Assessing Biocompatibility of Ethylene Vinyl Acetate
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
EVA Biocompatibility Background and Objectives
Ethylene Vinyl Acetate (EVA) has emerged as a versatile polymer with widespread applications in various industries, particularly in the medical and pharmaceutical sectors. The assessment of EVA's biocompatibility has become increasingly crucial as its use in medical devices and drug delivery systems continues to expand. This technological evolution has been driven by EVA's unique properties, including flexibility, transparency, and resistance to stress-cracking.
The history of EVA in biomedical applications dates back to the 1970s when it was first explored for controlled drug release. Since then, the polymer has undergone significant developments, with researchers and manufacturers continuously refining its composition and properties to enhance its biocompatibility. The journey of EVA in the medical field has been marked by milestones such as its approval for use in various implantable devices and its incorporation into advanced drug delivery systems.
In recent years, the focus on EVA biocompatibility has intensified due to growing concerns about the long-term effects of implantable materials on the human body. This has led to a surge in research activities aimed at understanding the interactions between EVA and biological systems at the molecular level. The technological trend is moving towards developing EVA formulations with improved biocompatibility profiles, reduced inflammatory responses, and enhanced integration with host tissues.
The primary objective of assessing EVA biocompatibility is to ensure the safety and efficacy of medical devices and pharmaceutical products that incorporate this material. This involves comprehensive evaluation of EVA's potential to elicit adverse biological responses, such as inflammation, toxicity, or immunogenicity. Additionally, researchers aim to optimize EVA's surface properties to promote favorable interactions with cells and tissues, thereby improving its performance in various biomedical applications.
Another critical goal is to develop standardized testing protocols and regulatory guidelines specific to EVA biocompatibility. This is essential for streamlining the approval process for EVA-based medical products and ensuring consistency in safety evaluations across different applications. The technological objectives also include exploring novel modification techniques to enhance EVA's biocompatibility without compromising its desirable mechanical and chemical properties.
As the field progresses, there is a growing emphasis on understanding the long-term effects of EVA implants and developing predictive models for biocompatibility assessment. This involves leveraging advanced analytical techniques and in vitro models to simulate the complex interactions between EVA and biological systems over extended periods. The ultimate aim is to establish EVA as a gold standard biomaterial for a wide range of medical applications, supported by robust scientific evidence of its biocompatibility and safety profile.
The history of EVA in biomedical applications dates back to the 1970s when it was first explored for controlled drug release. Since then, the polymer has undergone significant developments, with researchers and manufacturers continuously refining its composition and properties to enhance its biocompatibility. The journey of EVA in the medical field has been marked by milestones such as its approval for use in various implantable devices and its incorporation into advanced drug delivery systems.
In recent years, the focus on EVA biocompatibility has intensified due to growing concerns about the long-term effects of implantable materials on the human body. This has led to a surge in research activities aimed at understanding the interactions between EVA and biological systems at the molecular level. The technological trend is moving towards developing EVA formulations with improved biocompatibility profiles, reduced inflammatory responses, and enhanced integration with host tissues.
The primary objective of assessing EVA biocompatibility is to ensure the safety and efficacy of medical devices and pharmaceutical products that incorporate this material. This involves comprehensive evaluation of EVA's potential to elicit adverse biological responses, such as inflammation, toxicity, or immunogenicity. Additionally, researchers aim to optimize EVA's surface properties to promote favorable interactions with cells and tissues, thereby improving its performance in various biomedical applications.
Another critical goal is to develop standardized testing protocols and regulatory guidelines specific to EVA biocompatibility. This is essential for streamlining the approval process for EVA-based medical products and ensuring consistency in safety evaluations across different applications. The technological objectives also include exploring novel modification techniques to enhance EVA's biocompatibility without compromising its desirable mechanical and chemical properties.
As the field progresses, there is a growing emphasis on understanding the long-term effects of EVA implants and developing predictive models for biocompatibility assessment. This involves leveraging advanced analytical techniques and in vitro models to simulate the complex interactions between EVA and biological systems over extended periods. The ultimate aim is to establish EVA as a gold standard biomaterial for a wide range of medical applications, supported by robust scientific evidence of its biocompatibility and safety profile.
Market Demand for Biocompatible EVA
The market demand for biocompatible Ethylene Vinyl Acetate (EVA) has been steadily increasing in recent years, driven by its versatile applications in medical devices, pharmaceutical packaging, and other healthcare-related products. This growth is primarily attributed to EVA's unique properties, including flexibility, transparency, and resistance to chemicals and UV radiation.
In the medical device sector, biocompatible EVA is extensively used in the production of catheters, tubing, and drug delivery systems. The rising prevalence of chronic diseases and the aging population have led to an increased demand for these medical devices, consequently boosting the market for biocompatible EVA. Additionally, the growing trend towards minimally invasive surgical procedures has further accelerated the adoption of EVA-based medical devices.
The pharmaceutical packaging industry has also contributed significantly to the market demand for biocompatible EVA. EVA copolymers are widely used in blister packaging, parenteral containers, and other drug delivery systems due to their excellent barrier properties and compatibility with various pharmaceutical formulations. The global expansion of the pharmaceutical industry, coupled with stringent regulations on drug packaging materials, has fueled the demand for high-quality, biocompatible EVA.
Furthermore, the increasing focus on personalized medicine and advanced drug delivery systems has opened new avenues for biocompatible EVA applications. The material's ability to be customized for specific drug release profiles and its compatibility with a wide range of active pharmaceutical ingredients make it an attractive choice for innovative drug delivery platforms.
The orthopedic and dental sectors have also shown a growing interest in biocompatible EVA. Its use in the production of custom-made orthotic devices, mouthguards, and dental appliances has gained traction due to its flexibility, durability, and non-toxic nature. The rising awareness of sports-related injuries and the increasing demand for comfortable, personalized dental solutions have contributed to this market segment's growth.
In the context of global market trends, the Asia-Pacific region is expected to witness the highest growth rate in the biocompatible EVA market. This can be attributed to the rapid expansion of healthcare infrastructure, increasing healthcare expenditure, and the growing medical device manufacturing industry in countries like China and India.
However, the market demand for biocompatible EVA is not without challenges. Stringent regulatory requirements for medical-grade materials and the need for extensive biocompatibility testing can lead to longer product development cycles and increased costs. Additionally, the growing emphasis on sustainable and biodegradable materials in healthcare applications may pose a potential threat to the long-term growth of the EVA market.
In the medical device sector, biocompatible EVA is extensively used in the production of catheters, tubing, and drug delivery systems. The rising prevalence of chronic diseases and the aging population have led to an increased demand for these medical devices, consequently boosting the market for biocompatible EVA. Additionally, the growing trend towards minimally invasive surgical procedures has further accelerated the adoption of EVA-based medical devices.
The pharmaceutical packaging industry has also contributed significantly to the market demand for biocompatible EVA. EVA copolymers are widely used in blister packaging, parenteral containers, and other drug delivery systems due to their excellent barrier properties and compatibility with various pharmaceutical formulations. The global expansion of the pharmaceutical industry, coupled with stringent regulations on drug packaging materials, has fueled the demand for high-quality, biocompatible EVA.
Furthermore, the increasing focus on personalized medicine and advanced drug delivery systems has opened new avenues for biocompatible EVA applications. The material's ability to be customized for specific drug release profiles and its compatibility with a wide range of active pharmaceutical ingredients make it an attractive choice for innovative drug delivery platforms.
The orthopedic and dental sectors have also shown a growing interest in biocompatible EVA. Its use in the production of custom-made orthotic devices, mouthguards, and dental appliances has gained traction due to its flexibility, durability, and non-toxic nature. The rising awareness of sports-related injuries and the increasing demand for comfortable, personalized dental solutions have contributed to this market segment's growth.
In the context of global market trends, the Asia-Pacific region is expected to witness the highest growth rate in the biocompatible EVA market. This can be attributed to the rapid expansion of healthcare infrastructure, increasing healthcare expenditure, and the growing medical device manufacturing industry in countries like China and India.
However, the market demand for biocompatible EVA is not without challenges. Stringent regulatory requirements for medical-grade materials and the need for extensive biocompatibility testing can lead to longer product development cycles and increased costs. Additionally, the growing emphasis on sustainable and biodegradable materials in healthcare applications may pose a potential threat to the long-term growth of the EVA market.
Current Challenges in EVA Biocompatibility
Despite the widespread use of Ethylene Vinyl Acetate (EVA) in medical devices and pharmaceutical applications, several challenges persist in assessing and ensuring its biocompatibility. One of the primary concerns is the potential leaching of additives and residual monomers from EVA materials. These compounds can migrate into the surrounding biological environment, potentially causing adverse reactions or altering the efficacy of drugs in contact with the material.
Another significant challenge lies in the variability of EVA compositions. The ratio of ethylene to vinyl acetate can vary widely, affecting the material's properties and, consequently, its biocompatibility profile. This variability makes it difficult to establish standardized testing protocols that can accurately predict the biocompatibility of all EVA formulations.
The long-term stability of EVA in biological environments presents another hurdle. While EVA is generally considered stable, prolonged exposure to bodily fluids or harsh sterilization processes can lead to degradation. This degradation may not only compromise the material's mechanical properties but also release potentially harmful byproducts, complicating long-term biocompatibility assessments.
Furthermore, the interaction between EVA and specific drugs or biological substances remains a complex area of study. Some drugs may be absorbed by the EVA matrix, altering their release profiles and potentially affecting treatment efficacy. Conversely, certain biological substances may cause unexpected changes in the EVA material, impacting its performance and biocompatibility over time.
The development of more sensitive and specific biocompatibility testing methods for EVA is an ongoing challenge. Current standardized tests may not fully capture the nuanced interactions between EVA and living tissues, particularly in specialized applications such as drug-eluting devices or implants.
Additionally, the increasing focus on environmentally friendly materials poses a challenge for EVA biocompatibility. As the industry moves towards more sustainable options, balancing the biocompatibility of EVA with eco-friendly alternatives or modifications becomes crucial.
Lastly, regulatory challenges persist in the assessment of EVA biocompatibility. Different regulatory bodies may have varying requirements and interpretations of biocompatibility data, making it difficult for manufacturers to navigate global markets with consistent standards. This regulatory landscape adds complexity to the already challenging task of comprehensively assessing EVA biocompatibility across diverse applications and environments.
Another significant challenge lies in the variability of EVA compositions. The ratio of ethylene to vinyl acetate can vary widely, affecting the material's properties and, consequently, its biocompatibility profile. This variability makes it difficult to establish standardized testing protocols that can accurately predict the biocompatibility of all EVA formulations.
The long-term stability of EVA in biological environments presents another hurdle. While EVA is generally considered stable, prolonged exposure to bodily fluids or harsh sterilization processes can lead to degradation. This degradation may not only compromise the material's mechanical properties but also release potentially harmful byproducts, complicating long-term biocompatibility assessments.
Furthermore, the interaction between EVA and specific drugs or biological substances remains a complex area of study. Some drugs may be absorbed by the EVA matrix, altering their release profiles and potentially affecting treatment efficacy. Conversely, certain biological substances may cause unexpected changes in the EVA material, impacting its performance and biocompatibility over time.
The development of more sensitive and specific biocompatibility testing methods for EVA is an ongoing challenge. Current standardized tests may not fully capture the nuanced interactions between EVA and living tissues, particularly in specialized applications such as drug-eluting devices or implants.
Additionally, the increasing focus on environmentally friendly materials poses a challenge for EVA biocompatibility. As the industry moves towards more sustainable options, balancing the biocompatibility of EVA with eco-friendly alternatives or modifications becomes crucial.
Lastly, regulatory challenges persist in the assessment of EVA biocompatibility. Different regulatory bodies may have varying requirements and interpretations of biocompatibility data, making it difficult for manufacturers to navigate global markets with consistent standards. This regulatory landscape adds complexity to the already challenging task of comprehensively assessing EVA biocompatibility across diverse applications and environments.
Existing EVA Biocompatibility Assessment Methods
01 Biocompatible EVA compositions
Ethylene Vinyl Acetate (EVA) copolymers can be formulated to enhance biocompatibility for medical applications. These compositions often include specific additives or modifications to improve their compatibility with biological systems, making them suitable for use in medical devices, implants, or drug delivery systems.- Biocompatible EVA compositions: Ethylene Vinyl Acetate (EVA) copolymers can be formulated to enhance biocompatibility for medical applications. These compositions often include specific additives or modifications to improve their compatibility with biological systems, making them suitable for use in medical devices, implants, or drug delivery systems.
- EVA blends for improved properties: Blending EVA with other polymers or materials can enhance its biocompatibility and overall performance. These blends may offer improved mechanical properties, better drug release profiles, or increased resistance to degradation in biological environments, making them suitable for various biomedical applications.
- Surface modification of EVA: Surface treatments or modifications of EVA materials can significantly improve their biocompatibility. These modifications may include plasma treatment, chemical grafting, or coating with biocompatible substances to enhance cell adhesion, reduce protein adsorption, or prevent adverse immune responses.
- EVA-based drug delivery systems: EVA copolymers are utilized in controlled drug delivery systems due to their biocompatibility and tunable release properties. The polymer's composition and processing can be optimized to achieve desired drug release profiles while maintaining compatibility with biological tissues.
- Biocompatibility testing of EVA materials: Various methods and standards are employed to assess the biocompatibility of EVA materials. These tests evaluate cytotoxicity, sensitization, irritation, and long-term effects to ensure the safety and suitability of EVA-based products for biomedical applications.
02 EVA blends for improved properties
Blending EVA with other polymers or materials can enhance its biocompatibility and overall performance. These blends may offer improved mechanical properties, better drug release profiles, or increased resistance to degradation in biological environments, making them suitable for various biomedical applications.Expand Specific Solutions03 Surface modification of EVA
Surface treatments or modifications of EVA can improve its biocompatibility. These modifications may include plasma treatment, chemical grafting, or coating with biocompatible materials to enhance cell adhesion, reduce protein adsorption, or prevent bacterial colonization on the surface of EVA-based medical devices.Expand Specific Solutions04 EVA-based drug delivery systems
EVA copolymers can be used in controlled drug delivery systems due to their biocompatibility and tunable release properties. The composition and structure of EVA can be optimized to achieve desired drug release profiles, making it suitable for various therapeutic applications, including long-term implantable devices.Expand Specific Solutions05 Biocompatibility testing of EVA materials
Various methods and protocols are employed to assess the biocompatibility of EVA materials. These tests may include in vitro cytotoxicity assays, in vivo implantation studies, and evaluation of inflammatory responses to ensure the safety and suitability of EVA-based products for biomedical applications.Expand Specific Solutions
Key Players in EVA Biocompatibility Research
The biocompatibility assessment of Ethylene Vinyl Acetate (EVA) is currently in a mature stage of development, with a growing market driven by increasing applications in medical devices and drug delivery systems. The global EVA market size is projected to reach significant growth in the coming years. Companies like Henkel AG & Co. KGaA, Kuraray Co., Ltd., and LG Chem Ltd. are leading players in this field, leveraging their expertise in polymer science and materials engineering to develop advanced EVA formulations with enhanced biocompatibility. Research institutions such as Queen's University of Belfast and Beijing University of Chemical Technology are contributing to the advancement of EVA biocompatibility through collaborative studies and innovative testing methodologies.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed a comprehensive approach to assessing the biocompatibility of Ethylene Vinyl Acetate (EVA). Their method involves a multi-step process including in vitro cytotoxicity tests, sensitization studies, and implantation tests[1]. They utilize advanced surface modification techniques to enhance the biocompatibility of EVA, such as plasma treatment and grafting of bioactive molecules[2]. Henkel's research also focuses on the long-term stability of EVA in biological environments, employing accelerated aging studies to predict material performance over extended periods[3]. Additionally, they have developed proprietary additives that can be incorporated into EVA to improve its resistance to bacterial colonization, addressing a key concern in medical applications[4].
Strengths: Comprehensive testing methodology, advanced surface modification techniques, focus on long-term stability. Weaknesses: Potential increased production costs due to extensive testing and modifications, may limit applications in cost-sensitive markets.
Kuraray Co., Ltd.
Technical Solution: Kuraray has pioneered a novel approach to enhancing the biocompatibility of Ethylene Vinyl Acetate through their proprietary "Bio-EVA" technology. This involves incorporating bioactive glass particles into the EVA matrix, which has been shown to promote cell adhesion and proliferation[1]. Their research has demonstrated a significant reduction in inflammatory responses when compared to standard EVA materials[2]. Kuraray's method also includes a unique surface treatment process that creates a hydrophilic layer on the EVA surface, improving its interaction with biological fluids and reducing protein adsorption[3]. They have developed a range of EVA grades with varying vinyl acetate content, allowing for fine-tuning of mechanical properties and biocompatibility for specific medical applications[4].
Strengths: Innovative Bio-EVA technology, reduced inflammatory response, customizable grades for specific applications. Weaknesses: Potential higher material costs, may require specialized processing equipment.
Core Innovations in EVA Biocompatibility
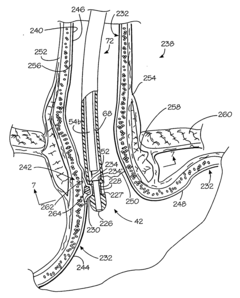
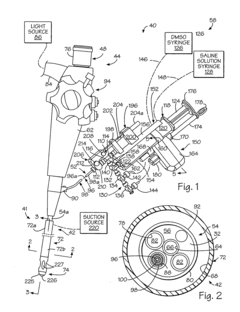
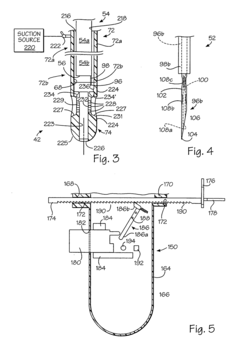
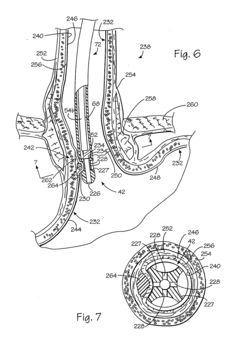
Kit for forming implants in wall of gastrointestinal tract
PatentInactiveUS20040249239A1
Innovation
- A minimally invasive apparatus comprising an elongate probe with a recess and a hollow needle that creates suction to draw the wall into the recess, allowing for the injection of a material to form consistent implants, guiding the needle to introduce the material into the wall and shape the tissue into protrusions for implant formation.
Bioactive implants for drug delivery
PatentWO2021053047A1
Innovation
- The use of eutectic mixtures in polymer-based medical devices, comprising biocompatible polymers like EVA with active agents, fatty acids, fatty alcohols, or fatty amines, to enhance drug loading, stability, and controlled release, facilitating easier implantation and removal with improved lubricity and sustained drug delivery.
Regulatory Framework for Biocompatible Materials
The regulatory framework for biocompatible materials plays a crucial role in ensuring the safety and efficacy of medical devices and implants. For Ethylene Vinyl Acetate (EVA), a polymer widely used in various biomedical applications, adherence to these regulations is paramount.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for biocompatible materials. The FDA's guidance document on the use of ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process," provides a comprehensive framework for assessing biocompatibility. This standard outlines the general principles for biological evaluation of medical devices and the categorization of devices based on the nature and duration of body contact.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern biocompatible materials. These regulations emphasize a risk-based approach to biocompatibility assessment, requiring manufacturers to demonstrate the safety of their materials through rigorous testing and documentation.
Japan's Pharmaceutical and Medical Device Agency (PMDA) follows similar principles, with specific guidelines for biological safety evaluation of medical devices. The Japanese Industrial Standards (JIS) T 0993 series, which is harmonized with ISO 10993, provides detailed requirements for biocompatibility testing.
For EVA specifically, manufacturers must conduct a range of tests to assess its biocompatibility. These typically include cytotoxicity, sensitization, irritation, systemic toxicity, and depending on the intended use, genotoxicity and implantation studies. The exact battery of tests required depends on the device's categorization and intended duration of contact with the body.
Regulatory bodies also require manufacturers to implement quality management systems, such as ISO 13485, to ensure consistent production of safe and effective medical devices. This includes maintaining detailed documentation of material sourcing, manufacturing processes, and quality control measures.
As the field of biomaterials advances, regulatory frameworks continue to evolve. Recent trends include increased focus on the long-term effects of implanted materials, consideration of nano-scale interactions, and the integration of computational modeling in biocompatibility assessments. Manufacturers working with EVA must stay abreast of these developments to ensure ongoing compliance and market access.
In conclusion, the regulatory framework for biocompatible materials, including EVA, is complex and multifaceted. It requires a thorough understanding of international standards, regional regulations, and specific testing requirements. Adherence to these regulations not only ensures patient safety but also facilitates global market access for medical devices incorporating EVA.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for biocompatible materials. The FDA's guidance document on the use of ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process," provides a comprehensive framework for assessing biocompatibility. This standard outlines the general principles for biological evaluation of medical devices and the categorization of devices based on the nature and duration of body contact.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern biocompatible materials. These regulations emphasize a risk-based approach to biocompatibility assessment, requiring manufacturers to demonstrate the safety of their materials through rigorous testing and documentation.
Japan's Pharmaceutical and Medical Device Agency (PMDA) follows similar principles, with specific guidelines for biological safety evaluation of medical devices. The Japanese Industrial Standards (JIS) T 0993 series, which is harmonized with ISO 10993, provides detailed requirements for biocompatibility testing.
For EVA specifically, manufacturers must conduct a range of tests to assess its biocompatibility. These typically include cytotoxicity, sensitization, irritation, systemic toxicity, and depending on the intended use, genotoxicity and implantation studies. The exact battery of tests required depends on the device's categorization and intended duration of contact with the body.
Regulatory bodies also require manufacturers to implement quality management systems, such as ISO 13485, to ensure consistent production of safe and effective medical devices. This includes maintaining detailed documentation of material sourcing, manufacturing processes, and quality control measures.
As the field of biomaterials advances, regulatory frameworks continue to evolve. Recent trends include increased focus on the long-term effects of implanted materials, consideration of nano-scale interactions, and the integration of computational modeling in biocompatibility assessments. Manufacturers working with EVA must stay abreast of these developments to ensure ongoing compliance and market access.
In conclusion, the regulatory framework for biocompatible materials, including EVA, is complex and multifaceted. It requires a thorough understanding of international standards, regional regulations, and specific testing requirements. Adherence to these regulations not only ensures patient safety but also facilitates global market access for medical devices incorporating EVA.
Environmental Impact of EVA in Biomedical Applications
The environmental impact of Ethylene Vinyl Acetate (EVA) in biomedical applications is a critical consideration as the material gains prominence in various medical devices and implants. EVA's biocompatibility and versatile properties have led to its widespread use, but its environmental implications require thorough examination.
One of the primary environmental concerns associated with EVA in biomedical applications is its end-of-life disposal. As medical devices and implants reach the end of their useful life, proper disposal methods are essential to minimize environmental contamination. EVA, being a thermoplastic material, can potentially be recycled. However, the recycling process for medical-grade EVA is complex due to potential contamination with biological materials and the need for stringent sterilization procedures.
The production of EVA for biomedical applications also raises environmental considerations. The manufacturing process involves the use of petrochemical resources, which contributes to carbon emissions and resource depletion. However, compared to some alternative materials, EVA production may have a lower environmental footprint due to its relatively simple manufacturing process and the ability to recycle production waste.
In terms of biodegradability, EVA is not readily biodegradable in natural environments. This characteristic ensures the longevity and stability of EVA-based medical devices during their intended use but poses challenges for environmental breakdown after disposal. Research into biodegradable variants of EVA or composite materials that incorporate EVA with biodegradable components is ongoing to address this issue.
The potential for EVA to leach chemicals into the environment, particularly in aquatic ecosystems, is another area of concern. While EVA is generally considered inert, long-term exposure to environmental factors may lead to the release of additives or degradation products. Studies on the ecotoxicological effects of EVA in various environmental compartments are necessary to fully understand its long-term impact.
On a positive note, the durability and long lifespan of EVA-based medical devices can contribute to reduced waste generation compared to single-use or short-lived alternatives. This aspect aligns with sustainability goals by potentially decreasing the overall environmental impact associated with medical device production and disposal.
The biomedical industry's increasing focus on sustainable practices has led to efforts to improve the environmental profile of EVA applications. These include developing more efficient production processes, exploring bio-based alternatives for EVA components, and implementing closed-loop recycling systems for medical-grade plastics.
In conclusion, while EVA offers significant benefits in biomedical applications, its environmental impact requires ongoing assessment and mitigation strategies. Balancing the material's performance in medical devices with environmental sustainability remains a key challenge for researchers and manufacturers in the biomedical field.
One of the primary environmental concerns associated with EVA in biomedical applications is its end-of-life disposal. As medical devices and implants reach the end of their useful life, proper disposal methods are essential to minimize environmental contamination. EVA, being a thermoplastic material, can potentially be recycled. However, the recycling process for medical-grade EVA is complex due to potential contamination with biological materials and the need for stringent sterilization procedures.
The production of EVA for biomedical applications also raises environmental considerations. The manufacturing process involves the use of petrochemical resources, which contributes to carbon emissions and resource depletion. However, compared to some alternative materials, EVA production may have a lower environmental footprint due to its relatively simple manufacturing process and the ability to recycle production waste.
In terms of biodegradability, EVA is not readily biodegradable in natural environments. This characteristic ensures the longevity and stability of EVA-based medical devices during their intended use but poses challenges for environmental breakdown after disposal. Research into biodegradable variants of EVA or composite materials that incorporate EVA with biodegradable components is ongoing to address this issue.
The potential for EVA to leach chemicals into the environment, particularly in aquatic ecosystems, is another area of concern. While EVA is generally considered inert, long-term exposure to environmental factors may lead to the release of additives or degradation products. Studies on the ecotoxicological effects of EVA in various environmental compartments are necessary to fully understand its long-term impact.
On a positive note, the durability and long lifespan of EVA-based medical devices can contribute to reduced waste generation compared to single-use or short-lived alternatives. This aspect aligns with sustainability goals by potentially decreasing the overall environmental impact associated with medical device production and disposal.
The biomedical industry's increasing focus on sustainable practices has led to efforts to improve the environmental profile of EVA applications. These include developing more efficient production processes, exploring bio-based alternatives for EVA components, and implementing closed-loop recycling systems for medical-grade plastics.
In conclusion, while EVA offers significant benefits in biomedical applications, its environmental impact requires ongoing assessment and mitigation strategies. Balancing the material's performance in medical devices with environmental sustainability remains a key challenge for researchers and manufacturers in the biomedical field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!