideration in reactor configuration design. Closed-loop electrolyte systems can reduce freshwater consumption by 70-85% compared to conventional processes. Advanced filtration and purification technologies integrated into reactor systems enable effective electrolyte recycling while minimizing discharge of contaminated process waters.
The selection of construction materials for reactor components also impacts sustainability profiles. Utilizing corrosion-resistant materials extends reactor lifespan and reduces maintenance requirements, while modular designs facilitate component replacement rather than complete system overhauls. Recent innovations in 3D-printed reactor components offer potential for optimized flow dynamics and reduced material usage, further enhancing sustainability metrics.
Waste valorization opportunities present additional sustainability advantages in continuous iron electrowinning. Spent electrolytes and electrode residues can be processed to recover valuable metals and chemicals, creating secondary value streams while minimizing waste disposal requirements. Some advanced reactor configurations incorporate in-situ regeneration capabilities, further reducing resource consumption and waste generation.
Membrane technology integration in reactor configurations offers another avenue for enhancing energy efficiency. Selective ion exchange membranes can reduce parasitic reactions and minimize energy losses, potentially decreasing overall energy consumption by 8-12%. Additionally, optimized electrolyte circulation systems that maintain uniform concentration gradients near electrode surfaces have shown improvements in current efficiency exceeding 10%.
Temperature management within continuous electrowinning reactors presents another critical sustainability consideration. Research indicates that maintaining optimal operating temperatures between 40-60°C can significantly reduce energy requirements while extending electrode lifespan. Heat recovery systems integrated into reactor designs can recapture up to 30% of thermal energy, further enhancing overall process efficiency.
From a sustainability perspective, continuous iron electrowinning offers substantial advantages over traditional pyrometallurgical processes. Life cycle assessments indicate potential reductions in carbon emissions by 40-60% when compared to conventional blast furnace operations. This reduction stems primarily from the elimination of carbon-based reducing agents and lower operating temperatures.
Water management represents another key sustainability consideration in reactor configuration design. Closed-loop electrolyte systems can reduce freshwater consumption by 70-85% compared to conventional processes. Advanced filtration and purification technologies integrated into reactor systems enable effective electrolyte recycling while minimizing discharge of contaminated process waters.
The selection of construction materials for reactor components also impacts sustainability profiles. Utilizing corrosion-resistant materials extends reactor lifespan and reduces maintenance requirements, while modular designs facilitate component replacement rather than complete system overhauls. Recent innovations in 3D-printed reactor components offer potential for optimized flow dynamics and reduced material usage, further enhancing sustainability metrics.
Waste valorization opportunities present additional sustainability advantages in continuous iron electrowinning. Spent electrolytes and electrode residues can be processed to recover valuable metals and chemicals, creating secondary value streams while minimizing waste disposal requirements. Some advanced reactor configurations incorporate in-situ regeneration capabilities, further reducing resource consumption and waste generation.
Scale-up Challenges and Industrial Implementation Strategies
Scaling up continuous iron electrowinning processes from laboratory to industrial scale presents significant engineering challenges that must be systematically addressed. The transition requires careful consideration of reactor geometry, electrode configuration, and process parameters to maintain efficiency and product quality at larger scales.
The primary challenge in scaling up electrowinning reactors is maintaining uniform current distribution across larger electrode surfaces. Laboratory-scale reactors typically feature well-controlled electrolyte flow and current distribution, but these parameters become increasingly difficult to manage in industrial settings. Non-uniform current distribution can lead to inconsistent iron deposition, reduced current efficiency, and increased energy consumption.
Heat management emerges as another critical factor during scale-up. Industrial electrowinning operations generate substantial heat that must be effectively dissipated to prevent electrolyte degradation and maintain optimal operating conditions. Implementation of efficient cooling systems and thermal management strategies becomes essential for continuous operation at scale.
Material selection for industrial-scale reactors requires careful consideration of corrosion resistance, durability, and cost-effectiveness. While laboratory reactors may utilize premium materials, industrial implementation demands economically viable alternatives that can withstand harsh operating conditions over extended periods without compromising performance.
Successful industrial implementation strategies typically involve a phased approach, beginning with pilot-scale testing to validate key process parameters before full-scale deployment. This intermediate step allows for identification and resolution of scale-dependent issues while minimizing financial risk. Modular reactor designs have proven particularly effective, enabling incremental capacity expansion and facilitating maintenance operations.
Process control systems represent another crucial element of industrial implementation. Advanced monitoring and control technologies, including real-time analysis of electrolyte composition, current efficiency, and product quality, enable responsive adjustment of operating parameters to maintain optimal performance. Integration of automation and digital technologies further enhances operational reliability and reduces labor requirements.
Economic considerations ultimately drive industrial implementation decisions. Capital expenditure requirements must be balanced against operational costs, with particular attention to energy efficiency, maintenance needs, and process reliability. Successful implementations typically prioritize robust designs that may have higher initial costs but offer superior long-term performance and reduced operational expenses.
Environmental and safety considerations must also be integrated into scale-up strategies, with particular attention to electrolyte handling, hydrogen management, and waste treatment. Regulatory compliance requirements vary by jurisdiction and must be addressed early in the implementation planning process.
The primary challenge in scaling up electrowinning reactors is maintaining uniform current distribution across larger electrode surfaces. Laboratory-scale reactors typically feature well-controlled electrolyte flow and current distribution, but these parameters become increasingly difficult to manage in industrial settings. Non-uniform current distribution can lead to inconsistent iron deposition, reduced current efficiency, and increased energy consumption.
Heat management emerges as another critical factor during scale-up. Industrial electrowinning operations generate substantial heat that must be effectively dissipated to prevent electrolyte degradation and maintain optimal operating conditions. Implementation of efficient cooling systems and thermal management strategies becomes essential for continuous operation at scale.
Material selection for industrial-scale reactors requires careful consideration of corrosion resistance, durability, and cost-effectiveness. While laboratory reactors may utilize premium materials, industrial implementation demands economically viable alternatives that can withstand harsh operating conditions over extended periods without compromising performance.
Successful industrial implementation strategies typically involve a phased approach, beginning with pilot-scale testing to validate key process parameters before full-scale deployment. This intermediate step allows for identification and resolution of scale-dependent issues while minimizing financial risk. Modular reactor designs have proven particularly effective, enabling incremental capacity expansion and facilitating maintenance operations.
Process control systems represent another crucial element of industrial implementation. Advanced monitoring and control technologies, including real-time analysis of electrolyte composition, current efficiency, and product quality, enable responsive adjustment of operating parameters to maintain optimal performance. Integration of automation and digital technologies further enhances operational reliability and reduces labor requirements.
Economic considerations ultimately drive industrial implementation decisions. Capital expenditure requirements must be balanced against operational costs, with particular attention to energy efficiency, maintenance needs, and process reliability. Successful implementations typically prioritize robust designs that may have higher initial costs but offer superior long-term performance and reduced operational expenses.
Environmental and safety considerations must also be integrated into scale-up strategies, with particular attention to electrolyte handling, hydrogen management, and waste treatment. Regulatory compliance requirements vary by jurisdiction and must be addressed early in the implementation planning process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!