Membrane Selection For Alkaline Iron Electrowinning Cells
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkaline Iron Electrowinning Membrane Technology Background
Alkaline iron electrowinning represents a significant advancement in sustainable metal extraction technologies, emerging as an environmentally friendly alternative to traditional acidic electrowinning processes. The development of this technology can be traced back to the early 1970s when researchers began exploring alkaline solutions as electrolytes for iron recovery. However, substantial progress in this field only materialized in the late 1990s with the advent of improved membrane technologies and electrode materials.
The fundamental principle of alkaline iron electrowinning involves the electrolytic recovery of iron from alkaline solutions, typically with pH values ranging from 12 to 14. This process offers several advantages over conventional acidic methods, including reduced energy consumption, minimized hydrogen evolution, and decreased environmental impact due to the absence of acid mist formation.
Membrane technology plays a crucial role in alkaline iron electrowinning cells, serving as a separator between the anodic and cathodic compartments while facilitating selective ion transport. The evolution of membrane materials has been pivotal in addressing historical challenges associated with alkaline electrowinning, such as membrane fouling, degradation in highly caustic environments, and insufficient ion selectivity.
Early membrane systems employed in alkaline electrowinning primarily consisted of asbestos diaphragms, which presented significant health and environmental concerns. The 1980s witnessed the introduction of polymeric ion-exchange membranes, marking a substantial improvement in performance and safety. By the early 2000s, composite membranes combining organic and inorganic materials emerged, offering enhanced chemical stability and ionic conductivity in highly alkaline conditions.
Recent technological advancements have focused on developing specialized membranes capable of withstanding the harsh alkaline environment while maintaining high selectivity for iron ions. These include perfluorinated sulfonic acid membranes modified for alkaline stability, hydrocarbon-based anion exchange membranes, and ceramic-polymer hybrid membranes with tailored pore structures.
The global research landscape has witnessed significant contributions from institutions in China, Germany, Canada, and Australia, with each developing unique approaches to membrane design for alkaline electrowinning applications. Industry-academia collaborations have accelerated innovation in this field, resulting in membranes with increasingly optimized properties for iron electrowinning.
Current technological trends indicate a shift toward multifunctional membranes that not only separate electrode compartments but also actively participate in the electrochemical process through catalytic functionalities. Additionally, there is growing interest in developing membranes with self-cleaning properties to mitigate fouling issues that have historically plagued alkaline electrowinning operations.
The fundamental principle of alkaline iron electrowinning involves the electrolytic recovery of iron from alkaline solutions, typically with pH values ranging from 12 to 14. This process offers several advantages over conventional acidic methods, including reduced energy consumption, minimized hydrogen evolution, and decreased environmental impact due to the absence of acid mist formation.
Membrane technology plays a crucial role in alkaline iron electrowinning cells, serving as a separator between the anodic and cathodic compartments while facilitating selective ion transport. The evolution of membrane materials has been pivotal in addressing historical challenges associated with alkaline electrowinning, such as membrane fouling, degradation in highly caustic environments, and insufficient ion selectivity.
Early membrane systems employed in alkaline electrowinning primarily consisted of asbestos diaphragms, which presented significant health and environmental concerns. The 1980s witnessed the introduction of polymeric ion-exchange membranes, marking a substantial improvement in performance and safety. By the early 2000s, composite membranes combining organic and inorganic materials emerged, offering enhanced chemical stability and ionic conductivity in highly alkaline conditions.
Recent technological advancements have focused on developing specialized membranes capable of withstanding the harsh alkaline environment while maintaining high selectivity for iron ions. These include perfluorinated sulfonic acid membranes modified for alkaline stability, hydrocarbon-based anion exchange membranes, and ceramic-polymer hybrid membranes with tailored pore structures.
The global research landscape has witnessed significant contributions from institutions in China, Germany, Canada, and Australia, with each developing unique approaches to membrane design for alkaline electrowinning applications. Industry-academia collaborations have accelerated innovation in this field, resulting in membranes with increasingly optimized properties for iron electrowinning.
Current technological trends indicate a shift toward multifunctional membranes that not only separate electrode compartments but also actively participate in the electrochemical process through catalytic functionalities. Additionally, there is growing interest in developing membranes with self-cleaning properties to mitigate fouling issues that have historically plagued alkaline electrowinning operations.
Market Analysis for Alkaline Iron Electrowinning Applications
The alkaline iron electrowinning market is experiencing significant growth driven by increasing demand for sustainable energy storage solutions and the global push toward decarbonization. The market size for iron-based flow batteries, which rely on alkaline iron electrowinning technology, is projected to reach $2.7 billion by 2030, with a compound annual growth rate of 26% from 2023 to 2030.
Key market drivers include the expanding renewable energy sector, which requires efficient and cost-effective energy storage systems to manage intermittent power generation. Iron-based systems offer advantages over traditional vanadium flow batteries due to their lower material costs and environmental sustainability. The iron electrowinning process specifically benefits from iron's abundance, non-toxicity, and stable electrochemical properties.
Geographically, North America and Europe currently lead the market adoption, with significant research investments and commercial deployments. However, Asia-Pacific regions, particularly China and Australia, are rapidly expanding their market share through aggressive manufacturing scale-up and favorable government policies supporting green energy technologies.
By application segment, utility-scale energy storage represents the largest market share (approximately 45%), followed by industrial applications (30%) and microgrid/off-grid solutions (15%). The remaining market consists of specialized applications including backup power systems and remote area power supplies.
Customer requirements are increasingly focused on membrane performance metrics including ionic conductivity, chemical stability in highly alkaline environments (pH >13), mechanical durability, and cost-effectiveness. End users typically demand membranes with operational lifespans exceeding 10 years to ensure economic viability of electrowinning systems.
Price sensitivity varies by market segment, with utility-scale applications prioritizing long-term performance and total cost of ownership, while smaller industrial applications tend to be more sensitive to upfront capital costs. The average price point for high-performance alkaline-resistant membranes ranges from $200-500 per square meter, depending on specifications and volume.
Market challenges include competition from alternative energy storage technologies such as lithium-ion batteries, which benefit from established supply chains and manufacturing economies of scale. Additionally, technical barriers related to membrane fouling and degradation in industrial environments continue to limit widespread adoption in certain applications.
Future market growth will likely be driven by technological innovations that improve membrane selectivity and durability while reducing costs. Emerging applications in green hydrogen production and carbon capture technologies are expected to create new market opportunities for specialized alkaline-resistant membrane solutions in electrowinning processes.
Key market drivers include the expanding renewable energy sector, which requires efficient and cost-effective energy storage systems to manage intermittent power generation. Iron-based systems offer advantages over traditional vanadium flow batteries due to their lower material costs and environmental sustainability. The iron electrowinning process specifically benefits from iron's abundance, non-toxicity, and stable electrochemical properties.
Geographically, North America and Europe currently lead the market adoption, with significant research investments and commercial deployments. However, Asia-Pacific regions, particularly China and Australia, are rapidly expanding their market share through aggressive manufacturing scale-up and favorable government policies supporting green energy technologies.
By application segment, utility-scale energy storage represents the largest market share (approximately 45%), followed by industrial applications (30%) and microgrid/off-grid solutions (15%). The remaining market consists of specialized applications including backup power systems and remote area power supplies.
Customer requirements are increasingly focused on membrane performance metrics including ionic conductivity, chemical stability in highly alkaline environments (pH >13), mechanical durability, and cost-effectiveness. End users typically demand membranes with operational lifespans exceeding 10 years to ensure economic viability of electrowinning systems.
Price sensitivity varies by market segment, with utility-scale applications prioritizing long-term performance and total cost of ownership, while smaller industrial applications tend to be more sensitive to upfront capital costs. The average price point for high-performance alkaline-resistant membranes ranges from $200-500 per square meter, depending on specifications and volume.
Market challenges include competition from alternative energy storage technologies such as lithium-ion batteries, which benefit from established supply chains and manufacturing economies of scale. Additionally, technical barriers related to membrane fouling and degradation in industrial environments continue to limit widespread adoption in certain applications.
Future market growth will likely be driven by technological innovations that improve membrane selectivity and durability while reducing costs. Emerging applications in green hydrogen production and carbon capture technologies are expected to create new market opportunities for specialized alkaline-resistant membrane solutions in electrowinning processes.
Current Membrane Technologies and Challenges
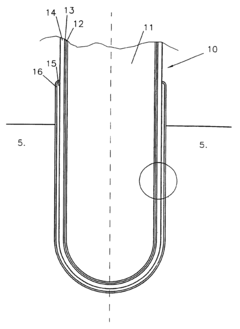
The membrane is a critical component in alkaline iron electrowinning cells, serving as a separator between the anolyte and catholyte compartments while allowing selective ion transport. Current membrane technologies for this application primarily include anion exchange membranes (AEMs), cation exchange membranes (CEMs), and bipolar membranes (BPMs), each with distinct properties and performance characteristics.
AEMs facilitate the transport of anions while blocking cations, making them particularly suitable for alkaline environments where hydroxide ion conductivity is essential. Leading commercial AEMs include Fumasep FAA-3, Sustainion, and Neosepta AMX. These membranes typically exhibit hydroxide conductivities ranging from 10-30 mS/cm under alkaline conditions, with varying degrees of chemical stability in high pH environments.
CEMs, which allow cation transport while blocking anions, are also employed in certain alkaline iron electrowinning configurations. Notable examples include Nafion, Fumasep FKS, and Neosepta CMX. While these membranes offer excellent proton conductivity in acidic media, their performance characteristics change significantly in alkaline environments, often resulting in reduced ionic conductivity.
Bipolar membranes, consisting of laminated anion and cation exchange layers, represent an emerging technology with potential benefits for electrowinning applications. These membranes can facilitate water dissociation at the interface, potentially enhancing system efficiency through local pH management.
Despite advances in membrane technology, significant challenges persist in alkaline iron electrowinning applications. Chemical stability remains a primary concern, as the highly alkaline environment (pH >14) combined with elevated temperatures (40-60°C) accelerates membrane degradation through hydrolysis of functional groups and polymer backbone deterioration. Commercial membranes typically demonstrate stability limitations above pH 13, particularly at elevated temperatures.
Iron fouling presents another substantial challenge, as iron hydroxide precipitates can accumulate on and within membrane structures, progressively reducing ionic conductivity and increasing cell resistance. This phenomenon is particularly problematic in systems with high iron concentrations or fluctuating pH conditions.
Mechanical integrity under pressure differentials and dimensional stability during hydration/dehydration cycles also remain significant engineering challenges. Membrane swelling in alkaline media can compromise sealing systems and lead to increased crossover of electrolyte components.
Additionally, the trade-off between selectivity and conductivity continues to challenge membrane development. Highly selective membranes typically exhibit lower ionic conductivity, necessitating careful optimization based on specific electrowinning parameters and objectives.
AEMs facilitate the transport of anions while blocking cations, making them particularly suitable for alkaline environments where hydroxide ion conductivity is essential. Leading commercial AEMs include Fumasep FAA-3, Sustainion, and Neosepta AMX. These membranes typically exhibit hydroxide conductivities ranging from 10-30 mS/cm under alkaline conditions, with varying degrees of chemical stability in high pH environments.
CEMs, which allow cation transport while blocking anions, are also employed in certain alkaline iron electrowinning configurations. Notable examples include Nafion, Fumasep FKS, and Neosepta CMX. While these membranes offer excellent proton conductivity in acidic media, their performance characteristics change significantly in alkaline environments, often resulting in reduced ionic conductivity.
Bipolar membranes, consisting of laminated anion and cation exchange layers, represent an emerging technology with potential benefits for electrowinning applications. These membranes can facilitate water dissociation at the interface, potentially enhancing system efficiency through local pH management.
Despite advances in membrane technology, significant challenges persist in alkaline iron electrowinning applications. Chemical stability remains a primary concern, as the highly alkaline environment (pH >14) combined with elevated temperatures (40-60°C) accelerates membrane degradation through hydrolysis of functional groups and polymer backbone deterioration. Commercial membranes typically demonstrate stability limitations above pH 13, particularly at elevated temperatures.
Iron fouling presents another substantial challenge, as iron hydroxide precipitates can accumulate on and within membrane structures, progressively reducing ionic conductivity and increasing cell resistance. This phenomenon is particularly problematic in systems with high iron concentrations or fluctuating pH conditions.
Mechanical integrity under pressure differentials and dimensional stability during hydration/dehydration cycles also remain significant engineering challenges. Membrane swelling in alkaline media can compromise sealing systems and lead to increased crossover of electrolyte components.
Additionally, the trade-off between selectivity and conductivity continues to challenge membrane development. Highly selective membranes typically exhibit lower ionic conductivity, necessitating careful optimization based on specific electrowinning parameters and objectives.
Comparative Analysis of Membrane Solutions
01 Anion exchange membranes for alkaline iron electrowinning
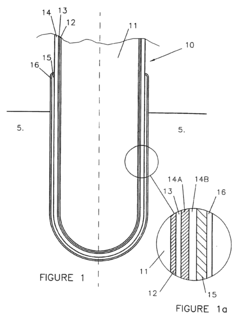
Anion exchange membranes are specifically designed for use in alkaline iron electrowinning cells to facilitate selective ion transport while preventing unwanted species migration. These membranes feature quaternary ammonium functional groups that provide high ionic conductivity and chemical stability in alkaline environments. The selective permeability of these membranes helps maintain electrolyte balance and improves current efficiency in the electrowinning process.- Anion exchange membranes for alkaline iron electrowinning: Anion exchange membranes are specifically designed for use in alkaline iron electrowinning cells to facilitate selective ion transport while preventing unwanted species migration. These membranes typically feature quaternary ammonium functional groups that provide high ionic conductivity and chemical stability in alkaline environments. The selective permeability of these membranes helps maintain electrolyte composition and improves current efficiency in the electrowinning process.
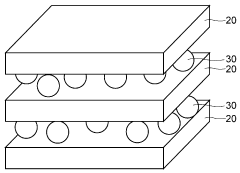
- Composite membranes with enhanced durability: Composite membranes combining multiple materials offer enhanced durability and performance in the harsh conditions of alkaline iron electrowinning cells. These membranes typically consist of a reinforcing substrate coated with ion-selective polymers that provide mechanical strength while maintaining desired electrochemical properties. The composite structure helps resist degradation from high pH environments and temperature fluctuations, extending membrane lifetime and reducing system maintenance requirements.
- Fluoropolymer-based membranes for chemical resistance: Fluoropolymer-based membranes offer exceptional chemical resistance in alkaline iron electrowinning applications. These membranes, often based on perfluorinated sulfonic acid polymers or modified PTFE structures, withstand the highly corrosive conditions present in electrowinning cells. Their chemical inertness prevents degradation from hydroxide ions and transition metal complexes, ensuring stable performance and minimal contamination of the electrolyte solution during the electrowinning process.
- Ceramic and inorganic membranes for high-temperature operation: Ceramic and inorganic membranes are designed for high-temperature operation in alkaline iron electrowinning cells. These membranes, typically composed of materials like zirconia, alumina, or silica, offer thermal stability and mechanical strength under extreme conditions. Their porous structure can be tailored to achieve specific ion selectivity while resisting fouling and scaling that commonly affect polymer-based membranes in high-temperature alkaline environments.
- Modified polymer membranes with iron-selective transport properties: Modified polymer membranes with iron-selective transport properties are specifically engineered for iron electrowinning applications. These membranes incorporate functional groups that facilitate selective transport of iron ions while blocking competing species. Surface modifications and polymer blending techniques are employed to enhance selectivity, reduce membrane fouling, and improve overall electrowinning efficiency. The tailored selectivity helps maintain electrolyte purity and increases the quality of the electrodeposited iron.
02 Polymer-based composite membranes with enhanced durability
Composite membranes combining polymeric materials with inorganic components offer superior mechanical strength and chemical resistance for alkaline iron electrowinning applications. These membranes typically incorporate fluoropolymers or polyethylene bases modified with ceramic particles or metal oxides to withstand the harsh alkaline conditions. The composite structure provides longer service life and maintains consistent performance under high pH and temperature conditions typical in iron electrowinning cells.Expand Specific Solutions03 Ion-selective membranes with reduced fouling properties
Specialized membranes designed to minimize fouling during alkaline iron electrowinning incorporate surface modifications or anti-fouling additives. These membranes feature hydrophilic coatings or charged surface groups that repel precipitates and prevent iron hydroxide deposition. The anti-fouling properties extend membrane lifetime and maintain consistent ion transport efficiency, reducing the need for frequent cleaning or replacement in industrial electrowinning operations.Expand Specific Solutions04 Membranes for high-efficiency iron recovery systems
Advanced membrane technologies specifically optimized for iron recovery in electrowinning cells focus on maximizing current efficiency and metal deposition rates. These membranes feature precisely controlled pore structures and thickness profiles to balance ion transport with mechanical integrity. The optimized design allows for higher current densities while maintaining selectivity, resulting in improved iron recovery rates and reduced energy consumption in industrial applications.Expand Specific Solutions05 Novel membrane materials for next-generation electrowinning cells
Emerging membrane materials for alkaline iron electrowinning include advanced polymers, nanomaterial composites, and biomimetic structures. These innovative materials offer unprecedented combinations of ionic conductivity, chemical stability, and mechanical strength. Research focuses on developing membranes with self-healing properties, temperature-responsive behavior, or integrated sensing capabilities to enable more efficient and intelligent electrowinning processes for iron recovery applications.Expand Specific Solutions
Key Manufacturers and Research Institutions
The alkaline iron electrowinning membrane selection market is currently in a growth phase, with increasing demand driven by sustainable metallurgical processing needs. The competitive landscape features established electrochemical technology leaders like Industrie De Nora and Asahi Kasei, who leverage their expertise in membrane technologies alongside mining giants such as Rio Tinto Alcan and Freeport-McMoRan. Academic institutions including MIT and Wuhan University of Technology are advancing fundamental research, while specialized players like AGC and Tosoh focus on high-performance membrane materials. The technology is approaching commercial maturity, with companies like TotalEnergies and Norsk Hydro investing in alkaline electrowinning to support green metal production initiatives, though optimization challenges remain in membrane durability and efficiency under industrial conditions.
Industrie De Nora SpA
Technical Solution: De Nora has developed advanced membrane technology specifically for alkaline iron electrowinning cells, focusing on ion-selective membranes that enhance efficiency while minimizing contamination. Their proprietary DSA (Dimensionally Stable Anode) technology, when combined with specialized cation exchange membranes, creates a system that significantly reduces energy consumption in iron electrowinning processes. The company's membrane selection approach prioritizes materials with high ionic conductivity and excellent chemical stability in highly alkaline environments (pH >14). Their membranes feature reinforced PTFE structures with specialized functional groups that allow for selective ion transport while maintaining mechanical integrity under the harsh conditions of iron electrowinning. De Nora's membrane technology also incorporates special surface treatments to minimize fouling and scaling, extending operational lifetimes in industrial settings.
Strengths: Superior chemical stability in highly alkaline environments; excellent selectivity for iron ions; reduced energy consumption; proven industrial durability. Weaknesses: Higher initial capital costs compared to non-membrane systems; requires specialized installation expertise; periodic maintenance to prevent membrane fouling in long-term operations.
Rio Tinto Alcan International Ltd.
Technical Solution: Rio Tinto Alcan has developed a comprehensive membrane selection framework for alkaline iron electrowinning cells that integrates with their broader hydrometallurgical expertise. Their approach focuses on perfluorinated sulfonic acid (PFSA) membranes modified specifically for iron electrowinning applications. The company has engineered membrane systems that can withstand the challenging conditions of iron electrowinning while maintaining high current efficiency. Their technology incorporates specialized membrane coatings that resist iron hydroxide precipitation and fouling, a common challenge in alkaline iron electrowinning. Rio Tinto's membrane selection process involves extensive testing under accelerated aging conditions to ensure long-term performance stability. Their systems typically employ composite membranes with reinforced structures to withstand the mechanical stresses of industrial electrowinning cells while maintaining optimal ion selectivity and conductivity properties.
Strengths: Excellent integration with existing hydrometallurgical processes; proven long-term stability in industrial settings; optimized for high current efficiency; reduced contamination of cathode products. Weaknesses: Higher implementation costs compared to traditional diaphragm cells; requires specialized operational expertise; performance can degrade in the presence of certain impurities in the electrolyte.
Critical Patents and Research in Ion-Exchange Membranes
Cells for the electrowinning of aluminium having dimensionally stable metal-based anodes
PatentInactiveUS6800192B2
Innovation
- The use of anodes with a metal-based substrate coated with an electrochemically active iron oxide-based layer, particularly hematite, operating at a lower temperature to limit iron solubility and contamination in the electrolyte, while maintaining dimensional stability and reducing thermal losses by controlling iron species concentration in the electrolyte.
Alkaline electrolyte membrane, electrode assembly and direct alcohol fuel cell
PatentWO2010109670A1
Innovation
- An inorganic alkaline electrolyte membrane composed of a layered double hydroxide, represented by the formula M2+1−xM3+x(OH)2Axn−x/mH2O, where M2+ is a divalent metal ion, M3+ is a trivalent metal ion, A^n− is a monovalent or divalent anion, and x ranges from 0.1 to 0.8, providing high heat resistance, durability, and anion conductivity, and can be used with non-noble metal catalysts in the cathode and anode layers.
Environmental Impact Assessment
The environmental impact of membrane selection for alkaline iron electrowinning cells extends across multiple ecological dimensions. Membrane materials and their manufacturing processes contribute significantly to the overall environmental footprint of electrowinning operations. Perfluorinated membranes, commonly used in these applications, involve production methods that release persistent organic pollutants and greenhouse gases. The manufacturing of these specialized membranes typically requires energy-intensive processes and hazardous chemicals, resulting in substantial carbon emissions and potential contamination of water sources.
During operational phases, membrane performance directly influences energy efficiency of the electrowinning process. Higher-quality membranes with optimal ion selectivity and conductivity reduce energy consumption by minimizing resistance and preventing unwanted side reactions. This energy reduction translates to lower greenhouse gas emissions, particularly in regions where electricity generation relies heavily on fossil fuels. Studies indicate that advanced composite membranes can reduce energy requirements by 15-20% compared to conventional options, representing significant environmental savings in large-scale operations.
Membrane durability and lifespan constitute another critical environmental consideration. Longer-lasting membranes reduce replacement frequency, thereby decreasing waste generation and resource consumption associated with manufacturing replacement components. Certain ceramic-polymer hybrid membranes demonstrate operational lifespans up to three times longer than traditional membranes, substantially reducing waste streams and associated environmental impacts over facility lifetimes.
Wastewater management represents a significant environmental challenge in alkaline iron electrowinning. Membrane selection directly affects the quality and quantity of wastewater produced, with implications for downstream treatment requirements and potential environmental contamination. Membranes with superior selectivity minimize cross-contamination between electrolyte compartments, reducing the concentration of harmful substances in wastewater streams and simplifying treatment processes.
End-of-life considerations for membranes present additional environmental challenges. Most current membrane materials have limited recyclability, often ending up in landfills after their operational life. Emerging biodegradable and recyclable membrane technologies offer promising alternatives, though these typically face performance limitations in the harsh chemical environment of alkaline iron electrowinning cells. Research into environmentally benign membrane disposal methods and circular economy approaches remains an active area of development within the industry.
During operational phases, membrane performance directly influences energy efficiency of the electrowinning process. Higher-quality membranes with optimal ion selectivity and conductivity reduce energy consumption by minimizing resistance and preventing unwanted side reactions. This energy reduction translates to lower greenhouse gas emissions, particularly in regions where electricity generation relies heavily on fossil fuels. Studies indicate that advanced composite membranes can reduce energy requirements by 15-20% compared to conventional options, representing significant environmental savings in large-scale operations.
Membrane durability and lifespan constitute another critical environmental consideration. Longer-lasting membranes reduce replacement frequency, thereby decreasing waste generation and resource consumption associated with manufacturing replacement components. Certain ceramic-polymer hybrid membranes demonstrate operational lifespans up to three times longer than traditional membranes, substantially reducing waste streams and associated environmental impacts over facility lifetimes.
Wastewater management represents a significant environmental challenge in alkaline iron electrowinning. Membrane selection directly affects the quality and quantity of wastewater produced, with implications for downstream treatment requirements and potential environmental contamination. Membranes with superior selectivity minimize cross-contamination between electrolyte compartments, reducing the concentration of harmful substances in wastewater streams and simplifying treatment processes.
End-of-life considerations for membranes present additional environmental challenges. Most current membrane materials have limited recyclability, often ending up in landfills after their operational life. Emerging biodegradable and recyclable membrane technologies offer promising alternatives, though these typically face performance limitations in the harsh chemical environment of alkaline iron electrowinning cells. Research into environmentally benign membrane disposal methods and circular economy approaches remains an active area of development within the industry.
Cost-Benefit Analysis of Membrane Options
The economic viability of membrane implementation in alkaline iron electrowinning cells requires thorough cost-benefit analysis across multiple dimensions. Initial acquisition costs vary significantly among membrane types, with anion exchange membranes typically ranging from $100-300/m², cation exchange membranes between $80-250/m², and composite bipolar membranes commanding premium prices of $200-500/m². These base costs must be evaluated against performance characteristics in alkaline environments.
Operational lifetime represents a critical economic factor, as membrane degradation in highly alkaline conditions directly impacts replacement frequency. Premium fluoropolymer-based membranes demonstrate superior chemical stability with operational lifespans of 3-5 years in pH>14 environments, justifying their 30-50% price premium over standard hydrocarbon membranes that typically require replacement every 12-18 months under similar conditions.
Energy efficiency gains constitute a major benefit offsetting membrane costs. High-selectivity membranes can reduce cell voltage by 0.2-0.4V, translating to energy savings of 10-20% in typical iron electrowinning operations. For industrial-scale facilities consuming 5-10 MW, this represents annual savings of $350,000-700,000 at average industrial electricity rates.
Maintenance requirements and downtime costs vary substantially between membrane options. Fouling-resistant membranes with modified surface properties may command a 15-25% price premium but reduce cleaning frequency by 40-60%, minimizing production interruptions valued at $5,000-15,000 per day for mid-sized operations.
Product quality improvements delivered by superior membrane selectivity generate significant downstream value. Enhanced iron purity from 98.5% to 99.5% can increase product value by $50-150 per ton, while reduced contamination decreases post-processing costs by 5-15%.
Environmental compliance benefits must also factor into economic calculations. Advanced membranes that minimize waste generation can reduce effluent treatment costs by $2-5 per cubic meter and potentially avoid regulatory penalties ranging from $10,000-100,000 for non-compliance incidents.
The total cost of ownership analysis reveals that despite higher initial investment, premium membranes optimized for alkaline iron electrowinning typically achieve return on investment within 8-14 months through combined operational savings. Sensitivity analysis indicates that facilities with higher energy costs or stricter product specifications benefit most from investing in advanced membrane technologies, with potential five-year net present value advantages of 30-45% compared to basic membrane options.
Operational lifetime represents a critical economic factor, as membrane degradation in highly alkaline conditions directly impacts replacement frequency. Premium fluoropolymer-based membranes demonstrate superior chemical stability with operational lifespans of 3-5 years in pH>14 environments, justifying their 30-50% price premium over standard hydrocarbon membranes that typically require replacement every 12-18 months under similar conditions.
Energy efficiency gains constitute a major benefit offsetting membrane costs. High-selectivity membranes can reduce cell voltage by 0.2-0.4V, translating to energy savings of 10-20% in typical iron electrowinning operations. For industrial-scale facilities consuming 5-10 MW, this represents annual savings of $350,000-700,000 at average industrial electricity rates.
Maintenance requirements and downtime costs vary substantially between membrane options. Fouling-resistant membranes with modified surface properties may command a 15-25% price premium but reduce cleaning frequency by 40-60%, minimizing production interruptions valued at $5,000-15,000 per day for mid-sized operations.
Product quality improvements delivered by superior membrane selectivity generate significant downstream value. Enhanced iron purity from 98.5% to 99.5% can increase product value by $50-150 per ton, while reduced contamination decreases post-processing costs by 5-15%.
Environmental compliance benefits must also factor into economic calculations. Advanced membranes that minimize waste generation can reduce effluent treatment costs by $2-5 per cubic meter and potentially avoid regulatory penalties ranging from $10,000-100,000 for non-compliance incidents.
The total cost of ownership analysis reveals that despite higher initial investment, premium membranes optimized for alkaline iron electrowinning typically achieve return on investment within 8-14 months through combined operational savings. Sensitivity analysis indicates that facilities with higher energy costs or stricter product specifications benefit most from investing in advanced membrane technologies, with potential five-year net present value advantages of 30-45% compared to basic membrane options.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!