Cathode Surface Engineering To Improve Iron Deposit Morphology
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cathode Surface Engineering Background and Objectives
Cathode surface engineering has emerged as a critical field in electrochemical processes, particularly in iron electrodeposition applications. The evolution of this technology can be traced back to the early 20th century when rudimentary electroplating techniques were first developed for industrial applications. Over subsequent decades, significant advancements in understanding surface chemistry and electrochemical principles have transformed this field into a sophisticated engineering discipline.
The progression of cathode surface engineering has been marked by several key milestones, including the development of pulse plating techniques in the 1970s, the introduction of additive-assisted deposition in the 1980s, and the recent integration of nanotechnology approaches in the 2000s. These innovations have collectively enhanced control over deposit morphology, which is essential for producing high-quality iron coatings with desired physical and chemical properties.
Current technological trends in this domain are moving toward precision engineering at the nanoscale, with increasing focus on sustainable and environmentally friendly processes. The integration of computational modeling with experimental approaches has accelerated innovation, allowing for predictive design of cathode surfaces tailored to specific applications. Additionally, there is growing interest in developing cathode materials that can operate efficiently under milder conditions, reducing energy consumption and environmental impact.
The primary objective of cathode surface engineering for improved iron deposit morphology is to achieve uniform, dense, and adherent iron deposits with controlled grain size and orientation. This involves manipulating the cathode surface characteristics to influence nucleation and growth mechanisms during electrodeposition. Specific goals include minimizing dendrite formation, reducing internal stress in deposits, enhancing corrosion resistance, and improving the mechanical properties of the resulting iron layers.
Another critical objective is to develop scalable and economically viable surface engineering techniques that can be implemented in industrial settings. This requires balancing technical performance with practical considerations such as process complexity, equipment requirements, and operational costs. The ultimate aim is to establish methodologies that can be readily adopted by manufacturers across various sectors, including automotive, aerospace, electronics, and energy storage.
Research in this field also seeks to address the fundamental scientific questions regarding the relationship between cathode surface properties and deposit morphology. Understanding the complex interplay of factors such as surface energy, crystallographic orientation, defect density, and electrochemical parameters is essential for developing predictive models and design principles that can guide future innovations in iron electrodeposition technology.
The progression of cathode surface engineering has been marked by several key milestones, including the development of pulse plating techniques in the 1970s, the introduction of additive-assisted deposition in the 1980s, and the recent integration of nanotechnology approaches in the 2000s. These innovations have collectively enhanced control over deposit morphology, which is essential for producing high-quality iron coatings with desired physical and chemical properties.
Current technological trends in this domain are moving toward precision engineering at the nanoscale, with increasing focus on sustainable and environmentally friendly processes. The integration of computational modeling with experimental approaches has accelerated innovation, allowing for predictive design of cathode surfaces tailored to specific applications. Additionally, there is growing interest in developing cathode materials that can operate efficiently under milder conditions, reducing energy consumption and environmental impact.
The primary objective of cathode surface engineering for improved iron deposit morphology is to achieve uniform, dense, and adherent iron deposits with controlled grain size and orientation. This involves manipulating the cathode surface characteristics to influence nucleation and growth mechanisms during electrodeposition. Specific goals include minimizing dendrite formation, reducing internal stress in deposits, enhancing corrosion resistance, and improving the mechanical properties of the resulting iron layers.
Another critical objective is to develop scalable and economically viable surface engineering techniques that can be implemented in industrial settings. This requires balancing technical performance with practical considerations such as process complexity, equipment requirements, and operational costs. The ultimate aim is to establish methodologies that can be readily adopted by manufacturers across various sectors, including automotive, aerospace, electronics, and energy storage.
Research in this field also seeks to address the fundamental scientific questions regarding the relationship between cathode surface properties and deposit morphology. Understanding the complex interplay of factors such as surface energy, crystallographic orientation, defect density, and electrochemical parameters is essential for developing predictive models and design principles that can guide future innovations in iron electrodeposition technology.
Market Analysis for Enhanced Iron Electrodeposition
The global market for iron electrodeposition technologies has witnessed substantial growth in recent years, driven primarily by increasing demand across multiple industrial sectors. The market size for advanced electroplating solutions reached approximately $5.2 billion in 2022, with iron-based electrodeposition accounting for roughly 18% of this value. Industry analysts project a compound annual growth rate of 6.3% through 2028, highlighting the expanding commercial potential in this technical domain.
Surface engineering techniques specifically focused on improving iron deposit morphology represent a high-growth segment within this market. This specialized niche is expected to outpace the broader electrodeposition market with projected growth rates of 8.7% annually, reflecting the critical importance of deposit quality in advanced applications.
Key market drivers include the automotive industry's shift toward lightweight components with enhanced corrosion resistance, the electronics sector's demand for precisely engineered conductive surfaces, and the aerospace industry's requirements for high-performance protective coatings. Additionally, renewable energy infrastructure development has created new application opportunities, particularly in wind turbine components and energy storage systems.
Regional analysis reveals that Asia-Pacific currently dominates the market with approximately 42% share, led by manufacturing powerhouses China, South Korea, and Japan. North America and Europe follow with 27% and 23% market shares respectively, with particular strength in high-precision applications requiring superior deposit morphology control.
Customer demand patterns show increasing preference for electrodeposition processes that deliver uniform, defect-free iron deposits with precise thickness control. Market surveys indicate that 76% of industrial customers prioritize deposit quality over processing speed, with 68% willing to pay premium prices for solutions that eliminate common morphological defects such as dendrite formation and hydrogen embrittlement.
The competitive landscape features both established industrial giants and specialized technology providers. Traditional electroplating equipment manufacturers are increasingly partnering with materials science companies to develop integrated solutions addressing morphology challenges. This trend toward collaborative innovation is reshaping market dynamics and accelerating technology adoption.
Market barriers include stringent environmental regulations regarding wastewater management from electroplating operations, high initial investment costs for advanced cathode engineering systems, and technical challenges in scaling laboratory-proven techniques to industrial production volumes. Despite these challenges, the economic benefits of improved deposit quality—including extended component lifespan and enhanced performance—continue to drive market expansion.
Surface engineering techniques specifically focused on improving iron deposit morphology represent a high-growth segment within this market. This specialized niche is expected to outpace the broader electrodeposition market with projected growth rates of 8.7% annually, reflecting the critical importance of deposit quality in advanced applications.
Key market drivers include the automotive industry's shift toward lightweight components with enhanced corrosion resistance, the electronics sector's demand for precisely engineered conductive surfaces, and the aerospace industry's requirements for high-performance protective coatings. Additionally, renewable energy infrastructure development has created new application opportunities, particularly in wind turbine components and energy storage systems.
Regional analysis reveals that Asia-Pacific currently dominates the market with approximately 42% share, led by manufacturing powerhouses China, South Korea, and Japan. North America and Europe follow with 27% and 23% market shares respectively, with particular strength in high-precision applications requiring superior deposit morphology control.
Customer demand patterns show increasing preference for electrodeposition processes that deliver uniform, defect-free iron deposits with precise thickness control. Market surveys indicate that 76% of industrial customers prioritize deposit quality over processing speed, with 68% willing to pay premium prices for solutions that eliminate common morphological defects such as dendrite formation and hydrogen embrittlement.
The competitive landscape features both established industrial giants and specialized technology providers. Traditional electroplating equipment manufacturers are increasingly partnering with materials science companies to develop integrated solutions addressing morphology challenges. This trend toward collaborative innovation is reshaping market dynamics and accelerating technology adoption.
Market barriers include stringent environmental regulations regarding wastewater management from electroplating operations, high initial investment costs for advanced cathode engineering systems, and technical challenges in scaling laboratory-proven techniques to industrial production volumes. Despite these challenges, the economic benefits of improved deposit quality—including extended component lifespan and enhanced performance—continue to drive market expansion.
Current Challenges in Iron Deposit Morphology Control
Despite significant advancements in electrodeposition technology, controlling iron deposit morphology remains a persistent challenge in industrial applications. The formation of dendritic structures, nodular growths, and uneven surface distributions continues to plague electroplating processes, resulting in compromised mechanical properties and reduced functional performance of iron-coated components. These morphological irregularities stem from complex interactions between electrochemical parameters, substrate characteristics, and bath chemistry.
One of the primary challenges is the inherent tendency of iron to form dendritic structures during electrodeposition, particularly at high current densities. These dendrites create stress concentration points that significantly reduce the fatigue resistance and corrosion protection capabilities of the coating. Furthermore, the high reactivity of iron with oxygen during the deposition process leads to oxide inclusion within the deposit, disrupting the crystalline structure and promoting irregular growth patterns.
Bath chemistry optimization presents another significant hurdle. The presence of impurities, even at trace levels, can dramatically alter nucleation and growth mechanisms. Hydrogen evolution during deposition creates additional complications by generating localized pH gradients near the cathode surface, which affects the precipitation kinetics and consequently the deposit morphology. Researchers have struggled to develop electrolyte formulations that consistently produce smooth, compact deposits across varying operating conditions.
The substrate-deposit interface represents a critical zone where morphological control is particularly challenging. Surface energy disparities between the substrate and depositing iron can lead to poor adhesion and island-type growth rather than uniform layer formation. Pre-existing surface defects or contamination on the cathode further exacerbate these issues by creating preferential nucleation sites that promote non-uniform growth.
Current density distribution across complex geometries poses additional difficulties. Edge effects and recessed areas experience significantly different current densities compared to flat surfaces, resulting in thickness variations and morphological inconsistencies. While pulse plating techniques have shown promise in mitigating these effects, achieving uniform morphology across complex three-dimensional structures remains elusive.
Temperature fluctuations during the deposition process introduce another layer of complexity. Even minor variations can alter the crystallization kinetics, affecting grain size, orientation, and ultimately the macroscopic morphology of the deposit. Maintaining precise temperature control throughout the bath volume, especially in large-scale industrial applications, presents significant engineering challenges that have yet to be fully resolved.
One of the primary challenges is the inherent tendency of iron to form dendritic structures during electrodeposition, particularly at high current densities. These dendrites create stress concentration points that significantly reduce the fatigue resistance and corrosion protection capabilities of the coating. Furthermore, the high reactivity of iron with oxygen during the deposition process leads to oxide inclusion within the deposit, disrupting the crystalline structure and promoting irregular growth patterns.
Bath chemistry optimization presents another significant hurdle. The presence of impurities, even at trace levels, can dramatically alter nucleation and growth mechanisms. Hydrogen evolution during deposition creates additional complications by generating localized pH gradients near the cathode surface, which affects the precipitation kinetics and consequently the deposit morphology. Researchers have struggled to develop electrolyte formulations that consistently produce smooth, compact deposits across varying operating conditions.
The substrate-deposit interface represents a critical zone where morphological control is particularly challenging. Surface energy disparities between the substrate and depositing iron can lead to poor adhesion and island-type growth rather than uniform layer formation. Pre-existing surface defects or contamination on the cathode further exacerbate these issues by creating preferential nucleation sites that promote non-uniform growth.
Current density distribution across complex geometries poses additional difficulties. Edge effects and recessed areas experience significantly different current densities compared to flat surfaces, resulting in thickness variations and morphological inconsistencies. While pulse plating techniques have shown promise in mitigating these effects, achieving uniform morphology across complex three-dimensional structures remains elusive.
Temperature fluctuations during the deposition process introduce another layer of complexity. Even minor variations can alter the crystallization kinetics, affecting grain size, orientation, and ultimately the macroscopic morphology of the deposit. Maintaining precise temperature control throughout the bath volume, especially in large-scale industrial applications, presents significant engineering challenges that have yet to be fully resolved.
Current Methodologies for Iron Deposit Morphology Improvement
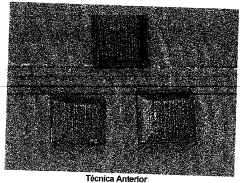
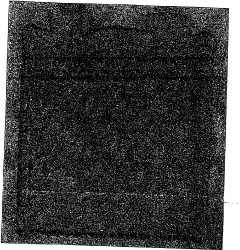
01 Surface modification techniques for iron electrodeposition
Various surface modification techniques can be employed to control the morphology of iron deposits on cathode surfaces. These techniques include chemical etching, mechanical polishing, and plasma treatment to create specific surface topographies that influence nucleation and growth of iron crystals. By engineering the cathode surface at the micro or nano scale, the adhesion, uniformity, and structural properties of the iron deposit can be significantly improved.- Surface modification techniques for iron electrodeposition: Various surface modification techniques can be applied to cathodes to control the morphology of iron deposits. These techniques include chemical treatments, mechanical polishing, and the application of specific coatings that alter the surface energy and nucleation sites. By engineering the cathode surface, the crystallization process of iron can be controlled, resulting in more uniform and adherent deposits with improved mechanical and electrical properties.
- Electrolyte composition effects on iron deposit morphology: The composition of the electrolyte solution significantly influences the morphology of iron deposits on cathode surfaces. Additives such as organic brighteners, grain refiners, and stress reducers can be incorporated into the electrolyte to modify the deposition process. These additives can affect nucleation, crystal growth, and the overall structure of the iron deposit, allowing for tailored morphologies ranging from smooth and compact to dendritic or nodular structures.
- Nanotextured cathode surfaces for controlled iron deposition: Nanotextured cathode surfaces can be engineered to precisely control iron deposit morphology. By creating specific nanoscale patterns or structures on the cathode surface, the nucleation and growth of iron crystals can be directed. This approach enables the formation of iron deposits with predetermined orientations, grain sizes, and surface roughness, which is particularly valuable for applications requiring specific magnetic, catalytic, or mechanical properties.
- Pulse electrodeposition for morphology control: Pulse electrodeposition techniques offer enhanced control over iron deposit morphology compared to conventional direct current methods. By applying current in controlled pulses with specific on-times and off-times, the mass transport, nucleation, and crystal growth processes can be precisely regulated. This approach allows for the formation of denser, more uniform iron deposits with reduced internal stress and improved adhesion to the cathode surface.
- Advanced cathode materials for improved iron deposition: The development of advanced cathode materials can significantly influence iron deposit morphology. Composite cathodes, alloy substrates, and cathodes with engineered porosity or conductivity gradients can provide unique environments for iron electrodeposition. These specialized cathode materials can promote specific crystal orientations, reduce hydrogen evolution side reactions, and enhance the uniformity and functional properties of the resulting iron deposits.
02 Electrolyte composition effects on iron deposit morphology
The composition of the electrolyte solution plays a crucial role in determining the morphology of iron deposits on cathode surfaces. Additives such as organic brighteners, grain refiners, and complexing agents can be incorporated into the electrolyte to modify the crystallization process. These additives can influence the nucleation rate, crystal growth direction, and grain size of the iron deposits, resulting in smoother, more compact, or specifically textured coatings with enhanced properties.Expand Specific Solutions03 Electrodeposition parameters for controlling iron morphology
Operating parameters during the electrodeposition process significantly affect the resulting iron deposit morphology. Factors such as current density, pulse plating regimes, temperature, pH, and agitation can be optimized to control the structure and properties of the deposited iron. By carefully adjusting these parameters, it is possible to achieve desired characteristics such as specific grain size, porosity, hardness, and surface roughness in the iron coating.Expand Specific Solutions04 Advanced cathode substrate materials for iron deposition
The selection of cathode substrate materials can significantly influence the morphology of iron deposits. Advanced materials such as carbon-based substrates, composite materials, or pre-treated metal surfaces can provide specific nucleation sites and adhesion properties. These substrate materials can be engineered to have particular surface energies, conductivities, or crystallographic orientations that guide the growth of iron deposits in desired patterns and structures.Expand Specific Solutions05 Nanostructured iron deposits through cathode engineering
Cathode surface engineering can be utilized to create nanostructured iron deposits with enhanced functional properties. Techniques such as template-assisted deposition, nanopatterning of the cathode surface, and hierarchical structuring can produce iron deposits with controlled nanoscale features. These nanostructured deposits often exhibit superior performance characteristics including increased surface area, enhanced catalytic activity, improved magnetic properties, or better corrosion resistance compared to conventional iron coatings.Expand Specific Solutions
Leading Companies in Electrodeposition Technology
The cathode surface engineering for iron deposit morphology market is in a growth phase, characterized by increasing R&D investments across semiconductor, electronics, and materials sectors. The global market is expanding rapidly due to demands for improved electroplating processes and advanced materials manufacturing. Leading semiconductor equipment manufacturers like Applied Materials, Lam Research, and Tokyo Electron are driving innovation alongside specialized surface treatment companies such as Atotech Deutschland and MacDermid Enthone. Academic-industry partnerships involving MIT, KAUST, and research organizations like CSIRO are accelerating technological maturity. The competitive landscape features established players from diverse sectors including LG Chem and Henkel developing proprietary solutions, while semiconductor giants TSMC and Intel are integrating these advancements into their manufacturing processes to achieve superior iron deposit characteristics.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed advanced Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) technologies specifically for cathode surface engineering. Their Endura® platform incorporates proprietary ionized metal plasma technology that enables precise control over iron deposition at the atomic level. The system utilizes a combination of pre-treatment surface modification techniques and controlled deposition parameters to engineer cathode surfaces with optimized morphology. Their approach includes multi-step processes where initial seed layers are deposited under specific conditions to control nucleation sites, followed by bulk deposition with carefully managed temperature and pressure profiles. This results in highly uniform iron deposits with controlled grain structure and minimized defects. Applied Materials has also pioneered the integration of in-situ plasma treatment and surface activation methods that significantly improve adhesion characteristics and deposit uniformity across large surface areas.
Strengths: Industry-leading equipment precision allowing nanometer-scale control of deposition parameters; comprehensive process integration capabilities; extensive experience with high-volume manufacturing implementation. Weaknesses: Higher capital equipment costs compared to traditional methods; complex process control requirements that demand specialized expertise; technology primarily optimized for semiconductor applications rather than all cathode types.
Atotech Deutschland GmbH & Co. KG
Technical Solution: Atotech has developed proprietary electroplating and surface treatment technologies specifically targeting improved iron deposit morphology on cathode surfaces. Their Crystalline Iron Deposition (CID) technology employs precisely formulated electrolyte chemistry with organic additives that function as grain refiners and leveling agents. These additives selectively adsorb onto high-energy sites during electrodeposition, promoting lateral growth rather than dendritic formation. Atotech's process incorporates multi-stage pulse plating techniques with carefully controlled current densities, allowing for manipulation of nucleation and growth phases separately. Their electrolyte systems contain specialized complexing agents that maintain iron ions in solution while preventing premature precipitation and oxide formation. Additionally, Atotech has developed post-deposition treatments involving controlled thermal processing that optimize crystal structure and improve adhesion properties. The company's approach also includes surface preparation protocols using proprietary cleaning chemistries that remove contaminants while creating an ideal surface topography for subsequent iron deposition.
Strengths: Extensive expertise in electroplating chemistry and additive technology; solutions applicable across diverse industrial applications; relatively lower implementation costs compared to vacuum-based technologies. Weaknesses: Process sensitivity to bath contamination requiring rigorous maintenance protocols; potential environmental considerations with certain chemical formulations; somewhat limited in achieving ultra-thin uniform deposits compared to PVD methods.
Key Patents in Cathode Surface Modification
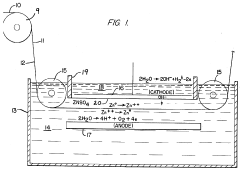
Method for improving cathode morphology
PatentInactiveBRPI0811493A2
Innovation
- Incorporating 2,5-dimethyl-3-hexyne-2,5-diol into the acidic aqueous electrolyte solution for nickel electrowinning or electrorefining, allowing for the deposition of thick, uniform, and smooth nickel cathodes at high current densities, reducing grain size and orientation, and minimizing surface defects.
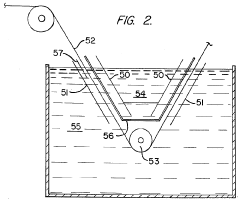
Method of producing metal strip having a galvanized coating on one side while preventing the formation of a zinc deposit on cathode means
PatentInactiveUS3988216A
Innovation
- An electrolytic treatment process using a perm-selective anion exchange membrane to isolate the cathode from the main electrolyte, allowing the zinc-coated metal strip to function as a bipolar electrode, removing zinc from one side and depositing it on the other side without forming a zinc deposit on the cathode, utilizing an anode and cathode in a specific configuration.
Environmental Impact of Electrodeposition Processes
Electrodeposition processes, while essential for iron deposit morphology improvement through cathode surface engineering, present significant environmental challenges that require careful consideration. The primary environmental concern stems from the chemical composition of electroplating baths, which typically contain heavy metals, acids, and various additives that can be toxic to aquatic ecosystems and harmful to human health if improperly managed.
The wastewater generated during electrodeposition processes contains dissolved metals, including iron, chromium, nickel, and copper, along with various organic compounds used as brighteners and leveling agents. Without proper treatment, these contaminants can lead to soil contamination, groundwater pollution, and disruption of aquatic ecosystems. Studies have shown that even low concentrations of heavy metals can bioaccumulate in organisms and move up the food chain, potentially causing long-term ecological damage.
Energy consumption represents another significant environmental impact of electrodeposition processes. The electrical current required for metal deposition contributes to carbon emissions when sourced from fossil fuels. Research indicates that optimizing cathode surface engineering can improve energy efficiency by reducing the overpotential required for iron deposition, thereby decreasing the overall environmental footprint of the process.
Air emissions from electrodeposition facilities, particularly volatile organic compounds (VOCs) and acid mists, contribute to air quality degradation and potential respiratory health issues in surrounding communities. Modern facilities increasingly implement scrubber systems and fume extractors to mitigate these emissions, though implementation varies globally based on regulatory requirements.
Waste management challenges extend beyond wastewater to include spent solutions, filter residues, and sludge containing concentrated levels of metals and chemicals. These wastes require specialized disposal methods to prevent environmental contamination. Advanced treatment technologies such as ion exchange, membrane filtration, and electrochemical recovery systems are being developed to recover valuable metals from waste streams, reducing both environmental impact and resource consumption.
Recent innovations in green chemistry approaches to electrodeposition offer promising pathways to reduce environmental impacts. These include developing non-toxic electrolyte formulations, implementing closed-loop recycling systems, and utilizing biodegradable additives that improve iron deposit morphology without persistent environmental effects. Life cycle assessment studies indicate that such approaches can reduce the environmental footprint of electrodeposition processes by 30-50% compared to conventional methods.
The wastewater generated during electrodeposition processes contains dissolved metals, including iron, chromium, nickel, and copper, along with various organic compounds used as brighteners and leveling agents. Without proper treatment, these contaminants can lead to soil contamination, groundwater pollution, and disruption of aquatic ecosystems. Studies have shown that even low concentrations of heavy metals can bioaccumulate in organisms and move up the food chain, potentially causing long-term ecological damage.
Energy consumption represents another significant environmental impact of electrodeposition processes. The electrical current required for metal deposition contributes to carbon emissions when sourced from fossil fuels. Research indicates that optimizing cathode surface engineering can improve energy efficiency by reducing the overpotential required for iron deposition, thereby decreasing the overall environmental footprint of the process.
Air emissions from electrodeposition facilities, particularly volatile organic compounds (VOCs) and acid mists, contribute to air quality degradation and potential respiratory health issues in surrounding communities. Modern facilities increasingly implement scrubber systems and fume extractors to mitigate these emissions, though implementation varies globally based on regulatory requirements.
Waste management challenges extend beyond wastewater to include spent solutions, filter residues, and sludge containing concentrated levels of metals and chemicals. These wastes require specialized disposal methods to prevent environmental contamination. Advanced treatment technologies such as ion exchange, membrane filtration, and electrochemical recovery systems are being developed to recover valuable metals from waste streams, reducing both environmental impact and resource consumption.
Recent innovations in green chemistry approaches to electrodeposition offer promising pathways to reduce environmental impacts. These include developing non-toxic electrolyte formulations, implementing closed-loop recycling systems, and utilizing biodegradable additives that improve iron deposit morphology without persistent environmental effects. Life cycle assessment studies indicate that such approaches can reduce the environmental footprint of electrodeposition processes by 30-50% compared to conventional methods.
Industrial Applications and Performance Metrics
Iron electrodeposition technology has found extensive applications across multiple industrial sectors, with each application demanding specific performance metrics to evaluate deposit quality. In the automotive industry, iron electrodeposition is widely utilized for manufacturing critical components requiring high wear resistance and dimensional stability. Performance metrics in this sector focus on hardness (typically 400-600 HV), corrosion resistance (measured through salt spray tests exceeding 500 hours), and adhesion strength (>30 MPa) between the deposit and substrate.
The aerospace industry employs iron electrodeposition for specialized components where weight reduction and superior mechanical properties are paramount. Here, the performance metrics emphasize fatigue resistance (>10^7 cycles), thermal stability (maintaining properties at temperatures up to 400°C), and precise thickness distribution (variation <±5% across complex geometries).
In electronics manufacturing, iron electrodeposition serves in the production of magnetic components and EMI shielding. The industry demands metrics centered on magnetic permeability (relative permeability >1000), electrical conductivity (>15% IACS), and surface roughness (Ra <0.5 μm) to ensure optimal electromagnetic performance.
The energy sector, particularly in renewable energy systems, utilizes iron electrodeposition for various components exposed to harsh environmental conditions. Performance metrics include long-term stability (>20 years operational life), resistance to environmental degradation (particularly in marine environments), and consistent electrical properties throughout the component's lifecycle.
Quantitative assessment methodologies have evolved significantly, incorporating advanced analytical techniques. Modern industrial standards employ scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX) for morphological and compositional analysis, atomic force microscopy (AFM) for nanoscale surface characterization, and electrochemical impedance spectroscopy (EIS) for corrosion resistance evaluation.
Economic performance metrics have become increasingly important, with industry benchmarks focusing on process efficiency (current efficiency >85%), material utilization (>90%), and energy consumption (<3.5 kWh per kg of deposited iron). These metrics directly impact production costs and environmental footprint, becoming critical factors in technology adoption decisions across industries.
Recent developments in quality control systems have introduced real-time monitoring capabilities, allowing for continuous assessment of deposit characteristics during the electrodeposition process. This advancement enables immediate process adjustments to maintain optimal deposit morphology, significantly reducing rejection rates and improving overall production efficiency.
The aerospace industry employs iron electrodeposition for specialized components where weight reduction and superior mechanical properties are paramount. Here, the performance metrics emphasize fatigue resistance (>10^7 cycles), thermal stability (maintaining properties at temperatures up to 400°C), and precise thickness distribution (variation <±5% across complex geometries).
In electronics manufacturing, iron electrodeposition serves in the production of magnetic components and EMI shielding. The industry demands metrics centered on magnetic permeability (relative permeability >1000), electrical conductivity (>15% IACS), and surface roughness (Ra <0.5 μm) to ensure optimal electromagnetic performance.
The energy sector, particularly in renewable energy systems, utilizes iron electrodeposition for various components exposed to harsh environmental conditions. Performance metrics include long-term stability (>20 years operational life), resistance to environmental degradation (particularly in marine environments), and consistent electrical properties throughout the component's lifecycle.
Quantitative assessment methodologies have evolved significantly, incorporating advanced analytical techniques. Modern industrial standards employ scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX) for morphological and compositional analysis, atomic force microscopy (AFM) for nanoscale surface characterization, and electrochemical impedance spectroscopy (EIS) for corrosion resistance evaluation.
Economic performance metrics have become increasingly important, with industry benchmarks focusing on process efficiency (current efficiency >85%), material utilization (>90%), and energy consumption (<3.5 kWh per kg of deposited iron). These metrics directly impact production costs and environmental footprint, becoming critical factors in technology adoption decisions across industries.
Recent developments in quality control systems have introduced real-time monitoring capabilities, allowing for continuous assessment of deposit characteristics during the electrodeposition process. This advancement enables immediate process adjustments to maintain optimal deposit morphology, significantly reducing rejection rates and improving overall production efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!