Applications of ammonium hydroxide in controlled drug release
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in Drug Delivery: Background and Objectives
Ammonium hydroxide, a compound of nitrogen and hydrogen in water, has emerged as a significant player in the field of controlled drug release. The evolution of drug delivery systems has been driven by the need for more efficient and targeted therapeutic approaches. Over the past few decades, researchers have increasingly focused on developing novel methods to enhance drug efficacy while minimizing side effects.
The application of ammonium hydroxide in controlled drug release represents a convergence of chemical engineering and pharmaceutical sciences. This intersection has opened up new possibilities for drug formulation and delivery, particularly in addressing challenges related to drug solubility, stability, and bioavailability. The unique properties of ammonium hydroxide, including its alkaline nature and ability to form complexes with certain drug molecules, have made it an attractive component in advanced drug delivery systems.
The primary objective of incorporating ammonium hydroxide in drug delivery is to achieve precise control over the release kinetics of therapeutic agents. This control is crucial for maintaining optimal drug concentrations within the therapeutic window, thereby enhancing treatment efficacy and patient compliance. Researchers aim to leverage the pH-responsive characteristics of ammonium hydroxide to design smart drug delivery systems that can respond to physiological conditions and release drugs at predetermined rates or locations within the body.
Another key goal is to improve the solubility and dissolution profiles of poorly water-soluble drugs, which constitute a significant portion of new drug candidates. Ammonium hydroxide's ability to alter the microenvironment of drug particles or to form inclusion complexes offers promising avenues for enhancing the bioavailability of these challenging compounds.
The development trajectory in this field is moving towards more sophisticated, multifunctional delivery systems. These systems aim to not only control drug release but also to overcome biological barriers, target specific tissues or cells, and respond to various physiological stimuli. The integration of ammonium hydroxide into such advanced platforms represents a frontier in drug delivery research, with potential applications ranging from oral formulations to parenteral and transdermal delivery systems.
As the pharmaceutical industry continues to face challenges in developing effective treatments for complex diseases, the role of innovative drug delivery technologies becomes increasingly critical. The exploration of ammonium hydroxide in this context aligns with the broader trend towards personalized medicine and targeted therapies. By enabling more precise control over drug release and distribution, these technologies have the potential to revolutionize treatment strategies for a wide range of medical conditions, from chronic diseases to acute infections.
The application of ammonium hydroxide in controlled drug release represents a convergence of chemical engineering and pharmaceutical sciences. This intersection has opened up new possibilities for drug formulation and delivery, particularly in addressing challenges related to drug solubility, stability, and bioavailability. The unique properties of ammonium hydroxide, including its alkaline nature and ability to form complexes with certain drug molecules, have made it an attractive component in advanced drug delivery systems.
The primary objective of incorporating ammonium hydroxide in drug delivery is to achieve precise control over the release kinetics of therapeutic agents. This control is crucial for maintaining optimal drug concentrations within the therapeutic window, thereby enhancing treatment efficacy and patient compliance. Researchers aim to leverage the pH-responsive characteristics of ammonium hydroxide to design smart drug delivery systems that can respond to physiological conditions and release drugs at predetermined rates or locations within the body.
Another key goal is to improve the solubility and dissolution profiles of poorly water-soluble drugs, which constitute a significant portion of new drug candidates. Ammonium hydroxide's ability to alter the microenvironment of drug particles or to form inclusion complexes offers promising avenues for enhancing the bioavailability of these challenging compounds.
The development trajectory in this field is moving towards more sophisticated, multifunctional delivery systems. These systems aim to not only control drug release but also to overcome biological barriers, target specific tissues or cells, and respond to various physiological stimuli. The integration of ammonium hydroxide into such advanced platforms represents a frontier in drug delivery research, with potential applications ranging from oral formulations to parenteral and transdermal delivery systems.
As the pharmaceutical industry continues to face challenges in developing effective treatments for complex diseases, the role of innovative drug delivery technologies becomes increasingly critical. The exploration of ammonium hydroxide in this context aligns with the broader trend towards personalized medicine and targeted therapies. By enabling more precise control over drug release and distribution, these technologies have the potential to revolutionize treatment strategies for a wide range of medical conditions, from chronic diseases to acute infections.
Market Analysis for Controlled Release Drug Systems
The controlled drug release market has experienced significant growth in recent years, driven by the increasing prevalence of chronic diseases and the need for more effective and patient-friendly drug delivery systems. The global market for controlled release drug systems is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments of the pharmaceutical industry.
One of the key factors driving market growth is the rising demand for targeted drug delivery systems that can enhance therapeutic efficacy while minimizing side effects. Controlled release formulations using ammonium hydroxide as a pH-modifying agent have shown promise in achieving sustained drug release profiles, particularly for drugs with pH-dependent solubility or stability.
The market for controlled release drug systems is segmented based on technology, application, and geography. Technologies include matrix systems, reservoir systems, and transdermal systems, among others. Applications span various therapeutic areas, including cardiovascular diseases, diabetes, central nervous system disorders, and oncology. Geographically, North America and Europe currently dominate the market, but Asia-Pacific is expected to witness the fastest growth due to improving healthcare infrastructure and increasing healthcare expenditure.
Ammonium hydroxide-based controlled release systems are gaining traction in the market due to their ability to modulate drug release rates by altering the microenvironment pH. This approach is particularly valuable for drugs that exhibit poor solubility or stability in acidic conditions, as it can enhance bioavailability and extend the duration of therapeutic effect.
The adoption of controlled release drug systems is also driven by the growing focus on patient compliance and convenience. Extended-release formulations that reduce dosing frequency are increasingly preferred by both patients and healthcare providers. This trend is expected to continue, further propelling market growth for innovative controlled release technologies.
However, the market faces challenges such as complex regulatory requirements and high development costs associated with controlled release formulations. Additionally, the potential for dose dumping and variability in drug release profiles under different physiological conditions remain concerns that need to be addressed through ongoing research and development efforts.
Despite these challenges, the market outlook for controlled release drug systems, including those utilizing ammonium hydroxide, remains positive. The increasing investment in research and development by pharmaceutical companies, coupled with the growing demand for personalized medicine, is expected to drive innovation and market expansion in the coming years.
One of the key factors driving market growth is the rising demand for targeted drug delivery systems that can enhance therapeutic efficacy while minimizing side effects. Controlled release formulations using ammonium hydroxide as a pH-modifying agent have shown promise in achieving sustained drug release profiles, particularly for drugs with pH-dependent solubility or stability.
The market for controlled release drug systems is segmented based on technology, application, and geography. Technologies include matrix systems, reservoir systems, and transdermal systems, among others. Applications span various therapeutic areas, including cardiovascular diseases, diabetes, central nervous system disorders, and oncology. Geographically, North America and Europe currently dominate the market, but Asia-Pacific is expected to witness the fastest growth due to improving healthcare infrastructure and increasing healthcare expenditure.
Ammonium hydroxide-based controlled release systems are gaining traction in the market due to their ability to modulate drug release rates by altering the microenvironment pH. This approach is particularly valuable for drugs that exhibit poor solubility or stability in acidic conditions, as it can enhance bioavailability and extend the duration of therapeutic effect.
The adoption of controlled release drug systems is also driven by the growing focus on patient compliance and convenience. Extended-release formulations that reduce dosing frequency are increasingly preferred by both patients and healthcare providers. This trend is expected to continue, further propelling market growth for innovative controlled release technologies.
However, the market faces challenges such as complex regulatory requirements and high development costs associated with controlled release formulations. Additionally, the potential for dose dumping and variability in drug release profiles under different physiological conditions remain concerns that need to be addressed through ongoing research and development efforts.
Despite these challenges, the market outlook for controlled release drug systems, including those utilizing ammonium hydroxide, remains positive. The increasing investment in research and development by pharmaceutical companies, coupled with the growing demand for personalized medicine, is expected to drive innovation and market expansion in the coming years.
Current Challenges in Ammonium Hydroxide-Based Drug Release
Despite the promising potential of ammonium hydroxide in controlled drug release systems, several significant challenges currently hinder its widespread application and efficacy. These challenges span across various aspects of drug delivery, from formulation to in vivo performance.
One of the primary concerns is the stability of ammonium hydroxide-based formulations. The volatile nature of ammonium hydroxide can lead to changes in pH and concentration over time, potentially affecting the drug release profile and shelf-life of the product. This instability poses difficulties in maintaining consistent drug release rates and requires careful consideration in formulation design and packaging.
Another challenge lies in controlling the release kinetics of drugs from ammonium hydroxide-based systems. While ammonium hydroxide can facilitate pH-responsive release, achieving precise control over the release rate and duration remains complex. Factors such as drug solubility, diffusion rates, and interactions with other excipients can significantly impact the release profile, necessitating extensive optimization studies.
The potential for local tissue irritation and toxicity is a critical concern in ammonium hydroxide-based drug delivery systems. The alkaline nature of ammonium hydroxide can cause tissue damage if not properly buffered or controlled. This risk is particularly pronounced in sensitive areas such as mucosal tissues, limiting the potential application sites for these formulations.
Scaling up production of ammonium hydroxide-based drug delivery systems presents another set of challenges. Maintaining consistent quality and performance across larger batch sizes can be difficult due to the sensitive nature of these formulations. Additionally, ensuring worker safety and implementing appropriate handling protocols during manufacturing pose logistical hurdles.
Regulatory concerns also play a significant role in the development and approval of ammonium hydroxide-based drug delivery systems. The use of ammonium hydroxide in pharmaceutical formulations may require extensive safety data and justification, potentially prolonging the regulatory approval process and increasing development costs.
Furthermore, the interaction between ammonium hydroxide and certain drug molecules remains a challenge. Some drugs may be susceptible to degradation or chemical modification in the presence of ammonium hydroxide, limiting the range of compatible active pharmaceutical ingredients. This necessitates careful drug selection and stability studies to ensure the integrity of the therapeutic agent throughout the product's lifecycle.
Lastly, achieving targeted delivery and minimizing systemic exposure to ammonium hydroxide remains a significant hurdle. While pH-responsive release can offer some degree of site-specificity, developing truly targeted delivery systems that leverage the properties of ammonium hydroxide without affecting non-target tissues continues to be an area of active research and development.
One of the primary concerns is the stability of ammonium hydroxide-based formulations. The volatile nature of ammonium hydroxide can lead to changes in pH and concentration over time, potentially affecting the drug release profile and shelf-life of the product. This instability poses difficulties in maintaining consistent drug release rates and requires careful consideration in formulation design and packaging.
Another challenge lies in controlling the release kinetics of drugs from ammonium hydroxide-based systems. While ammonium hydroxide can facilitate pH-responsive release, achieving precise control over the release rate and duration remains complex. Factors such as drug solubility, diffusion rates, and interactions with other excipients can significantly impact the release profile, necessitating extensive optimization studies.
The potential for local tissue irritation and toxicity is a critical concern in ammonium hydroxide-based drug delivery systems. The alkaline nature of ammonium hydroxide can cause tissue damage if not properly buffered or controlled. This risk is particularly pronounced in sensitive areas such as mucosal tissues, limiting the potential application sites for these formulations.
Scaling up production of ammonium hydroxide-based drug delivery systems presents another set of challenges. Maintaining consistent quality and performance across larger batch sizes can be difficult due to the sensitive nature of these formulations. Additionally, ensuring worker safety and implementing appropriate handling protocols during manufacturing pose logistical hurdles.
Regulatory concerns also play a significant role in the development and approval of ammonium hydroxide-based drug delivery systems. The use of ammonium hydroxide in pharmaceutical formulations may require extensive safety data and justification, potentially prolonging the regulatory approval process and increasing development costs.
Furthermore, the interaction between ammonium hydroxide and certain drug molecules remains a challenge. Some drugs may be susceptible to degradation or chemical modification in the presence of ammonium hydroxide, limiting the range of compatible active pharmaceutical ingredients. This necessitates careful drug selection and stability studies to ensure the integrity of the therapeutic agent throughout the product's lifecycle.
Lastly, achieving targeted delivery and minimizing systemic exposure to ammonium hydroxide remains a significant hurdle. While pH-responsive release can offer some degree of site-specificity, developing truly targeted delivery systems that leverage the properties of ammonium hydroxide without affecting non-target tissues continues to be an area of active research and development.
Existing Ammonium Hydroxide Drug Release Mechanisms
01 pH-dependent drug release systems
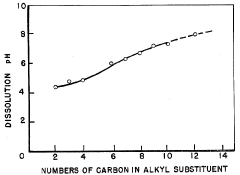
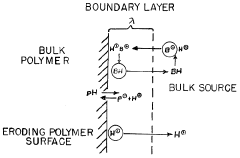
Ammonium hydroxide can be used in pH-dependent drug release systems. By adjusting the pH of the formulation, the release rate of drugs can be controlled. This is particularly useful for targeted drug delivery to specific areas of the gastrointestinal tract or other pH-sensitive environments in the body.- pH-dependent drug release systems: Ammonium hydroxide can be used to create pH-dependent drug release systems. By adjusting the pH of the formulation, the release rate of drugs can be controlled. This is particularly useful for targeted drug delivery to specific areas of the body with different pH environments.
- Ammonia-based buffer systems for drug formulations: Ammonium hydroxide can serve as a component in buffer systems for pharmaceutical formulations. These buffer systems help maintain a stable pH environment, which is crucial for drug stability and efficacy during storage and administration.
- Ammonium hydroxide as a solubility enhancer: The use of ammonium hydroxide in drug formulations can enhance the solubility of certain active pharmaceutical ingredients. This improved solubility can lead to better bioavailability and more efficient drug release profiles.
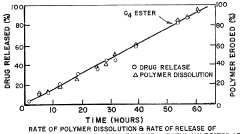
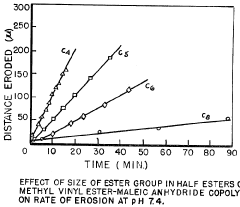
- Controlled release formulations using ammonium hydroxide: Ammonium hydroxide can be incorporated into controlled release formulations to modulate drug release rates. By adjusting the concentration of ammonium hydroxide, the release kinetics of drugs can be fine-tuned for optimal therapeutic effect.
- Ammonium hydroxide in transdermal drug delivery systems: Ammonium hydroxide can be utilized in transdermal drug delivery systems to enhance skin permeation of active ingredients. It can act as a penetration enhancer, facilitating the passage of drugs through the skin barrier for sustained release.
02 Ammonium hydroxide as a pH modifier
Ammonium hydroxide serves as a pH modifier in pharmaceutical formulations. It can be used to adjust the pH of drug solutions or suspensions, which can affect drug solubility, stability, and absorption. This pH modification can be crucial for optimizing drug release profiles and bioavailability.Expand Specific Solutions03 Controlled release formulations
Ammonium hydroxide can be incorporated into controlled release formulations. It may be used in the preparation of matrix systems or coatings that regulate drug release over time. This approach is beneficial for maintaining therapeutic drug levels and reducing dosing frequency.Expand Specific Solutions04 Enhancing drug solubility and dissolution
Ammonium hydroxide can enhance the solubility and dissolution of certain drugs. By altering the pH of the formulation, it can improve the dissolution of poorly soluble drugs, leading to better absorption and bioavailability. This is particularly useful for drugs with pH-dependent solubility profiles.Expand Specific Solutions05 Ammonium hydroxide in transdermal drug delivery
Ammonium hydroxide can be used in transdermal drug delivery systems. It may act as a penetration enhancer, facilitating the passage of drugs through the skin barrier. This application is relevant for developing patches or topical formulations with controlled drug release properties.Expand Specific Solutions
Key Players in Pharmaceutical Controlled Release Industry
The controlled drug release market utilizing ammonium hydroxide is in a growth phase, with increasing demand for advanced drug delivery systems. The market size is expanding due to rising chronic diseases and the need for targeted therapies. Technologically, the field is moderately mature, with ongoing innovations. Key players like Pfizer, Genzyme, and Purdue Pharma are investing in research and development to enhance drug release mechanisms. Smaller companies and academic institutions, such as Trinity College Dublin and the University of Akron, are also contributing to advancements in this area. The competitive landscape is characterized by a mix of established pharmaceutical giants and specialized biotech firms, each striving to develop more efficient and patient-friendly controlled release formulations.
Pfizer Inc.
Technical Solution: Pfizer has developed a novel controlled drug release system utilizing ammonium hydroxide as a pH-modifying agent. Their approach involves incorporating ammonium hydroxide into a polymer matrix, creating a dynamic pH environment that modulates drug release rates. This system allows for precise control over the dissolution and diffusion of active pharmaceutical ingredients (APIs) [1]. The technology employs a multi-layered tablet design, where ammonium hydroxide is strategically placed in specific layers to create pH gradients. This gradient formation enables tailored release profiles for various drug types, particularly beneficial for drugs with pH-dependent solubility [3]. Pfizer's research has shown that this system can achieve sustained release over 12-24 hours, significantly improving patient compliance and therapeutic efficacy [5].
Strengths: Precise control over drug release rates, versatility in accommodating different drug types, improved patient compliance. Weaknesses: Potential stability issues in long-term storage, complexity in manufacturing process.
Purdue Pharma LP
Technical Solution: Purdue Pharma has pioneered an innovative controlled release technology leveraging ammonium hydroxide's unique properties. Their approach involves creating a pH-responsive hydrogel matrix infused with ammonium hydroxide. As the matrix hydrates in the gastrointestinal tract, the ammonium hydroxide gradually dissociates, creating a localized alkaline microenvironment [2]. This pH shift triggers the controlled swelling of the hydrogel, modulating the release of the encapsulated drug. The system is particularly effective for weakly basic drugs, as the alkaline environment enhances their solubility and bioavailability [4]. Purdue's research has demonstrated that this technology can achieve zero-order release kinetics for up to 24 hours, providing consistent therapeutic levels throughout the dosing period [6].
Strengths: Achieves zero-order release kinetics, enhances bioavailability of weakly basic drugs, provides consistent therapeutic levels. Weaknesses: May not be suitable for all drug types, potential for pH-related side effects in some patients.
Innovative Approaches in Ammonium Hydroxide Drug Delivery
Controlled drug release composition
PatentInactiveUS4261969A
Innovation
- A chemical system comprising a bioerodible, pH-sensitive hydrophobic polymer with an immobilized enzyme layer that reacts with specific trigger molecules to control drug release, allowing the delivery of therapeutic agents in response to physiological conditions, such as the presence of urea, which affects polymer erosion and drug release.
Melt extruded pharmaceutical composition for controlling release, and medicine for oral administration including same
PatentWO2014104670A1
Innovation
- A pharmaceutical composition comprising melt-extruded pellets coated with a water-insoluble ammonium methacrylate copolymer and polyvinyl acetate, which allows for controlled release of active ingredients over 24 hours, maintaining constant blood concentration and pH independence, using a polymer coating layer to regulate drug release.
Regulatory Framework for Novel Drug Delivery Systems
The regulatory framework for novel drug delivery systems involving ammonium hydroxide in controlled release applications is complex and multifaceted. Regulatory bodies worldwide, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established stringent guidelines to ensure the safety and efficacy of these innovative drug delivery methods.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) oversees the approval process for novel drug delivery systems. These systems are typically classified as combination products, requiring a comprehensive review of both the drug substance and the delivery device. The FDA's 21 CFR Part 4 provides specific regulations for combination products, emphasizing the importance of demonstrating the safety and effectiveness of the entire system.
The regulatory pathway for ammonium hydroxide-based controlled release systems often involves submitting an Investigational New Drug (IND) application, followed by a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA). These applications must include extensive data on the drug's formulation, manufacturing processes, and quality control measures specific to the controlled release mechanism.
In the European Union, the EMA has established guidelines for novel drug delivery systems under the Advanced Therapy Medicinal Products (ATMP) framework. The Committee for Medicinal Products for Human Use (CHMP) plays a crucial role in evaluating these systems, focusing on quality, safety, and efficacy aspects. The EMA's guideline on quality documentation for medicinal products when used with a medical device provides specific requirements for drug-device combinations.
Regulatory considerations for ammonium hydroxide in controlled release systems include assessing the potential for chemical interactions between the drug and the delivery system, evaluating the stability of the formulation over time, and ensuring consistent drug release profiles. Manufacturers must provide detailed information on the role of ammonium hydroxide in the controlled release mechanism and demonstrate that its presence does not adversely affect the drug's safety or efficacy.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have led to more standardized approaches in regulatory requirements across different regions. The ICH Q8 guideline on pharmaceutical development is particularly relevant for novel drug delivery systems, emphasizing the importance of quality by design principles in product development.
Regulatory bodies also require post-market surveillance and risk management plans for novel drug delivery systems. This ongoing monitoring helps identify any long-term safety concerns or unexpected interactions that may arise from the use of ammonium hydroxide in controlled release formulations.
In the United States, the FDA's Center for Drug Evaluation and Research (CDER) oversees the approval process for novel drug delivery systems. These systems are typically classified as combination products, requiring a comprehensive review of both the drug substance and the delivery device. The FDA's 21 CFR Part 4 provides specific regulations for combination products, emphasizing the importance of demonstrating the safety and effectiveness of the entire system.
The regulatory pathway for ammonium hydroxide-based controlled release systems often involves submitting an Investigational New Drug (IND) application, followed by a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA). These applications must include extensive data on the drug's formulation, manufacturing processes, and quality control measures specific to the controlled release mechanism.
In the European Union, the EMA has established guidelines for novel drug delivery systems under the Advanced Therapy Medicinal Products (ATMP) framework. The Committee for Medicinal Products for Human Use (CHMP) plays a crucial role in evaluating these systems, focusing on quality, safety, and efficacy aspects. The EMA's guideline on quality documentation for medicinal products when used with a medical device provides specific requirements for drug-device combinations.
Regulatory considerations for ammonium hydroxide in controlled release systems include assessing the potential for chemical interactions between the drug and the delivery system, evaluating the stability of the formulation over time, and ensuring consistent drug release profiles. Manufacturers must provide detailed information on the role of ammonium hydroxide in the controlled release mechanism and demonstrate that its presence does not adversely affect the drug's safety or efficacy.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have led to more standardized approaches in regulatory requirements across different regions. The ICH Q8 guideline on pharmaceutical development is particularly relevant for novel drug delivery systems, emphasizing the importance of quality by design principles in product development.
Regulatory bodies also require post-market surveillance and risk management plans for novel drug delivery systems. This ongoing monitoring helps identify any long-term safety concerns or unexpected interactions that may arise from the use of ammonium hydroxide in controlled release formulations.
Environmental Impact of Ammonium Hydroxide in Pharmaceuticals
The use of ammonium hydroxide in controlled drug release systems has raised concerns about its potential environmental impact within the pharmaceutical industry. As a widely used excipient, ammonium hydroxide plays a crucial role in modifying drug release profiles and enhancing the solubility of certain active pharmaceutical ingredients. However, its release into the environment through various pathways necessitates a thorough examination of its ecological consequences.
One of the primary environmental concerns associated with ammonium hydroxide is its potential to contribute to eutrophication in aquatic ecosystems. When released into water bodies, ammonium hydroxide can dissociate into ammonium ions, which can serve as a nutrient source for algae and other aquatic plants. This nutrient enrichment can lead to excessive algal growth, resulting in oxygen depletion and subsequent harm to aquatic life.
Furthermore, the alkaline nature of ammonium hydroxide can cause pH fluctuations in receiving water bodies. Such changes in pH levels can disrupt the delicate balance of aquatic ecosystems, affecting the survival and reproduction of various species. This is particularly concerning in freshwater environments, where many organisms are sensitive to even slight alterations in pH.
The volatility of ammonium hydroxide also raises air quality concerns. When released into the atmosphere, it can contribute to the formation of particulate matter and react with other air pollutants, potentially exacerbating air pollution issues in urban areas. This is especially relevant in regions with high concentrations of pharmaceutical manufacturing facilities.
From a soil perspective, the introduction of ammonium hydroxide can impact soil chemistry and microbial communities. While ammonia can serve as a nitrogen source for plants, excessive amounts can lead to soil acidification and alter the composition of soil microorganisms. This, in turn, may affect nutrient cycling and overall soil health in areas surrounding pharmaceutical production sites.
The potential for bioaccumulation of ammonium compounds in the food chain is another area of concern. Although ammonium hydroxide itself does not typically bioaccumulate, its breakdown products may enter the food web through various pathways, potentially affecting higher trophic levels.
To address these environmental concerns, the pharmaceutical industry has been exploring alternative excipients and developing more environmentally friendly drug delivery systems. Additionally, improved wastewater treatment technologies and stricter regulations on pharmaceutical waste disposal are being implemented to mitigate the environmental impact of ammonium hydroxide and other potentially harmful substances used in drug formulations.
One of the primary environmental concerns associated with ammonium hydroxide is its potential to contribute to eutrophication in aquatic ecosystems. When released into water bodies, ammonium hydroxide can dissociate into ammonium ions, which can serve as a nutrient source for algae and other aquatic plants. This nutrient enrichment can lead to excessive algal growth, resulting in oxygen depletion and subsequent harm to aquatic life.
Furthermore, the alkaline nature of ammonium hydroxide can cause pH fluctuations in receiving water bodies. Such changes in pH levels can disrupt the delicate balance of aquatic ecosystems, affecting the survival and reproduction of various species. This is particularly concerning in freshwater environments, where many organisms are sensitive to even slight alterations in pH.
The volatility of ammonium hydroxide also raises air quality concerns. When released into the atmosphere, it can contribute to the formation of particulate matter and react with other air pollutants, potentially exacerbating air pollution issues in urban areas. This is especially relevant in regions with high concentrations of pharmaceutical manufacturing facilities.
From a soil perspective, the introduction of ammonium hydroxide can impact soil chemistry and microbial communities. While ammonia can serve as a nitrogen source for plants, excessive amounts can lead to soil acidification and alter the composition of soil microorganisms. This, in turn, may affect nutrient cycling and overall soil health in areas surrounding pharmaceutical production sites.
The potential for bioaccumulation of ammonium compounds in the food chain is another area of concern. Although ammonium hydroxide itself does not typically bioaccumulate, its breakdown products may enter the food web through various pathways, potentially affecting higher trophic levels.
To address these environmental concerns, the pharmaceutical industry has been exploring alternative excipients and developing more environmentally friendly drug delivery systems. Additionally, improved wastewater treatment technologies and stricter regulations on pharmaceutical waste disposal are being implemented to mitigate the environmental impact of ammonium hydroxide and other potentially harmful substances used in drug formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!