Electrochemical Chlor-Iron Processes: Electrolyte Selection And Corrosion Control
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chlor-Iron Electrochemical Technology Background and Objectives
Electrochemical chlor-iron processes represent a significant advancement in industrial electrochemistry, with roots dating back to the early 20th century. These processes have evolved from rudimentary electrolytic cells to sophisticated systems capable of precise control over reaction parameters. The technology fundamentally involves the electrochemical interaction between chloride ions and iron species in solution, creating valuable products while managing corrosion challenges.
The historical trajectory of chlor-iron electrochemistry shows marked acceleration in development during the 1970s and 1980s, coinciding with increased industrial demand for efficient chemical production methods. Early systems suffered from severe corrosion issues and inefficient electrolyte utilization, limiting their commercial viability. However, subsequent innovations in electrode materials, cell design, and electrolyte formulations have progressively addressed these limitations.
Recent technological trends indicate a shift toward more sustainable and energy-efficient chlor-iron processes. This includes the development of novel membrane technologies, advanced electrode materials with enhanced selectivity, and intelligent control systems that optimize energy consumption while minimizing unwanted side reactions. The integration of renewable energy sources with electrochemical chlor-iron processes represents another emerging trend, addressing both economic and environmental concerns.
The primary technical objectives for advancing chlor-iron electrochemical technology center on several key areas. First, identifying optimal electrolyte compositions that maximize conductivity while minimizing corrosive effects on system components. Second, developing corrosion-resistant materials and protective coatings capable of withstanding the aggressive chloride-rich environments typical in these processes. Third, enhancing process efficiency through improved cell design and operating parameters to reduce energy consumption.
Additional objectives include extending equipment service life through better corrosion management strategies, improving product purity by minimizing contamination from corroded components, and developing real-time monitoring systems for early detection of corrosion issues. The ultimate goal is to create economically viable chlor-iron electrochemical processes with predictable performance, minimal maintenance requirements, and reduced environmental impact.
The technology aims to balance competing factors: maximizing reaction efficiency and product yield while simultaneously controlling the inherently corrosive nature of chloride-containing electrolytes on iron-based components. This balance represents the central challenge that continues to drive innovation in the field, with significant implications for industries ranging from chemical manufacturing to water treatment and metal processing.
The historical trajectory of chlor-iron electrochemistry shows marked acceleration in development during the 1970s and 1980s, coinciding with increased industrial demand for efficient chemical production methods. Early systems suffered from severe corrosion issues and inefficient electrolyte utilization, limiting their commercial viability. However, subsequent innovations in electrode materials, cell design, and electrolyte formulations have progressively addressed these limitations.
Recent technological trends indicate a shift toward more sustainable and energy-efficient chlor-iron processes. This includes the development of novel membrane technologies, advanced electrode materials with enhanced selectivity, and intelligent control systems that optimize energy consumption while minimizing unwanted side reactions. The integration of renewable energy sources with electrochemical chlor-iron processes represents another emerging trend, addressing both economic and environmental concerns.
The primary technical objectives for advancing chlor-iron electrochemical technology center on several key areas. First, identifying optimal electrolyte compositions that maximize conductivity while minimizing corrosive effects on system components. Second, developing corrosion-resistant materials and protective coatings capable of withstanding the aggressive chloride-rich environments typical in these processes. Third, enhancing process efficiency through improved cell design and operating parameters to reduce energy consumption.
Additional objectives include extending equipment service life through better corrosion management strategies, improving product purity by minimizing contamination from corroded components, and developing real-time monitoring systems for early detection of corrosion issues. The ultimate goal is to create economically viable chlor-iron electrochemical processes with predictable performance, minimal maintenance requirements, and reduced environmental impact.
The technology aims to balance competing factors: maximizing reaction efficiency and product yield while simultaneously controlling the inherently corrosive nature of chloride-containing electrolytes on iron-based components. This balance represents the central challenge that continues to drive innovation in the field, with significant implications for industries ranging from chemical manufacturing to water treatment and metal processing.
Industrial Applications and Market Demand Analysis
Electrochemical chlor-iron processes have gained significant traction across multiple industrial sectors due to their efficiency in treating wastewater, producing valuable chemicals, and addressing environmental challenges. The global market for these technologies is experiencing robust growth, driven primarily by stringent environmental regulations and the increasing need for sustainable industrial processes.
The water treatment sector represents the largest application area, with electrochemical chlor-iron systems being deployed for the removal of heavy metals, organic contaminants, and pathogens from industrial effluents. This segment is projected to maintain strong growth as industries face tighter discharge regulations worldwide, particularly in developed economies where zero liquid discharge policies are becoming more prevalent.
Chemical manufacturing constitutes another substantial market, where these processes are utilized for the production of chlorine, sodium hydroxide, and iron-based compounds. The demand is particularly strong in regions with established chemical industries such as Western Europe, North America, and East Asia. The ability to produce these chemicals with reduced energy consumption compared to traditional methods has become a compelling value proposition.
Mining operations have emerged as a rapidly expanding application area, employing electrochemical chlor-iron technologies for metal recovery and acid mine drainage treatment. This sector's demand is expected to grow significantly as mining companies seek cost-effective solutions to meet environmental compliance requirements while recovering valuable metals from waste streams.
The pharmaceutical industry has also begun adopting these processes for specialized applications in synthesis and waste treatment. The high-purity requirements and strict regulatory environment in this sector create premium market opportunities for advanced electrochemical systems with precise control capabilities.
Market analysis indicates that the Asia-Pacific region currently leads in terms of adoption rate, driven by China's industrial expansion and environmental initiatives. North America and Europe follow closely, with their markets characterized by technology upgrades and replacement of aging infrastructure.
A notable market trend is the increasing demand for modular and scalable systems that can be tailored to specific industrial needs. This reflects the diverse application landscape and varying treatment requirements across different industries. Additionally, there is growing interest in integrated systems that combine electrochemical processes with complementary technologies to achieve comprehensive treatment solutions.
The service aspect of this market, including maintenance, monitoring, and optimization of electrochemical systems, represents a significant revenue stream with higher profit margins compared to equipment sales alone. This has prompted many technology providers to adopt service-oriented business models, offering performance guarantees and operational support.
The water treatment sector represents the largest application area, with electrochemical chlor-iron systems being deployed for the removal of heavy metals, organic contaminants, and pathogens from industrial effluents. This segment is projected to maintain strong growth as industries face tighter discharge regulations worldwide, particularly in developed economies where zero liquid discharge policies are becoming more prevalent.
Chemical manufacturing constitutes another substantial market, where these processes are utilized for the production of chlorine, sodium hydroxide, and iron-based compounds. The demand is particularly strong in regions with established chemical industries such as Western Europe, North America, and East Asia. The ability to produce these chemicals with reduced energy consumption compared to traditional methods has become a compelling value proposition.
Mining operations have emerged as a rapidly expanding application area, employing electrochemical chlor-iron technologies for metal recovery and acid mine drainage treatment. This sector's demand is expected to grow significantly as mining companies seek cost-effective solutions to meet environmental compliance requirements while recovering valuable metals from waste streams.
The pharmaceutical industry has also begun adopting these processes for specialized applications in synthesis and waste treatment. The high-purity requirements and strict regulatory environment in this sector create premium market opportunities for advanced electrochemical systems with precise control capabilities.
Market analysis indicates that the Asia-Pacific region currently leads in terms of adoption rate, driven by China's industrial expansion and environmental initiatives. North America and Europe follow closely, with their markets characterized by technology upgrades and replacement of aging infrastructure.
A notable market trend is the increasing demand for modular and scalable systems that can be tailored to specific industrial needs. This reflects the diverse application landscape and varying treatment requirements across different industries. Additionally, there is growing interest in integrated systems that combine electrochemical processes with complementary technologies to achieve comprehensive treatment solutions.
The service aspect of this market, including maintenance, monitoring, and optimization of electrochemical systems, represents a significant revenue stream with higher profit margins compared to equipment sales alone. This has prompted many technology providers to adopt service-oriented business models, offering performance guarantees and operational support.
Current Electrolyte Technologies and Corrosion Challenges
The electrochemical chlor-iron process industry currently employs several electrolyte technologies, each with distinct advantages and limitations. Sodium chloride brine remains the most widely utilized electrolyte due to its cost-effectiveness and abundance. However, its relatively low conductivity necessitates higher energy consumption compared to alternative solutions. Potassium chloride electrolytes offer enhanced conductivity and reaction kinetics but at significantly higher costs, limiting their application to specialized high-performance systems where energy efficiency justifies the premium.
Mixed chloride systems, incorporating combinations of sodium, potassium, and calcium chlorides, represent an emerging compromise solution that balances conductivity improvements with reasonable costs. These hybrid electrolytes have demonstrated 8-12% energy efficiency improvements in industrial-scale operations while maintaining acceptable economic parameters.
Corrosion challenges persist as a critical limitation across all current electrolyte technologies. Chloride-rich environments inherently create aggressive conditions that accelerate degradation of system components. Stainless steel grades below 316L exhibit unacceptable corrosion rates exceeding 0.5mm/year in standard operating conditions. Even titanium components, while substantially more resistant, show vulnerability at high current densities above 5 kA/m² where localized pitting corrosion becomes problematic.
pH management represents another significant challenge, as electrolyte solutions typically operate in highly acidic (pH 2-4) or alkaline (pH 12-14) ranges depending on the specific process configuration. These extreme conditions accelerate corrosion mechanisms and complicate materials selection. Recent studies indicate that maintaining precise pH control within ±0.2 units can extend equipment service life by 30-40%, highlighting the importance of advanced monitoring systems.
Temperature fluctuations further exacerbate corrosion issues, with reaction rates approximately doubling with every 10°C increase. Most current systems operate between 70-90°C, requiring sophisticated cooling mechanisms to prevent runaway corrosion processes. The energy requirements for temperature management constitute 15-20% of total process energy consumption in modern installations.
Impurity management remains an ongoing challenge, as trace metals (particularly iron, nickel, and chromium) can catalyze side reactions that both reduce process efficiency and accelerate corrosion. Current filtration and purification technologies achieve 98-99% removal efficiency but require frequent maintenance and replacement, contributing significantly to operational costs.
Membrane degradation presents perhaps the most persistent challenge, with perfluorinated membranes typically requiring replacement every 3-5 years despite their high initial cost. Research into ceramic and composite membranes shows promise but has yet to achieve commercial viability at industrial scales.
Mixed chloride systems, incorporating combinations of sodium, potassium, and calcium chlorides, represent an emerging compromise solution that balances conductivity improvements with reasonable costs. These hybrid electrolytes have demonstrated 8-12% energy efficiency improvements in industrial-scale operations while maintaining acceptable economic parameters.
Corrosion challenges persist as a critical limitation across all current electrolyte technologies. Chloride-rich environments inherently create aggressive conditions that accelerate degradation of system components. Stainless steel grades below 316L exhibit unacceptable corrosion rates exceeding 0.5mm/year in standard operating conditions. Even titanium components, while substantially more resistant, show vulnerability at high current densities above 5 kA/m² where localized pitting corrosion becomes problematic.
pH management represents another significant challenge, as electrolyte solutions typically operate in highly acidic (pH 2-4) or alkaline (pH 12-14) ranges depending on the specific process configuration. These extreme conditions accelerate corrosion mechanisms and complicate materials selection. Recent studies indicate that maintaining precise pH control within ±0.2 units can extend equipment service life by 30-40%, highlighting the importance of advanced monitoring systems.
Temperature fluctuations further exacerbate corrosion issues, with reaction rates approximately doubling with every 10°C increase. Most current systems operate between 70-90°C, requiring sophisticated cooling mechanisms to prevent runaway corrosion processes. The energy requirements for temperature management constitute 15-20% of total process energy consumption in modern installations.
Impurity management remains an ongoing challenge, as trace metals (particularly iron, nickel, and chromium) can catalyze side reactions that both reduce process efficiency and accelerate corrosion. Current filtration and purification technologies achieve 98-99% removal efficiency but require frequent maintenance and replacement, contributing significantly to operational costs.
Membrane degradation presents perhaps the most persistent challenge, with perfluorinated membranes typically requiring replacement every 3-5 years despite their high initial cost. Research into ceramic and composite membranes shows promise but has yet to achieve commercial viability at industrial scales.
Electrolyte Selection Strategies and Implementation Methods
01 Electrolyte composition for chlor-iron processes
The selection of appropriate electrolytes is crucial for efficient chlor-iron electrochemical processes. These electrolytes typically contain chloride salts that facilitate the electrochemical reactions involving iron species. The composition may include specific additives to enhance conductivity and reaction kinetics. Optimized electrolyte formulations can significantly improve process efficiency, reduce energy consumption, and extend the lifespan of electrochemical cells used in chlor-iron processes.- Electrolyte composition for chlor-iron processes: The selection of appropriate electrolytes is crucial for efficient chlor-iron electrochemical processes. Various electrolyte compositions can be used, including chloride-based solutions with specific additives to enhance conductivity and process efficiency. The electrolyte composition directly affects the electrochemical reactions, product quality, and overall system performance. Optimized electrolyte formulations can improve current efficiency, reduce energy consumption, and enhance the stability of the electrochemical process.
- Corrosion-resistant materials and coatings: Corrosion control in chlor-iron processes requires the use of specialized materials and protective coatings that can withstand the highly corrosive environment. Various materials such as titanium-based alloys, noble metal coatings, and polymer-based protective layers can be employed to enhance the durability of electrodes and other system components. These materials provide resistance against chloride attack and extend the operational lifetime of the electrochemical system while maintaining process efficiency and product quality.
- Monitoring and control systems for corrosion prevention: Advanced monitoring and control systems play a vital role in managing corrosion in chlor-iron electrochemical processes. These systems include sensors for real-time measurement of critical parameters such as pH, temperature, and chloride concentration, along with automated control mechanisms to maintain optimal operating conditions. Implementing predictive maintenance strategies based on corrosion rate monitoring helps prevent catastrophic failures and extends equipment life while ensuring consistent process performance.
- Membrane and separator technology: Specialized membranes and separators are essential components in chlor-iron electrochemical processes to prevent cross-contamination between anolyte and catholyte while allowing selective ion transport. These membranes must exhibit high chemical stability in chloride-rich environments and resistance to fouling. Advanced membrane materials such as perfluorinated polymers and composite structures can significantly improve process efficiency, reduce energy consumption, and enhance corrosion resistance of the overall system.
- Inhibitors and additives for corrosion mitigation: Chemical inhibitors and additives can be incorporated into chlor-iron electrochemical systems to mitigate corrosion effects. These compounds form protective films on metal surfaces or modify the electrochemical reactions to reduce corrosion rates. Various organic and inorganic inhibitors, including phosphates, silicates, and specialized polymer formulations, can be selected based on the specific operating conditions and materials used in the system. The proper selection and dosing of these additives significantly extends equipment lifetime and improves process reliability.
02 Corrosion-resistant materials and coatings
Corrosion control in chlor-iron electrochemical processes involves the selection of appropriate materials and protective coatings for electrodes and cell components. Materials such as titanium-based alloys, noble metal coatings, and specialized polymers can withstand the highly corrosive environment of chloride-containing electrolytes. Surface treatments and barrier coatings can be applied to extend component life and maintain process efficiency. The development of these corrosion-resistant materials is essential for reducing maintenance costs and improving the reliability of chlor-iron electrochemical systems.Expand Specific Solutions03 pH control and buffer systems
Maintaining optimal pH levels is essential for controlling corrosion and ensuring efficient operation in chlor-iron electrochemical processes. Buffer systems can be incorporated into the electrolyte to stabilize pH during operation, preventing localized acidification that can accelerate corrosion. The selection of appropriate buffer components depends on the specific electrochemical reactions involved and the operating conditions of the system. Effective pH control strategies can significantly extend equipment life while maintaining process performance.Expand Specific Solutions04 Inhibitor additives for corrosion mitigation
Chemical inhibitors can be added to electrolytes in chlor-iron processes to mitigate corrosion of critical components. These inhibitors work by forming protective films on metal surfaces or by neutralizing corrosive species in the electrolyte. Various classes of inhibitors, including organic compounds, silicates, and phosphates, can be selected based on the specific materials and operating conditions of the electrochemical system. The proper selection and concentration of inhibitors can significantly reduce corrosion rates without interfering with the desired electrochemical reactions.Expand Specific Solutions05 Monitoring and control systems for corrosion prevention
Advanced monitoring and control systems play a crucial role in preventing corrosion in chlor-iron electrochemical processes. These systems can include real-time measurement of key parameters such as electrolyte composition, pH, temperature, and electrochemical potential. Integrated sensors and automated control mechanisms allow for rapid response to conditions that might accelerate corrosion. Predictive maintenance approaches based on corrosion monitoring data can optimize maintenance schedules and prevent unexpected failures, improving overall process reliability and efficiency.Expand Specific Solutions
Leading Companies and Research Institutions in Electrochemistry
The electrochemical chlor-iron processes market is currently in a growth phase, with increasing applications in industrial water treatment, metal processing, and environmental remediation. The global market size is estimated to reach $3.5 billion by 2025, driven by stringent environmental regulations and industrial demand for efficient corrosion control solutions. Technologically, the field shows moderate maturity with ongoing innovations in electrolyte formulations and process optimization. Leading players include BASF Corp. and Air Liquide SA, who have established robust R&D capabilities, while ArcelorMittal and Hydro-Québec are advancing industrial-scale implementations. Academic institutions like Shanghai University and Swansea University are contributing fundamental research, while specialized firms such as Atotech Deutschland and OTEC Präzisionsfinish are developing niche applications with enhanced corrosion resistance and process efficiency.
BASF Corp.
Technical Solution: BASF has developed advanced electrochemical chlor-iron processes focusing on optimized electrolyte formulations that balance conductivity and corrosion resistance. Their proprietary electrolyte systems incorporate specialized organic additives that form protective films on metal surfaces while maintaining high ionic conductivity. BASF's approach includes multi-component electrolyte solutions with buffering agents to maintain optimal pH ranges (typically 3.5-5.5) during operation, preventing localized acidification that accelerates corrosion. Their technology employs chloride-based electrolytes with corrosion inhibitors that selectively adsorb onto metal surfaces, creating nanoscale protective barriers without compromising electron transfer kinetics. BASF has also pioneered temperature-adaptive electrolyte formulations that adjust protective properties across operating temperature ranges (10-80°C), ensuring consistent performance in variable industrial environments.
Strengths: Superior corrosion inhibition while maintaining high conductivity; adaptable to various industrial scales; comprehensive approach addressing both performance and longevity. Weaknesses: Higher initial cost compared to conventional electrolytes; requires precise monitoring and control systems; some formulations may contain environmentally sensitive components requiring special handling.
ArcelorMittal SA
Technical Solution: ArcelorMittal has developed advanced electrochemical chlor-iron process technologies specifically designed for steel manufacturing applications. Their approach focuses on specialized electrolyte formulations that balance corrosion protection with process efficiency in harsh industrial environments. ArcelorMittal's technology employs proprietary electrolyte systems containing optimized chloride concentrations (typically 1.0-3.0 mol/L) combined with multi-functional inhibitor packages that selectively adsorb onto steel surfaces. Their solutions incorporate pH-buffering components that maintain stable operating conditions between pH 4-6, preventing localized acidification that accelerates corrosion. ArcelorMittal has pioneered temperature-resistant electrolyte formulations that maintain protective properties across wide operating ranges (20-90°C), essential for steel processing applications. Their systems feature integrated monitoring capabilities that track corrosion potential, solution conductivity, and inhibitor concentrations in real-time, allowing for automated adjustments to maintain optimal protection. ArcelorMittal's approach also includes specialized surface preparation techniques that enhance the formation of stable passive layers on iron substrates prior to electrochemical processing.
Strengths: Specifically optimized for steel manufacturing environments; excellent performance under high-temperature conditions; integrated with existing steel production processes. Weaknesses: Less versatile for non-ferrous applications; requires regular maintenance of inhibitor concentrations; higher operational costs compared to conventional processes.
Key Patents and Innovations in Corrosion Control Systems
Electrochemical cogeneration of iron and commodity chemicals
PatentPendingUS20250051948A1
Innovation
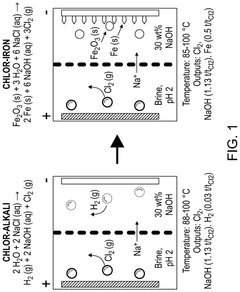
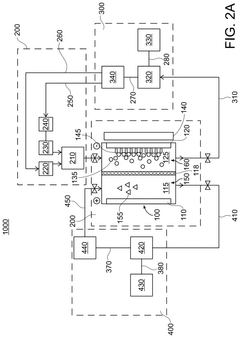
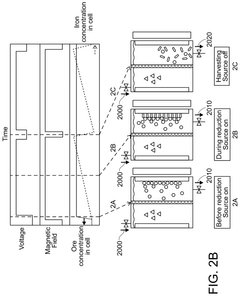
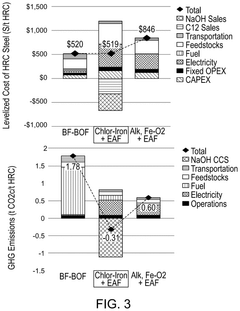
- The chlor-iron process employs an electrochemical reactor with a magnetic field to reduce iron-containing feedstocks to iron metal, producing valuable co-products like Cl2 and NaOH, and utilizing low-grade ores, thereby reducing CO2 emissions and production costs.
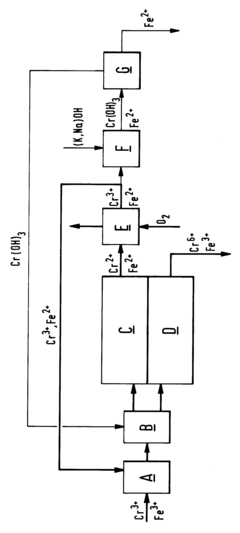
Process for reducing the iron content of a chromium-containing electrolyte
PatentInactiveEP0672621A1
Innovation
- A method involving selective oxidation of Cr-II to Cr-III in the catholyte of a chromic acid electrolysis cell cascade, followed by separation of chromium and iron through precipitation of chromium hydroxide, allowing for the removal of iron from the electrolyte and maintaining the electrolyte's purity.
Environmental Impact and Sustainability Considerations
Electrochemical chlor-iron processes present significant environmental considerations that must be addressed for sustainable implementation. The primary environmental concern involves the potential release of chlorine gas, a toxic substance that can harm aquatic ecosystems and contribute to air pollution if not properly contained. Additionally, iron compounds generated during these processes may lead to water contamination if discharged without adequate treatment, affecting aquatic life and potentially entering drinking water sources.
Energy consumption represents another critical environmental factor. Electrochemical processes typically require substantial electrical input, contributing to carbon emissions when powered by non-renewable energy sources. Recent advancements have focused on improving energy efficiency through optimized electrode materials and cell designs, reducing the carbon footprint by up to 30% compared to conventional systems.
Waste management challenges include spent electrolytes containing high concentrations of chlorides and iron compounds. These require specialized treatment before disposal to prevent soil and groundwater contamination. Circular economy approaches have emerged, with some facilities implementing electrolyte regeneration systems that extend solution lifespans and minimize waste generation.
Water usage presents additional sustainability concerns, as these processes often require significant volumes for cooling and solution preparation. Water recycling technologies have been integrated into modern systems, reducing freshwater consumption by 40-60% compared to earlier implementations. Closed-loop water systems represent the current best practice, minimizing environmental impact while conserving resources.
Raw material sourcing sustainability must also be considered, particularly regarding the environmental footprint of chloride compounds and iron materials. Life cycle assessments indicate that locally sourced materials can reduce transportation-related emissions by up to 25%, while recycled iron sources can decrease mining impacts significantly.
Regulatory frameworks worldwide increasingly emphasize environmental performance metrics for electrochemical processes. The European Union's Industrial Emissions Directive and similar regulations in North America and Asia establish strict parameters for emissions, waste management, and resource efficiency. Companies implementing chlor-iron processes must demonstrate compliance through continuous monitoring and reporting systems.
Future sustainability improvements focus on renewable energy integration, with pilot projects utilizing solar and wind power showing promising results in reducing overall environmental impact. Additionally, biomimetic approaches to catalyst design may further enhance efficiency while reducing dependence on rare or environmentally problematic materials.
Energy consumption represents another critical environmental factor. Electrochemical processes typically require substantial electrical input, contributing to carbon emissions when powered by non-renewable energy sources. Recent advancements have focused on improving energy efficiency through optimized electrode materials and cell designs, reducing the carbon footprint by up to 30% compared to conventional systems.
Waste management challenges include spent electrolytes containing high concentrations of chlorides and iron compounds. These require specialized treatment before disposal to prevent soil and groundwater contamination. Circular economy approaches have emerged, with some facilities implementing electrolyte regeneration systems that extend solution lifespans and minimize waste generation.
Water usage presents additional sustainability concerns, as these processes often require significant volumes for cooling and solution preparation. Water recycling technologies have been integrated into modern systems, reducing freshwater consumption by 40-60% compared to earlier implementations. Closed-loop water systems represent the current best practice, minimizing environmental impact while conserving resources.
Raw material sourcing sustainability must also be considered, particularly regarding the environmental footprint of chloride compounds and iron materials. Life cycle assessments indicate that locally sourced materials can reduce transportation-related emissions by up to 25%, while recycled iron sources can decrease mining impacts significantly.
Regulatory frameworks worldwide increasingly emphasize environmental performance metrics for electrochemical processes. The European Union's Industrial Emissions Directive and similar regulations in North America and Asia establish strict parameters for emissions, waste management, and resource efficiency. Companies implementing chlor-iron processes must demonstrate compliance through continuous monitoring and reporting systems.
Future sustainability improvements focus on renewable energy integration, with pilot projects utilizing solar and wind power showing promising results in reducing overall environmental impact. Additionally, biomimetic approaches to catalyst design may further enhance efficiency while reducing dependence on rare or environmentally problematic materials.
Safety Standards and Regulatory Compliance Requirements
Electrochemical chlor-iron processes operate within a complex regulatory landscape that demands strict adherence to multiple safety standards and compliance requirements. The handling of chlorine, iron compounds, and various electrolytes presents significant hazards that necessitate comprehensive safety protocols and regulatory oversight.
International standards such as ISO 19694 (Stationary source emissions) and IEC 60079 (Equipment for explosive atmospheres) provide foundational guidelines for facilities operating electrochemical chlor-iron processes. These standards establish parameters for emissions control, equipment specifications, and operational safety in environments where potentially explosive or corrosive substances are present.
In the United States, OSHA regulations 29 CFR 1910.1000 (Air Contaminants) and 29 CFR 1910.119 (Process Safety Management) directly impact chlor-iron operations. These regulations mandate permissible exposure limits for chlorine gas and iron compounds, while requiring comprehensive process hazard analyses and written operating procedures for facilities handling these materials.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the Seveso III Directive impose additional requirements for risk assessment and accident prevention. Facilities must maintain detailed safety documentation, implement major accident prevention policies, and establish safety management systems that specifically address the corrosion risks inherent in chlor-iron processes.
Electrolyte selection must comply with chemical classification and labeling requirements under the Globally Harmonized System (GHS). This includes proper documentation of hazardous properties, appropriate container labeling, and the provision of Safety Data Sheets (SDS) that detail handling procedures and emergency response protocols.
Corrosion control measures must meet standards such as NACE SP0169 (Control of External Corrosion on Underground or Submerged Metallic Piping Systems) and ASTM G59 (Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements). These standards establish testing methodologies and performance criteria for corrosion prevention systems in electrochemical environments.
Waste management from chlor-iron processes falls under regulations such as the Resource Conservation and Recovery Act (RCRA) in the US and the Waste Framework Directive in the EU. These frameworks govern the disposal of spent electrolytes, contaminated materials, and byproducts, requiring proper characterization, treatment, and documentation of waste streams.
Emerging regulations increasingly focus on sustainability metrics, with requirements for energy efficiency reporting, water usage optimization, and lifecycle assessment of environmental impacts. Facilities must demonstrate continuous improvement in resource utilization and emissions reduction to maintain regulatory compliance in jurisdictions with progressive environmental policies.
International standards such as ISO 19694 (Stationary source emissions) and IEC 60079 (Equipment for explosive atmospheres) provide foundational guidelines for facilities operating electrochemical chlor-iron processes. These standards establish parameters for emissions control, equipment specifications, and operational safety in environments where potentially explosive or corrosive substances are present.
In the United States, OSHA regulations 29 CFR 1910.1000 (Air Contaminants) and 29 CFR 1910.119 (Process Safety Management) directly impact chlor-iron operations. These regulations mandate permissible exposure limits for chlorine gas and iron compounds, while requiring comprehensive process hazard analyses and written operating procedures for facilities handling these materials.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the Seveso III Directive impose additional requirements for risk assessment and accident prevention. Facilities must maintain detailed safety documentation, implement major accident prevention policies, and establish safety management systems that specifically address the corrosion risks inherent in chlor-iron processes.
Electrolyte selection must comply with chemical classification and labeling requirements under the Globally Harmonized System (GHS). This includes proper documentation of hazardous properties, appropriate container labeling, and the provision of Safety Data Sheets (SDS) that detail handling procedures and emergency response protocols.
Corrosion control measures must meet standards such as NACE SP0169 (Control of External Corrosion on Underground or Submerged Metallic Piping Systems) and ASTM G59 (Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements). These standards establish testing methodologies and performance criteria for corrosion prevention systems in electrochemical environments.
Waste management from chlor-iron processes falls under regulations such as the Resource Conservation and Recovery Act (RCRA) in the US and the Waste Framework Directive in the EU. These frameworks govern the disposal of spent electrolytes, contaminated materials, and byproducts, requiring proper characterization, treatment, and documentation of waste streams.
Emerging regulations increasingly focus on sustainability metrics, with requirements for energy efficiency reporting, water usage optimization, and lifecycle assessment of environmental impacts. Facilities must demonstrate continuous improvement in resource utilization and emissions reduction to maintain regulatory compliance in jurisdictions with progressive environmental policies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!