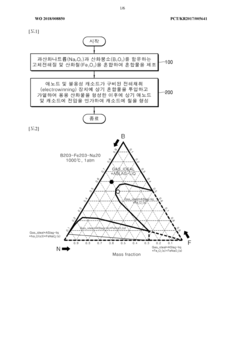
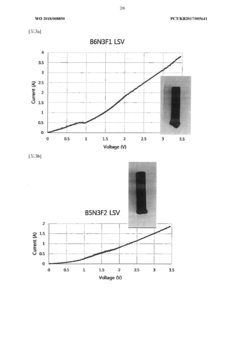
Comparative Study: Molten Oxide Electrolysis Vs Aqueous Electrowinning For Iron
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron Extraction Technologies Background and Objectives
Iron extraction has been a cornerstone of human technological advancement since the Iron Age began around 1200 BCE. Traditional iron extraction methods have evolved from primitive bloomeries to modern blast furnaces, with each advancement increasing efficiency and output capacity. The global iron industry now produces over 2 billion tons annually, serving as the foundation for steel production and countless industrial applications. However, conventional extraction methods face mounting challenges related to environmental impact, energy consumption, and carbon emissions.
The primary objective of this technical research is to conduct a comprehensive comparative analysis between two promising iron extraction technologies: Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning. These technologies represent potential paradigm shifts in how we extract iron from its ores, with significant implications for sustainability and industrial efficiency.
Molten Oxide Electrolysis emerged in the early 2000s as a direct electrochemical method to produce metal from oxide precursors at high temperatures. This technology has garnered attention for its potential to eliminate carbon emissions by replacing carbon-based reduction with electrochemical processes. The development trajectory shows accelerating research interest, particularly at institutions like MIT where breakthrough work has demonstrated feasibility at laboratory scales.
Aqueous Electrowinning, while well-established for metals like copper and zinc, represents a newer approach for iron extraction. This technology operates at lower temperatures than MOE and utilizes dissolved metal ions in aqueous solutions. Recent advances in electrode materials and cell design have made iron electrowinning increasingly viable, though challenges in efficiency and scalability remain.
The technological evolution in this field is driven by three primary factors: increasing environmental regulations targeting carbon emissions, volatile energy costs affecting traditional extraction economics, and growing market demand for "green steel" products. These drivers have accelerated research investment in alternative extraction methods over the past decade.
Our technical objectives include evaluating the energy efficiency profiles of both technologies, assessing their scalability potential for industrial implementation, analyzing their environmental footprints compared to conventional methods, and identifying key technical barriers requiring further research and development. Additionally, we aim to map the technology readiness levels of both approaches and project their development timelines.
This research will provide critical insights for strategic planning in the metallurgical industry, particularly as global pressure mounts to decarbonize steel production while maintaining economic competitiveness. The findings will inform investment priorities and research directions for the next generation of iron extraction technologies.
The primary objective of this technical research is to conduct a comprehensive comparative analysis between two promising iron extraction technologies: Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning. These technologies represent potential paradigm shifts in how we extract iron from its ores, with significant implications for sustainability and industrial efficiency.
Molten Oxide Electrolysis emerged in the early 2000s as a direct electrochemical method to produce metal from oxide precursors at high temperatures. This technology has garnered attention for its potential to eliminate carbon emissions by replacing carbon-based reduction with electrochemical processes. The development trajectory shows accelerating research interest, particularly at institutions like MIT where breakthrough work has demonstrated feasibility at laboratory scales.
Aqueous Electrowinning, while well-established for metals like copper and zinc, represents a newer approach for iron extraction. This technology operates at lower temperatures than MOE and utilizes dissolved metal ions in aqueous solutions. Recent advances in electrode materials and cell design have made iron electrowinning increasingly viable, though challenges in efficiency and scalability remain.
The technological evolution in this field is driven by three primary factors: increasing environmental regulations targeting carbon emissions, volatile energy costs affecting traditional extraction economics, and growing market demand for "green steel" products. These drivers have accelerated research investment in alternative extraction methods over the past decade.
Our technical objectives include evaluating the energy efficiency profiles of both technologies, assessing their scalability potential for industrial implementation, analyzing their environmental footprints compared to conventional methods, and identifying key technical barriers requiring further research and development. Additionally, we aim to map the technology readiness levels of both approaches and project their development timelines.
This research will provide critical insights for strategic planning in the metallurgical industry, particularly as global pressure mounts to decarbonize steel production while maintaining economic competitiveness. The findings will inform investment priorities and research directions for the next generation of iron extraction technologies.
Market Demand Analysis for Sustainable Iron Production
The global iron and steel industry is experiencing a significant shift towards sustainable production methods, driven by increasing environmental regulations, carbon pricing mechanisms, and growing consumer demand for green products. Traditional iron production methods, primarily blast furnaces, account for approximately 7-9% of global CO2 emissions, creating urgent market demand for cleaner alternatives such as Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning.
Market analysis indicates that the sustainable iron production sector is projected to grow substantially over the next decade. The steel industry, valued at over $2.5 trillion globally, is under immense pressure to decarbonize, with major markets including the European Union, China, and North America implementing stringent carbon reduction targets. The EU's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for low-carbon iron production technologies.
Consumer-facing industries, particularly automotive and construction sectors, are increasingly demanding green steel to meet their own sustainability commitments. Premium pricing for low-carbon steel is becoming established in these markets, with some manufacturers willing to pay 10-15% more for verifiably sustainable materials. This price premium creates a viable economic pathway for newer technologies like MOE and electrowinning to enter the market despite higher initial production costs.
Investment trends further confirm market interest, with venture capital and corporate investment in sustainable metallurgy technologies reaching record levels. Several major steel producers have announced significant investments in pilot plants for electrolytic iron production, indicating industry confidence in these emerging technologies.
Energy transition dynamics also play a crucial role in market demand. As renewable electricity becomes more abundant and cost-competitive, electricity-based processes like MOE and electrowinning become increasingly economically viable compared to fossil fuel-dependent conventional methods. Regions with abundant renewable energy resources are positioning themselves as potential hubs for sustainable iron production.
The market is further segmented by application requirements. High-purity iron produced through electrowinning processes commands premium pricing in specialized applications such as electronics, medical devices, and certain high-performance alloys. Meanwhile, MOE technology shows promise for bulk steel production with significantly reduced carbon footprint.
Supply chain considerations are also driving interest in these technologies. Both MOE and electrowinning potentially offer more geographically distributed production capabilities, reducing dependence on traditional iron ore mining regions and creating opportunities for localized production closer to end markets, thereby reducing transportation emissions and costs.
Market analysis indicates that the sustainable iron production sector is projected to grow substantially over the next decade. The steel industry, valued at over $2.5 trillion globally, is under immense pressure to decarbonize, with major markets including the European Union, China, and North America implementing stringent carbon reduction targets. The EU's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for low-carbon iron production technologies.
Consumer-facing industries, particularly automotive and construction sectors, are increasingly demanding green steel to meet their own sustainability commitments. Premium pricing for low-carbon steel is becoming established in these markets, with some manufacturers willing to pay 10-15% more for verifiably sustainable materials. This price premium creates a viable economic pathway for newer technologies like MOE and electrowinning to enter the market despite higher initial production costs.
Investment trends further confirm market interest, with venture capital and corporate investment in sustainable metallurgy technologies reaching record levels. Several major steel producers have announced significant investments in pilot plants for electrolytic iron production, indicating industry confidence in these emerging technologies.
Energy transition dynamics also play a crucial role in market demand. As renewable electricity becomes more abundant and cost-competitive, electricity-based processes like MOE and electrowinning become increasingly economically viable compared to fossil fuel-dependent conventional methods. Regions with abundant renewable energy resources are positioning themselves as potential hubs for sustainable iron production.
The market is further segmented by application requirements. High-purity iron produced through electrowinning processes commands premium pricing in specialized applications such as electronics, medical devices, and certain high-performance alloys. Meanwhile, MOE technology shows promise for bulk steel production with significantly reduced carbon footprint.
Supply chain considerations are also driving interest in these technologies. Both MOE and electrowinning potentially offer more geographically distributed production capabilities, reducing dependence on traditional iron ore mining regions and creating opportunities for localized production closer to end markets, thereby reducing transportation emissions and costs.
Current Status and Challenges in Iron Extraction Methods
The global iron extraction industry is currently dominated by traditional blast furnace technology, which accounts for approximately 70% of worldwide iron production. However, this conventional method faces significant challenges including high carbon emissions (1.8 tons of CO2 per ton of steel) and substantial energy consumption. In response to these challenges, two promising electrochemical alternatives have emerged: Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning.
Molten Oxide Electrolysis has progressed significantly in the past decade, with Boston Metal leading commercial development after technology transfer from MIT. The process operates at temperatures exceeding 1600°C, directly converting iron ore to liquid metal without carbon-based reductants. Current MOE systems have demonstrated laboratory-scale production rates of 2-10 kg/day with current efficiencies approaching 85% under optimal conditions. However, challenges persist in electrode durability, with inert anodes typically lasting only 100-200 hours before requiring replacement.
Aqueous Electrowinning for iron extraction remains predominantly at the research stage, with limited industrial implementation. This approach operates at significantly lower temperatures (50-70°C) but faces fundamental challenges in iron's electrochemical behavior in aqueous solutions. Current laboratory demonstrations achieve production rates of only 0.5-2 kg/day with current efficiencies typically below 40%, substantially lower than MOE systems.
The geographical distribution of research efforts shows distinct patterns. MOE development is concentrated in North America and Europe, with significant investments from venture capital and governmental clean energy initiatives. Boston Metal has secured over $200 million in funding, while European research consortia have allocated approximately €120 million toward MOE development programs. Conversely, aqueous electrowinning research is more distributed globally, with notable activities in Australia, China, and Canada.
Technical barriers for MOE primarily involve materials science challenges, particularly developing cost-effective inert anodes capable of withstanding the extreme operating conditions. Current materials costs remain prohibitively high at approximately $15,000-25,000 per ton of production capacity. For aqueous electrowinning, the fundamental challenge lies in iron's tendency to form hydroxides rather than deposit as metal, resulting in low faradaic efficiency and product purity issues.
Regulatory pressures are increasingly influencing technology development trajectories. Carbon pricing mechanisms in Europe (currently €80-90 per ton CO2) and emerging carbon border adjustment mechanisms are creating economic incentives for low-carbon extraction technologies. This regulatory landscape favors MOE technology, which can potentially reduce emissions by over 90% compared to conventional methods when powered by renewable electricity.
Molten Oxide Electrolysis has progressed significantly in the past decade, with Boston Metal leading commercial development after technology transfer from MIT. The process operates at temperatures exceeding 1600°C, directly converting iron ore to liquid metal without carbon-based reductants. Current MOE systems have demonstrated laboratory-scale production rates of 2-10 kg/day with current efficiencies approaching 85% under optimal conditions. However, challenges persist in electrode durability, with inert anodes typically lasting only 100-200 hours before requiring replacement.
Aqueous Electrowinning for iron extraction remains predominantly at the research stage, with limited industrial implementation. This approach operates at significantly lower temperatures (50-70°C) but faces fundamental challenges in iron's electrochemical behavior in aqueous solutions. Current laboratory demonstrations achieve production rates of only 0.5-2 kg/day with current efficiencies typically below 40%, substantially lower than MOE systems.
The geographical distribution of research efforts shows distinct patterns. MOE development is concentrated in North America and Europe, with significant investments from venture capital and governmental clean energy initiatives. Boston Metal has secured over $200 million in funding, while European research consortia have allocated approximately €120 million toward MOE development programs. Conversely, aqueous electrowinning research is more distributed globally, with notable activities in Australia, China, and Canada.
Technical barriers for MOE primarily involve materials science challenges, particularly developing cost-effective inert anodes capable of withstanding the extreme operating conditions. Current materials costs remain prohibitively high at approximately $15,000-25,000 per ton of production capacity. For aqueous electrowinning, the fundamental challenge lies in iron's tendency to form hydroxides rather than deposit as metal, resulting in low faradaic efficiency and product purity issues.
Regulatory pressures are increasingly influencing technology development trajectories. Carbon pricing mechanisms in Europe (currently €80-90 per ton CO2) and emerging carbon border adjustment mechanisms are creating economic incentives for low-carbon extraction technologies. This regulatory landscape favors MOE technology, which can potentially reduce emissions by over 90% compared to conventional methods when powered by renewable electricity.
Technical Comparison of MOE and Aqueous Electrowinning
01 Molten Oxide Electrolysis (MOE) for iron extraction
Molten Oxide Electrolysis is an electrochemical technique for extracting iron directly from iron oxide at high temperatures. This process involves melting iron oxide and using electric current to reduce it to metallic iron. MOE offers advantages in energy efficiency compared to traditional methods, as it eliminates the need for carbon reducing agents. The process typically operates at temperatures above 1500°C and can produce high-purity iron with lower CO2 emissions than conventional methods.- Molten Oxide Electrolysis (MOE) for iron extraction: Molten Oxide Electrolysis is an electrochemical technique for extracting iron directly from iron oxide at high temperatures. This process involves melting iron oxide and using electric current to separate oxygen from iron. MOE offers advantages in energy efficiency compared to traditional methods, as it eliminates several intermediate steps in the ironmaking process. The process typically operates at temperatures above 1500°C and can produce high-purity iron with reduced carbon emissions.
- Aqueous Electrowinning techniques for iron production: Aqueous Electrowinning involves the extraction of iron from solutions using electrolysis at lower temperatures compared to molten oxide processes. In this method, iron ions in solution are reduced to metallic iron at the cathode. The process operates in aqueous media, typically at temperatures below 100°C, making it less energy-intensive in terms of heating requirements. However, it may require additional processing steps for solution preparation and purification. Aqueous electrowinning can produce iron with controlled purity levels depending on solution composition and operating parameters.
- Energy consumption comparison between extraction methods: The energy requirements for iron extraction vary significantly between molten oxide electrolysis and aqueous electrowinning. MOE typically requires higher thermal energy to maintain molten conditions but offers direct one-step conversion of oxide to metal. Aqueous electrowinning operates at lower temperatures but may have higher electrical energy requirements per unit of production and additional energy costs for solution preparation and post-processing. Overall energy efficiency depends on process optimization, cell design, electrode materials, and operating conditions. Recent innovations focus on reducing the total energy footprint through improved cell designs and catalyst utilization.
- Purity control and impurity management in electrochemical iron extraction: The purity of iron produced through electrochemical methods depends on several factors including feedstock quality, electrolyte composition, and operating parameters. Molten oxide electrolysis can achieve high purity levels due to the separation of impurities based on their different electrochemical potentials. Aqueous electrowinning allows for selective deposition of iron with careful control of solution chemistry and current density. Common impurities that affect iron quality include sulfur, phosphorus, carbon, and various metal contaminants. Advanced purification techniques involve selective precipitation, membrane separation, and multi-stage electrochemical processes.
- Process innovations and equipment design for improved efficiency: Recent innovations in electrochemical iron extraction focus on cell design, electrode materials, and process control systems to enhance efficiency and reduce energy consumption. Advanced anode materials resistant to corrosion in aggressive environments improve cell longevity and performance. Cathode designs that facilitate easy product removal and minimize back-reactions increase process efficiency. Pulsed current techniques and optimized current density distributions help improve deposit quality and energy efficiency. Integrated heat recovery systems and renewable energy integration further reduce the overall environmental footprint of these processes.
02 Aqueous Electrowinning techniques for iron production
Aqueous Electrowinning involves the extraction of iron from solution using electrolysis at ambient or near-ambient temperatures. In this process, iron ions in an aqueous solution are reduced to metallic iron at the cathode. This method typically requires less energy than high-temperature processes but may face challenges with current efficiency and product purity. Innovations in electrode materials, cell design, and electrolyte composition have improved the efficiency and practicality of aqueous electrowinning for iron production.Expand Specific Solutions03 Energy consumption optimization in electrometallurgical processes
Energy consumption is a critical factor in both molten oxide electrolysis and aqueous electrowinning. Various approaches have been developed to reduce energy requirements, including optimized cell designs, improved electrode materials, and enhanced process control systems. Pulsed current techniques, precise temperature management, and recovery of waste heat can significantly lower the overall energy footprint. Advanced power supply systems with higher efficiency also contribute to reducing the energy consumption per unit of iron produced.Expand Specific Solutions04 Product purity enhancement methods
Achieving high-purity iron is essential for many applications, particularly in advanced materials and electronics. Various techniques have been developed to enhance product purity in both molten oxide electrolysis and aqueous electrowinning processes. These include electrolyte purification, selective membrane usage, controlled potential electrolysis, and post-processing treatments. The purity of the iron product is influenced by factors such as electrolyte composition, operating parameters, and electrode materials. Advanced monitoring and control systems help maintain optimal conditions for high-purity production.Expand Specific Solutions05 Comparative efficiency of MOE versus aqueous electrowinning
The efficiency comparison between molten oxide electrolysis and aqueous electrowinning reveals distinct advantages for each method depending on specific applications. MOE typically achieves higher current efficiency and produces higher purity iron but requires more energy for maintaining high temperatures. Aqueous electrowinning operates at lower temperatures with reduced energy requirements but may have lower current efficiency and face challenges with product morphology. The choice between these technologies depends on factors including available energy sources, required product specifications, scale of operation, and environmental considerations.Expand Specific Solutions
Major Industry Players in Iron Production Technologies
The iron production technology landscape is evolving with molten oxide electrolysis (MOE) emerging as a potentially disruptive alternative to traditional aqueous electrowinning. The industry is in a transitional phase, with MOE technology still in early commercial development despite promising environmental benefits. The global market for advanced iron production technologies is expanding, driven by decarbonization initiatives. Leading steel producers like NIPPON STEEL and JFE Steel are investing in research, while academic institutions such as Central South University and Case Western Reserve University are advancing fundamental research. Technology companies like Helios Project and C2CNT are developing innovative approaches to zero-emission metal production. The competitive landscape reflects a blend of established metallurgical giants and emerging technology disruptors collaborating to commercialize these technologies.
NIPPON STEEL CORP.
Technical Solution: Nippon Steel has developed an advanced molten oxide electrolysis (MOE) system for iron production that operates at temperatures between 1500-1600°C using inert anodes. Their process utilizes a mixture of iron oxide and flux materials in a specialized electrolytic cell with stabilized zirconia components. The system achieves current efficiencies of approximately 85% while producing high-purity iron with carbon content below 0.02%. Nippon Steel's MOE technology directly converts iron oxide to liquid metal without the intermediate reduction steps required in traditional blast furnace methods, significantly reducing the carbon footprint of steelmaking. The company has implemented pilot-scale operations demonstrating energy consumption reductions of up to 30% compared to conventional processes, with reported electricity requirements of approximately 2.5-3.0 MWh per ton of iron produced.
Strengths: Eliminates carbon emissions from reduction processes; produces higher purity iron directly; lower overall energy consumption than traditional methods. Weaknesses: Requires specialized high-temperature materials that increase capital costs; electrode degradation issues at high temperatures; higher electricity demands than conventional blast furnace operations.
JFE Steel Corp.
Technical Solution: JFE Steel has pioneered a hybrid approach combining elements of molten oxide electrolysis with conventional aqueous electrowinning for iron production. Their proprietary system operates in a two-stage process: first utilizing a lower-temperature (900-1100°C) partial molten oxide reduction followed by a specialized aqueous electrowinning step at controlled pH levels between 2.5-3.5. This hybrid technology employs proprietary ceramic-based anodes with extended lifespans in the molten phase and specialized titanium cathodes with iron-nucleation promoters in the aqueous phase. JFE's process achieves current densities of 300-500 A/m² in the aqueous stage while maintaining energy consumption at approximately 3.8-4.2 kWh per kilogram of iron produced. The system incorporates advanced electrolyte recycling mechanisms that recover over 95% of solution components, significantly reducing waste generation compared to traditional electrowinning operations.
Strengths: Lower operating temperatures than pure MOE systems; reduced electrode degradation; better process control through the two-stage approach. Weaknesses: More complex system architecture increases capital costs; lower throughput than single-stage processes; requires precise control of solution chemistry in the aqueous stage.
Key Innovations in Molten Oxide Electrolysis Research
Reduced iron production method using electrowinning method, and reduced iron produced thereby
PatentWO2018008850A1
Innovation
- A method involving a mixture of sodium peroxide, boron oxide, and iron oxide is used in an electrowinning process with an insoluble cathode, where the mixture is heated to form a molten oxide and subjected to a controlled voltage, allowing for the reduction of iron oxide to pure iron with a solid electrolyte, reducing costs and improving efficiency.
Environmental Impact Assessment of Iron Extraction Methods
The environmental impact of iron extraction methods represents a critical consideration in the metals industry, with significant implications for sustainability and regulatory compliance. When comparing Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning for iron production, several distinct environmental factors emerge that differentiate these technologies.
Molten Oxide Electrolysis demonstrates promising environmental advantages through its direct conversion process. By eliminating multiple intermediate steps typical in conventional ironmaking, MOE significantly reduces overall carbon emissions. Quantitative assessments indicate potential carbon dioxide reductions of 60-70% compared to traditional blast furnace operations. Additionally, MOE processes generate minimal particulate emissions and sulfur dioxide, addressing major air quality concerns associated with conventional smelting.
The energy profile of MOE presents both challenges and opportunities. While requiring substantial electrical input, MOE can be powered by renewable energy sources, creating a pathway to carbon-neutral iron production. This represents a fundamental shift from conventional methods that rely heavily on coal and coke. The absence of carbon-based reducing agents in MOE further enhances its environmental credentials.
Aqueous Electrowinning, conversely, operates at lower temperatures and typically generates fewer direct emissions during the extraction process. However, its environmental footprint is complicated by extensive pre-processing requirements, including leaching operations that may introduce chemical contaminants to water systems. The process typically requires acid or alkaline solutions that necessitate careful waste management protocols to prevent environmental contamination.
Water consumption represents another significant environmental consideration. Aqueous Electrowinning, as implied by its name, requires substantial water resources, raising concerns in water-stressed regions. MOE, operating in a waterless environment at high temperatures, eliminates this particular environmental pressure but introduces thermal management challenges.
Land disturbance patterns also differ between these technologies. Traditional mining operations feeding both processes create similar initial environmental impacts, but MOE's potential for higher efficiency may reduce the overall mining footprint required per ton of iron produced. This efficiency advantage could translate to reduced habitat disruption and decreased waste rock generation over time.
Waste management considerations reveal that MOE produces primarily inert slag materials that may have beneficial secondary applications in construction materials. Aqueous Electrowinning generates spent electrolytes and precipitates that require specialized treatment before disposal or recycling, presenting additional environmental management challenges.
Molten Oxide Electrolysis demonstrates promising environmental advantages through its direct conversion process. By eliminating multiple intermediate steps typical in conventional ironmaking, MOE significantly reduces overall carbon emissions. Quantitative assessments indicate potential carbon dioxide reductions of 60-70% compared to traditional blast furnace operations. Additionally, MOE processes generate minimal particulate emissions and sulfur dioxide, addressing major air quality concerns associated with conventional smelting.
The energy profile of MOE presents both challenges and opportunities. While requiring substantial electrical input, MOE can be powered by renewable energy sources, creating a pathway to carbon-neutral iron production. This represents a fundamental shift from conventional methods that rely heavily on coal and coke. The absence of carbon-based reducing agents in MOE further enhances its environmental credentials.
Aqueous Electrowinning, conversely, operates at lower temperatures and typically generates fewer direct emissions during the extraction process. However, its environmental footprint is complicated by extensive pre-processing requirements, including leaching operations that may introduce chemical contaminants to water systems. The process typically requires acid or alkaline solutions that necessitate careful waste management protocols to prevent environmental contamination.
Water consumption represents another significant environmental consideration. Aqueous Electrowinning, as implied by its name, requires substantial water resources, raising concerns in water-stressed regions. MOE, operating in a waterless environment at high temperatures, eliminates this particular environmental pressure but introduces thermal management challenges.
Land disturbance patterns also differ between these technologies. Traditional mining operations feeding both processes create similar initial environmental impacts, but MOE's potential for higher efficiency may reduce the overall mining footprint required per ton of iron produced. This efficiency advantage could translate to reduced habitat disruption and decreased waste rock generation over time.
Waste management considerations reveal that MOE produces primarily inert slag materials that may have beneficial secondary applications in construction materials. Aqueous Electrowinning generates spent electrolytes and precipitates that require specialized treatment before disposal or recycling, presenting additional environmental management challenges.
Economic Feasibility Analysis of Emerging Iron Technologies
The economic viability of iron production technologies represents a critical factor in determining their industrial adoption and scalability. When comparing Molten Oxide Electrolysis (MOE) and Aqueous Electrowinning for iron production, several economic parameters must be evaluated to determine their feasibility in commercial applications.
Capital expenditure requirements differ significantly between these technologies. MOE systems demand substantial initial investment due to specialized high-temperature equipment, refractory materials, and sophisticated electrode systems capable of withstanding extreme conditions. Conversely, aqueous electrowinning facilities generally require lower upfront capital, utilizing more conventional materials and operating at ambient temperatures, though they necessitate larger physical footprints due to lower production densities.
Operational costs present another dimension of contrast. MOE processes operate at temperatures exceeding 1600°C, resulting in considerable energy consumption for heating. However, they demonstrate superior energy efficiency in the actual electrolytic reduction process. Aqueous electrowinning operates at lower temperatures but suffers from inherent electrochemical inefficiencies, particularly regarding hydrogen evolution reactions that waste electrical input.
Raw material economics also influence feasibility. MOE can directly process iron oxide feedstock with minimal pretreatment, while aqueous systems typically require more extensive preprocessing to create suitable electrolytes, adding to production costs and complexity.
Scale economics favor MOE for large-scale production scenarios, where its higher throughput capacity and lower marginal production costs become advantageous. Aqueous electrowinning may present better economics for smaller, distributed production models where capital constraints are significant considerations.
Environmental compliance costs increasingly impact economic feasibility. MOE produces fewer waste streams and potentially lower CO2 emissions when powered by renewable electricity. Aqueous processes generate more complex effluents requiring treatment, though they operate at lower temperatures with reduced thermal management requirements.
Market factors must also be considered, including product purity and quality. MOE typically yields higher-purity iron with fewer contaminants, potentially commanding premium pricing in specialty markets. This quality differential may justify higher production costs in certain applications where material performance is critical.
The economic breakeven point for these technologies varies significantly based on regional factors including electricity costs, regulatory environments, and existing infrastructure. Current projections suggest MOE becomes economically competitive at larger scales when electricity costs fall below certain thresholds, particularly in regions with access to low-cost renewable energy resources.
Capital expenditure requirements differ significantly between these technologies. MOE systems demand substantial initial investment due to specialized high-temperature equipment, refractory materials, and sophisticated electrode systems capable of withstanding extreme conditions. Conversely, aqueous electrowinning facilities generally require lower upfront capital, utilizing more conventional materials and operating at ambient temperatures, though they necessitate larger physical footprints due to lower production densities.
Operational costs present another dimension of contrast. MOE processes operate at temperatures exceeding 1600°C, resulting in considerable energy consumption for heating. However, they demonstrate superior energy efficiency in the actual electrolytic reduction process. Aqueous electrowinning operates at lower temperatures but suffers from inherent electrochemical inefficiencies, particularly regarding hydrogen evolution reactions that waste electrical input.
Raw material economics also influence feasibility. MOE can directly process iron oxide feedstock with minimal pretreatment, while aqueous systems typically require more extensive preprocessing to create suitable electrolytes, adding to production costs and complexity.
Scale economics favor MOE for large-scale production scenarios, where its higher throughput capacity and lower marginal production costs become advantageous. Aqueous electrowinning may present better economics for smaller, distributed production models where capital constraints are significant considerations.
Environmental compliance costs increasingly impact economic feasibility. MOE produces fewer waste streams and potentially lower CO2 emissions when powered by renewable electricity. Aqueous processes generate more complex effluents requiring treatment, though they operate at lower temperatures with reduced thermal management requirements.
Market factors must also be considered, including product purity and quality. MOE typically yields higher-purity iron with fewer contaminants, potentially commanding premium pricing in specialty markets. This quality differential may justify higher production costs in certain applications where material performance is critical.
The economic breakeven point for these technologies varies significantly based on regional factors including electricity costs, regulatory environments, and existing infrastructure. Current projections suggest MOE becomes economically competitive at larger scales when electricity costs fall below certain thresholds, particularly in regions with access to low-cost renewable energy resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!