Electrochemical Ironmaking: Managing Impurity Elements In Low-Grade Ores
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Ironmaking Background and Objectives
Electrochemical ironmaking represents a revolutionary approach to iron production that diverges significantly from traditional blast furnace methods. This technology has evolved over several decades, with initial conceptual work dating back to the 1970s, though substantial research acceleration has occurred only in the past 15-20 years. The fundamental principle involves using electricity to extract iron from its oxide form through an electrochemical cell, rather than relying on carbon-based reduction processes that dominate conventional ironmaking.
The evolution of this technology has been driven by increasing environmental pressures on the steel industry, which currently accounts for approximately 7-9% of global CO2 emissions. Traditional blast furnace operations require substantial carbon inputs, primarily in the form of metallurgical coal, resulting in significant carbon dioxide emissions. As global climate policies tighten, the steel industry faces mounting pressure to decarbonize its operations, creating a strong impetus for alternative production methods.
Recent technological advancements in electrochemistry, materials science, and renewable electricity generation have converged to make electrochemical ironmaking increasingly viable. The development trajectory has seen progress from laboratory-scale experiments to pilot demonstrations, with several research institutions and companies now operating small-scale proof-of-concept facilities. These developments suggest that the technology is approaching a critical inflection point in its maturity curve.
A particularly significant aspect of electrochemical ironmaking is its potential compatibility with low-grade iron ores. Traditional blast furnace operations typically require high-grade ores to maintain efficiency and product quality. However, as high-grade ore reserves diminish globally, the ability to process lower-grade resources becomes increasingly valuable. This presents both an opportunity and a challenge, as these ores contain higher concentrations of impurity elements that must be effectively managed.
The primary technical objective of current research is to develop efficient, scalable electrochemical processes capable of selectively extracting iron while managing impurity elements commonly found in low-grade ores, such as silicon, phosphorus, aluminum, and various trace metals. Secondary objectives include reducing energy consumption, increasing current efficiency, extending electrode lifespans, and designing cell configurations suitable for industrial-scale implementation.
Looking forward, the technology aims to achieve commercial viability within the next decade, with the ultimate goal of providing a carbon-neutral alternative to blast furnace ironmaking that can operate economically using increasingly abundant renewable electricity. Success in this domain could fundamentally transform the environmental footprint of the global steel industry while simultaneously expanding the resource base available for iron production.
The evolution of this technology has been driven by increasing environmental pressures on the steel industry, which currently accounts for approximately 7-9% of global CO2 emissions. Traditional blast furnace operations require substantial carbon inputs, primarily in the form of metallurgical coal, resulting in significant carbon dioxide emissions. As global climate policies tighten, the steel industry faces mounting pressure to decarbonize its operations, creating a strong impetus for alternative production methods.
Recent technological advancements in electrochemistry, materials science, and renewable electricity generation have converged to make electrochemical ironmaking increasingly viable. The development trajectory has seen progress from laboratory-scale experiments to pilot demonstrations, with several research institutions and companies now operating small-scale proof-of-concept facilities. These developments suggest that the technology is approaching a critical inflection point in its maturity curve.
A particularly significant aspect of electrochemical ironmaking is its potential compatibility with low-grade iron ores. Traditional blast furnace operations typically require high-grade ores to maintain efficiency and product quality. However, as high-grade ore reserves diminish globally, the ability to process lower-grade resources becomes increasingly valuable. This presents both an opportunity and a challenge, as these ores contain higher concentrations of impurity elements that must be effectively managed.
The primary technical objective of current research is to develop efficient, scalable electrochemical processes capable of selectively extracting iron while managing impurity elements commonly found in low-grade ores, such as silicon, phosphorus, aluminum, and various trace metals. Secondary objectives include reducing energy consumption, increasing current efficiency, extending electrode lifespans, and designing cell configurations suitable for industrial-scale implementation.
Looking forward, the technology aims to achieve commercial viability within the next decade, with the ultimate goal of providing a carbon-neutral alternative to blast furnace ironmaking that can operate economically using increasingly abundant renewable electricity. Success in this domain could fundamentally transform the environmental footprint of the global steel industry while simultaneously expanding the resource base available for iron production.
Market Demand Analysis for Low-Grade Ore Processing
The global iron and steel industry is experiencing a paradigm shift driven by environmental regulations, resource depletion, and economic pressures. Traditional ironmaking processes rely heavily on high-grade iron ores, which are becoming increasingly scarce and expensive. This scarcity has created a significant market opportunity for technologies capable of efficiently processing low-grade ores containing higher levels of impurities such as phosphorus, sulfur, silicon, and aluminum.
Market analysis indicates that the demand for low-grade ore processing technologies is projected to grow substantially over the next decade. This growth is primarily fueled by the steel industry's need to reduce production costs while meeting stringent environmental standards. Countries with abundant low-grade iron ore reserves, including China, India, Brazil, and Australia, represent particularly promising markets for electrochemical ironmaking technologies.
The economic incentive for developing effective low-grade ore processing methods is compelling. High-grade iron ore prices have shown significant volatility, with premium grades commanding substantial price premiums over lower grades. This price differential creates a strong business case for technologies that can upgrade or directly process lower-grade materials, potentially offering cost savings of 15-30% compared to conventional processes when factoring in reduced raw material costs.
Environmental regulations worldwide are becoming increasingly stringent regarding CO2 emissions and other pollutants associated with traditional ironmaking. The steel industry, responsible for approximately 7-9% of global CO2 emissions, faces mounting pressure to decarbonize. Technologies that can process low-grade ores while reducing carbon emissions have a distinct market advantage in this regulatory landscape.
Market research reveals growing interest from major steel producers in alternative ironmaking technologies. Several leading companies have announced investments in research and pilot projects focused on electrochemical processes that can handle higher impurity levels. This corporate interest signals recognition of both the economic and environmental benefits of such technologies.
Regional market analysis shows varying degrees of readiness for adoption. Developing economies with growing steel sectors and limited access to high-grade ores demonstrate the highest immediate demand. Meanwhile, developed economies are primarily driven by environmental considerations and the need to maintain competitiveness in a carbon-constrained future.
The market for technologies specifically addressing impurity management in electrochemical ironmaking is still nascent but shows promising growth potential. As traditional high-grade ore reserves continue to deplete, the ability to efficiently process lower-grade alternatives will become increasingly valuable, creating sustained demand for innovative solutions in this space.
Market analysis indicates that the demand for low-grade ore processing technologies is projected to grow substantially over the next decade. This growth is primarily fueled by the steel industry's need to reduce production costs while meeting stringent environmental standards. Countries with abundant low-grade iron ore reserves, including China, India, Brazil, and Australia, represent particularly promising markets for electrochemical ironmaking technologies.
The economic incentive for developing effective low-grade ore processing methods is compelling. High-grade iron ore prices have shown significant volatility, with premium grades commanding substantial price premiums over lower grades. This price differential creates a strong business case for technologies that can upgrade or directly process lower-grade materials, potentially offering cost savings of 15-30% compared to conventional processes when factoring in reduced raw material costs.
Environmental regulations worldwide are becoming increasingly stringent regarding CO2 emissions and other pollutants associated with traditional ironmaking. The steel industry, responsible for approximately 7-9% of global CO2 emissions, faces mounting pressure to decarbonize. Technologies that can process low-grade ores while reducing carbon emissions have a distinct market advantage in this regulatory landscape.
Market research reveals growing interest from major steel producers in alternative ironmaking technologies. Several leading companies have announced investments in research and pilot projects focused on electrochemical processes that can handle higher impurity levels. This corporate interest signals recognition of both the economic and environmental benefits of such technologies.
Regional market analysis shows varying degrees of readiness for adoption. Developing economies with growing steel sectors and limited access to high-grade ores demonstrate the highest immediate demand. Meanwhile, developed economies are primarily driven by environmental considerations and the need to maintain competitiveness in a carbon-constrained future.
The market for technologies specifically addressing impurity management in electrochemical ironmaking is still nascent but shows promising growth potential. As traditional high-grade ore reserves continue to deplete, the ability to efficiently process lower-grade alternatives will become increasingly valuable, creating sustained demand for innovative solutions in this space.
Current Challenges in Impurity Management Technologies
The management of impurity elements in electrochemical ironmaking processes presents significant technical challenges that continue to impede widespread industrial adoption. Current impurity management technologies face several critical limitations when processing low-grade iron ores, which typically contain elevated levels of silicon, phosphorus, sulfur, and various transition metals.
Conventional pyrometallurgical separation methods struggle with selective removal of impurities during electrochemical processes due to the fundamentally different reaction mechanisms involved. Unlike traditional blast furnace operations where slag formation facilitates impurity removal, electrochemical systems lack efficient in-situ separation mechanisms, resulting in impurity co-deposition or contamination of the iron product.
Membrane technology, while promising for selective ion transport, suffers from rapid fouling when exposed to complex ore compositions. Current ceramic and polymer-based membranes demonstrate insufficient durability under the harsh electrochemical conditions required for ironmaking, with membrane lifespans typically limited to 200-300 operational hours before performance degradation becomes unacceptable.
Pre-treatment leaching processes designed to remove impurities prior to electrochemical reduction achieve only partial success, with removal efficiencies ranging from 40-75% depending on the specific impurity element. These processes also generate significant volumes of acidic waste streams requiring additional treatment, offsetting some of the environmental benefits of electrochemical ironmaking.
Real-time monitoring and control systems for impurity levels remain underdeveloped for electrochemical ironmaking applications. Current sensor technologies cannot withstand the corrosive electrolyte environments or provide accurate measurements at the required timescales, limiting the implementation of adaptive process control strategies.
Energy consumption presents another critical challenge, as selective electrochemical separation of impurities typically requires precise potential control and often results in parasitic reactions that reduce overall energy efficiency. Studies indicate that impurity management can account for 15-30% of the total energy consumption in electrochemical ironmaking processes.
Scaling these technologies from laboratory to industrial scale introduces additional complexities. Bench-scale systems that demonstrate effective impurity management often encounter unforeseen challenges when scaled up, including uneven current distribution, heat management issues, and accelerated electrode degradation in the presence of impurities.
Economically viable solutions must balance technical performance with cost considerations, as many advanced separation technologies (such as functionalized electrodes or specialized ion-exchange materials) remain prohibitively expensive for large-scale implementation, particularly when processing low-value feedstocks like low-grade ores.
Conventional pyrometallurgical separation methods struggle with selective removal of impurities during electrochemical processes due to the fundamentally different reaction mechanisms involved. Unlike traditional blast furnace operations where slag formation facilitates impurity removal, electrochemical systems lack efficient in-situ separation mechanisms, resulting in impurity co-deposition or contamination of the iron product.
Membrane technology, while promising for selective ion transport, suffers from rapid fouling when exposed to complex ore compositions. Current ceramic and polymer-based membranes demonstrate insufficient durability under the harsh electrochemical conditions required for ironmaking, with membrane lifespans typically limited to 200-300 operational hours before performance degradation becomes unacceptable.
Pre-treatment leaching processes designed to remove impurities prior to electrochemical reduction achieve only partial success, with removal efficiencies ranging from 40-75% depending on the specific impurity element. These processes also generate significant volumes of acidic waste streams requiring additional treatment, offsetting some of the environmental benefits of electrochemical ironmaking.
Real-time monitoring and control systems for impurity levels remain underdeveloped for electrochemical ironmaking applications. Current sensor technologies cannot withstand the corrosive electrolyte environments or provide accurate measurements at the required timescales, limiting the implementation of adaptive process control strategies.
Energy consumption presents another critical challenge, as selective electrochemical separation of impurities typically requires precise potential control and often results in parasitic reactions that reduce overall energy efficiency. Studies indicate that impurity management can account for 15-30% of the total energy consumption in electrochemical ironmaking processes.
Scaling these technologies from laboratory to industrial scale introduces additional complexities. Bench-scale systems that demonstrate effective impurity management often encounter unforeseen challenges when scaled up, including uneven current distribution, heat management issues, and accelerated electrode degradation in the presence of impurities.
Economically viable solutions must balance technical performance with cost considerations, as many advanced separation technologies (such as functionalized electrodes or specialized ion-exchange materials) remain prohibitively expensive for large-scale implementation, particularly when processing low-value feedstocks like low-grade ores.
Existing Impurity Removal Solutions for Low-Grade Ores
01 Removal of impurities in electrochemical ironmaking processes
Various methods are employed to remove impurities during electrochemical ironmaking. These techniques focus on eliminating elements such as sulfur, phosphorus, and other contaminants that can negatively affect the quality of the final iron product. The processes may involve specific electrode configurations, electrolyte compositions, or post-processing treatments designed to selectively remove or neutralize impurity elements while maintaining the integrity of the iron being produced.- Removal of impurities in electrochemical ironmaking processes: Various techniques are employed to remove impurities during electrochemical ironmaking. These methods focus on eliminating elements such as sulfur, phosphorus, and silicon that can negatively affect the quality of the final iron product. The processes often involve specific electrode configurations, electrolyte compositions, or post-treatment steps designed to selectively separate impurities from the iron during or after the electrochemical reduction process.
- Electrolyte composition for controlling impurity elements: The composition of electrolytes plays a crucial role in controlling impurity elements during electrochemical ironmaking. Specific electrolyte formulations can help prevent the co-deposition of unwanted elements or facilitate their selective removal. These electrolytes may contain additives that form complexes with impurities, making them less likely to be incorporated into the iron product, or components that alter the reduction potential of certain impurity elements.
- Electrode materials and configurations for impurity management: The selection of electrode materials and their configurations significantly impacts the management of impurity elements in electrochemical ironmaking. Certain electrode materials demonstrate selectivity toward iron deposition while rejecting impurities. Advanced electrode designs can create localized conditions that favor the separation of iron from contaminants. Multi-electrode systems may also be employed to create sequential reduction zones that progressively purify the iron.
- Process parameters optimization for impurity control: Optimization of process parameters such as current density, temperature, pH, and potential can significantly influence the behavior of impurity elements during electrochemical ironmaking. By carefully controlling these parameters, it's possible to create conditions that minimize the co-deposition of impurities or enhance their removal. Pulsed current techniques, temperature gradients, and controlled potential methods are among the approaches used to achieve higher purity iron products.
- Post-treatment methods for impurity removal: Various post-treatment methods are applied to remove residual impurities from electrochemically produced iron. These techniques include heat treatment processes that cause impurities to segregate to grain boundaries or surfaces, secondary electrochemical treatments that selectively dissolve impurity elements, and chemical leaching processes designed to extract specific contaminants. The combination of electrochemical ironmaking with appropriate post-treatment steps can result in high-purity iron products suitable for specialized applications.
02 Electrolyte composition for controlling impurity elements
The composition of electrolytes used in electrochemical ironmaking significantly impacts the behavior of impurity elements. Specialized electrolyte formulations can help control the dissolution, migration, and deposition of various impurities during the electrochemical process. By adjusting parameters such as pH, ionic strength, and specific additives, the electrolyte can be optimized to minimize the incorporation of unwanted elements into the iron product while enhancing the removal of impurities from the system.Expand Specific Solutions03 Electrode materials and configurations for impurity management
The selection of electrode materials and their configurations plays a crucial role in managing impurity elements during electrochemical ironmaking. Specific electrode compositions can exhibit selective reactivity toward certain impurities, facilitating their removal from the system. Advanced electrode designs may incorporate catalytic materials or structured surfaces that enhance the separation of iron from contaminants. The arrangement of electrodes within the cell can also create zones that promote the segregation and removal of impurity elements.Expand Specific Solutions04 Process parameters affecting impurity behavior in electrochemical ironmaking
Operating parameters such as current density, temperature, and potential significantly influence the behavior of impurity elements during electrochemical ironmaking. By carefully controlling these parameters, it is possible to create conditions that favor the selective deposition of pure iron while rejecting impurities. Temperature management affects the solubility and mobility of various elements, while applied potential can be tuned to fall within ranges that minimize co-deposition of unwanted elements. Pulsed current techniques may also be employed to enhance purity by providing intervals for impurity desorption.Expand Specific Solutions05 Post-processing techniques for impurity removal in electrochemical iron products
After the primary electrochemical ironmaking process, various post-processing techniques can be applied to further reduce impurity content. These may include heat treatments that promote the migration of impurities to grain boundaries for easier removal, secondary electrochemical refining steps, or chemical treatments designed to selectively react with and extract specific impurity elements. Such post-processing approaches are particularly important for applications requiring high-purity iron with strictly controlled impurity profiles.Expand Specific Solutions
Key Industry Players in Electrochemical Metallurgy
Electrochemical ironmaking for low-grade ores is in an early development stage, with market growth driven by decarbonization initiatives in the steel industry. The technology shows promising potential but remains commercially immature, with an estimated market size of $2-5 billion by 2030. Key players demonstrate varying levels of technological readiness: Electra Steel leads with pilot-scale operations specifically targeting impurity management, while established entities like Form Energy, JFE Steel, and Midrex Technologies contribute significant R&D. Academic institutions (University of Science & Technology Beijing, Central South University) provide fundamental research support. The competitive landscape features both specialized startups and diversified industrial conglomerates (BASF, Kobe Steel, Panasonic) developing complementary technologies for electrochemical processing of impurity-rich iron ores.
Form Energy, Inc.
Technical Solution: Form Energy has developed an innovative iron-air battery technology that, while primarily focused on energy storage, has led to significant advancements in electrochemical ironmaking processes that address impurity management. Their "reversible rust" technology has been adapted to create a novel approach for producing high-purity iron from low-grade ores. The process utilizes specialized electrochemical cells where iron ions are selectively extracted from ore slurries containing multiple impurity elements. Form Energy's system employs proprietary electrode materials and carefully formulated electrolytes that preferentially interact with iron while rejecting common impurities like phosphorus, silicon, and manganese. The technology operates at ambient temperatures, dramatically reducing energy requirements compared to conventional pyrometallurgical methods. A key innovation is their multi-stage purification approach that progressively removes different impurity elements based on their electrochemical properties. The process includes a regenerative electrolyte management system that maintains optimal performance while minimizing waste generation. Form Energy's technology enables the production of iron with exceptionally high purity (>99.9%) from increasingly lower-grade ore sources that would be uneconomical with traditional methods.
Strengths: Extremely low energy consumption compared to conventional methods; ability to produce ultra-high purity iron; ambient temperature operation reducing infrastructure requirements; potential for integration with renewable energy sources. Weaknesses: Lower production rates compared to conventional methods; challenges with processing certain complex ore types; higher capital costs for specialized electrochemical equipment; limited demonstration at industrial scale.
ElectraSteel, Inc.
Technical Solution: ElectraSteel has pioneered a molten oxide electrolysis (MOE) approach specifically designed for low-grade iron ores with complex impurity profiles. Their proprietary technology utilizes specialized inert anodes and carefully formulated electrolytes that enable selective electrodeposition of iron while managing problematic impurities. The process operates at temperatures between 1400-1600°C, where the molten oxide electrolyte facilitates the separation of iron from impurities like phosphorus, sulfur, and silicon. ElectraSteel's innovation includes a multi-stage impurity management system that first removes volatile impurities through pre-treatment, followed by electrochemical separation during the electrolysis process. Their technology incorporates real-time monitoring of impurity concentrations and automated adjustment of electrochemical parameters to maintain product quality despite variations in feed composition. This adaptive approach allows for processing ores with impurity levels that would be prohibitive for conventional ironmaking methods, while producing high-purity iron suitable for demanding applications.
Strengths: Direct production of high-purity iron from low-grade ores; elimination of traditional coking and sintering steps; significant reduction in carbon emissions; ability to process ores with variable impurity profiles. Weaknesses: High energy requirements for maintaining molten electrolyte; challenges with electrode durability in harsh electrochemical environments; complex process control requirements; limited commercial-scale demonstration.
Critical Patents in Electrochemical Impurity Management
Removal of impurities contained in iron ores
PatentWO2025101838A1
Innovation
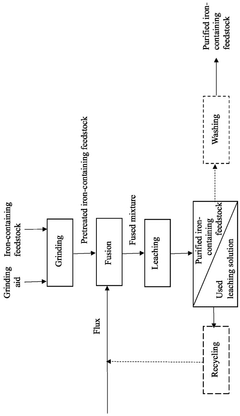
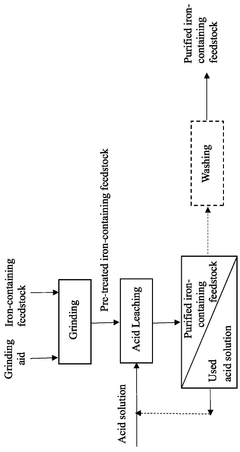
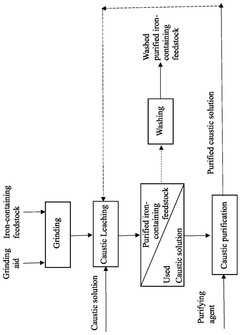
- A method involving grinding the iron-containing feedstock with an alkali metal chloride or fluoride salt as a grinding aid, followed by fusion with a metal borate flux and leaching with a suitable solution to remove impurities, resulting in a purified iron-containing feedstock.
Method of melt-removing impurity elements from iron
PatentInactiveUS6368380B1
Innovation
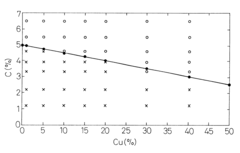
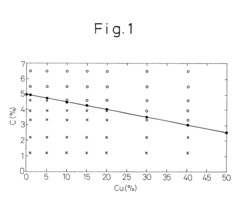
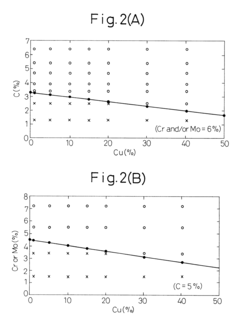
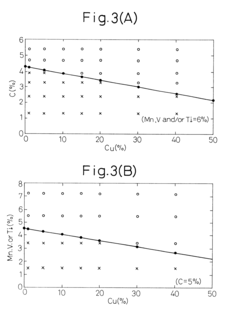
- A method involving melting iron scraps in an oxygen-containing atmosphere, adding C and suitable alloy elements to separate the molten iron into Fe-enriched and Cu-enriched layers, utilizing specific gravity differences to precipitate and remove Cu, with optional additions of Cr, Mo, Mn, V, Ti, and Ag to enhance separation efficiency.
Environmental Impact Assessment of Electrochemical Methods
The environmental impact assessment of electrochemical ironmaking methods reveals significant advantages over conventional blast furnace processes. These novel approaches substantially reduce carbon dioxide emissions by eliminating the need for coke and coal as reducing agents, instead utilizing electricity which can be sourced from renewable energy. Studies indicate potential CO2 emission reductions of 60-90% compared to traditional methods, depending on the electricity source.
Water usage patterns in electrochemical processes differ markedly from conventional methods. While traditional ironmaking requires substantial water for cooling and gas cleaning, electrochemical methods typically demand water for electrolyte preparation and maintenance. The closed-loop nature of many electrochemical systems allows for more efficient water recycling, potentially reducing overall freshwater consumption by 30-50%.
Waste generation profiles show promising improvements. Electrochemical methods produce significantly less solid waste, with some processes generating recoverable by-products rather than slag. The elimination of sintering and coking operations removes associated particulate emissions and hazardous air pollutants, including sulfur dioxide and volatile organic compounds, improving local air quality around production facilities.
Land use requirements for electrochemical facilities are generally smaller than traditional integrated steel plants, reducing habitat disruption and allowing for more flexible siting options, potentially closer to urban centers where electricity infrastructure is robust. This proximity can reduce transportation-related environmental impacts in the supply chain.
Energy efficiency assessments reveal complex trade-offs. While electrochemical processes eliminate combustion inefficiencies, they introduce electrical conversion losses. However, technological advancements in electrode materials and cell design are steadily improving efficiency metrics, with recent laboratory demonstrations achieving energy efficiencies of 70-80%.
Life cycle assessments indicate that managing impurities in low-grade ores through electrochemical methods may reduce the need for extensive pre-treatment processes that typically consume significant energy and chemicals. This advantage becomes particularly relevant as high-grade ore reserves diminish globally, making the environmental case for electrochemical processing of lower-grade resources increasingly compelling.
Regulatory compliance is generally more straightforward for electrochemical methods due to their reduced emission profiles, potentially accelerating permitting processes and reducing compliance costs compared to conventional ironmaking facilities that face increasingly stringent environmental regulations worldwide.
Water usage patterns in electrochemical processes differ markedly from conventional methods. While traditional ironmaking requires substantial water for cooling and gas cleaning, electrochemical methods typically demand water for electrolyte preparation and maintenance. The closed-loop nature of many electrochemical systems allows for more efficient water recycling, potentially reducing overall freshwater consumption by 30-50%.
Waste generation profiles show promising improvements. Electrochemical methods produce significantly less solid waste, with some processes generating recoverable by-products rather than slag. The elimination of sintering and coking operations removes associated particulate emissions and hazardous air pollutants, including sulfur dioxide and volatile organic compounds, improving local air quality around production facilities.
Land use requirements for electrochemical facilities are generally smaller than traditional integrated steel plants, reducing habitat disruption and allowing for more flexible siting options, potentially closer to urban centers where electricity infrastructure is robust. This proximity can reduce transportation-related environmental impacts in the supply chain.
Energy efficiency assessments reveal complex trade-offs. While electrochemical processes eliminate combustion inefficiencies, they introduce electrical conversion losses. However, technological advancements in electrode materials and cell design are steadily improving efficiency metrics, with recent laboratory demonstrations achieving energy efficiencies of 70-80%.
Life cycle assessments indicate that managing impurities in low-grade ores through electrochemical methods may reduce the need for extensive pre-treatment processes that typically consume significant energy and chemicals. This advantage becomes particularly relevant as high-grade ore reserves diminish globally, making the environmental case for electrochemical processing of lower-grade resources increasingly compelling.
Regulatory compliance is generally more straightforward for electrochemical methods due to their reduced emission profiles, potentially accelerating permitting processes and reducing compliance costs compared to conventional ironmaking facilities that face increasingly stringent environmental regulations worldwide.
Economic Viability Analysis of Low-Grade Ore Processing
The economic viability of processing low-grade iron ores through electrochemical ironmaking represents a critical consideration for industry stakeholders. Current economic analyses indicate that traditional pyrometallurgical processes become increasingly cost-prohibitive as ore grades decline below 30% iron content, primarily due to escalating energy requirements and flux consumption. Electrochemical approaches offer potential cost advantages through reduced energy intensity and elimination of coking coal dependency.
Capital expenditure comparisons reveal that electrochemical ironmaking facilities may require 15-20% lower initial investment than conventional blast furnaces when designed for processing low-grade ores. This advantage stems from simplified materials handling systems and reduced need for sintering plants. However, operational expenditures present a more complex picture, with electricity costs serving as the dominant variable in electrochemical processes.
Sensitivity analyses demonstrate that electrochemical ironmaking becomes economically competitive when electricity prices fall below $0.06/kWh, assuming current technology readiness levels. Regions with access to low-cost renewable energy sources therefore present particularly favorable conditions for implementation. The economic equation is further influenced by impurity management costs, which can represent 8-12% of total operational expenditures depending on ore composition.
Value chain considerations reveal additional economic benefits through potential recovery of valuable by-products. Selective electrochemical processes can isolate elements like vanadium, chromium, and titanium that are often present in low-grade ores, creating secondary revenue streams that improve overall project economics. This multi-product approach can enhance return on investment by 7-15% compared to single-product ironmaking operations.
Lifecycle cost assessments indicate that electrochemical processing of low-grade ores may achieve payback periods of 5-7 years under favorable market conditions, compared to 8-10 years for conventional technologies applied to similar resources. This improved financial performance is primarily attributed to lower energy costs and higher metal recovery rates, which can reach 95% compared to 80-85% in traditional processes.
Market risk factors include volatility in electricity pricing, regulatory uncertainties regarding carbon pricing, and potential supply chain disruptions affecting electrode materials. Mitigation strategies involve developing hybrid energy systems, securing long-term power purchase agreements, and investing in electrode recycling technologies to reduce dependency on critical materials.
The economic case strengthens significantly when environmental externalities are monetized. Carbon pricing mechanisms at $30-50 per ton of CO2 equivalent would improve the comparative economics of electrochemical ironmaking by an estimated 12-18%, potentially accelerating industry adoption despite higher technology risks.
Capital expenditure comparisons reveal that electrochemical ironmaking facilities may require 15-20% lower initial investment than conventional blast furnaces when designed for processing low-grade ores. This advantage stems from simplified materials handling systems and reduced need for sintering plants. However, operational expenditures present a more complex picture, with electricity costs serving as the dominant variable in electrochemical processes.
Sensitivity analyses demonstrate that electrochemical ironmaking becomes economically competitive when electricity prices fall below $0.06/kWh, assuming current technology readiness levels. Regions with access to low-cost renewable energy sources therefore present particularly favorable conditions for implementation. The economic equation is further influenced by impurity management costs, which can represent 8-12% of total operational expenditures depending on ore composition.
Value chain considerations reveal additional economic benefits through potential recovery of valuable by-products. Selective electrochemical processes can isolate elements like vanadium, chromium, and titanium that are often present in low-grade ores, creating secondary revenue streams that improve overall project economics. This multi-product approach can enhance return on investment by 7-15% compared to single-product ironmaking operations.
Lifecycle cost assessments indicate that electrochemical processing of low-grade ores may achieve payback periods of 5-7 years under favorable market conditions, compared to 8-10 years for conventional technologies applied to similar resources. This improved financial performance is primarily attributed to lower energy costs and higher metal recovery rates, which can reach 95% compared to 80-85% in traditional processes.
Market risk factors include volatility in electricity pricing, regulatory uncertainties regarding carbon pricing, and potential supply chain disruptions affecting electrode materials. Mitigation strategies involve developing hybrid energy systems, securing long-term power purchase agreements, and investing in electrode recycling technologies to reduce dependency on critical materials.
The economic case strengthens significantly when environmental externalities are monetized. Carbon pricing mechanisms at $30-50 per ton of CO2 equivalent would improve the comparative economics of electrochemical ironmaking by an estimated 12-18%, potentially accelerating industry adoption despite higher technology risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!