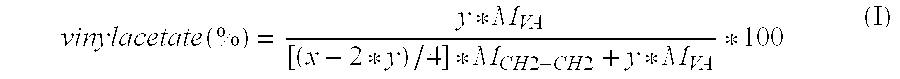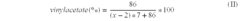Exploring Ethylene Vinyl Acetate in Drug Delivery Systems
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
EVA in Drug Delivery: Background and Objectives
Ethylene Vinyl Acetate (EVA) has emerged as a significant polymer in the field of drug delivery systems, marking a notable evolution in pharmaceutical technology. The journey of EVA in this domain began in the 1960s when its potential for controlled release of drugs was first recognized. Since then, EVA has undergone continuous development, adapting to the changing needs of the pharmaceutical industry and addressing various challenges in drug delivery.
The primary objective of exploring EVA in drug delivery systems is to enhance the efficacy and safety of pharmaceutical treatments. EVA's unique properties, including its biocompatibility, flexibility, and ability to control drug release rates, make it an ideal candidate for various drug delivery applications. Researchers aim to leverage these characteristics to develop innovative drug delivery systems that can improve patient compliance, reduce side effects, and optimize therapeutic outcomes.
One of the key trends in EVA-based drug delivery systems is the development of long-acting formulations. These formulations are designed to maintain therapeutic drug levels over extended periods, reducing the frequency of drug administration and improving patient adherence to treatment regimens. This trend aligns with the growing demand for patient-centric healthcare solutions and the need for more efficient drug delivery methods in chronic disease management.
Another significant trend is the exploration of EVA in targeted drug delivery. By modifying the surface properties of EVA-based systems or incorporating them into smart delivery devices, researchers aim to achieve site-specific drug release. This approach holds promise for enhancing the treatment of localized conditions while minimizing systemic exposure to drugs, potentially reducing side effects and improving overall treatment efficacy.
The evolution of EVA in drug delivery systems also reflects broader technological advancements in materials science and pharmaceutical engineering. Researchers are investigating ways to combine EVA with other materials or incorporate it into novel delivery platforms, such as 3D-printed devices or nanocarriers. These efforts are driven by the goal of creating more sophisticated, responsive, and personalized drug delivery systems that can adapt to individual patient needs and specific disease conditions.
As the field progresses, the objectives for EVA in drug delivery systems continue to expand. Current research aims to overcome existing limitations, such as improving the drug loading capacity of EVA-based systems and enhancing their compatibility with a wider range of pharmaceutical compounds. Additionally, there is a growing focus on developing EVA formulations that can respond to specific physiological triggers or external stimuli, enabling precise control over drug release profiles.
The primary objective of exploring EVA in drug delivery systems is to enhance the efficacy and safety of pharmaceutical treatments. EVA's unique properties, including its biocompatibility, flexibility, and ability to control drug release rates, make it an ideal candidate for various drug delivery applications. Researchers aim to leverage these characteristics to develop innovative drug delivery systems that can improve patient compliance, reduce side effects, and optimize therapeutic outcomes.
One of the key trends in EVA-based drug delivery systems is the development of long-acting formulations. These formulations are designed to maintain therapeutic drug levels over extended periods, reducing the frequency of drug administration and improving patient adherence to treatment regimens. This trend aligns with the growing demand for patient-centric healthcare solutions and the need for more efficient drug delivery methods in chronic disease management.
Another significant trend is the exploration of EVA in targeted drug delivery. By modifying the surface properties of EVA-based systems or incorporating them into smart delivery devices, researchers aim to achieve site-specific drug release. This approach holds promise for enhancing the treatment of localized conditions while minimizing systemic exposure to drugs, potentially reducing side effects and improving overall treatment efficacy.
The evolution of EVA in drug delivery systems also reflects broader technological advancements in materials science and pharmaceutical engineering. Researchers are investigating ways to combine EVA with other materials or incorporate it into novel delivery platforms, such as 3D-printed devices or nanocarriers. These efforts are driven by the goal of creating more sophisticated, responsive, and personalized drug delivery systems that can adapt to individual patient needs and specific disease conditions.
As the field progresses, the objectives for EVA in drug delivery systems continue to expand. Current research aims to overcome existing limitations, such as improving the drug loading capacity of EVA-based systems and enhancing their compatibility with a wider range of pharmaceutical compounds. Additionally, there is a growing focus on developing EVA formulations that can respond to specific physiological triggers or external stimuli, enabling precise control over drug release profiles.
Market Analysis for EVA-based Drug Delivery Systems
The market for Ethylene Vinyl Acetate (EVA) in drug delivery systems has shown significant growth and potential in recent years. This versatile copolymer has gained traction in the pharmaceutical industry due to its unique properties that make it suitable for various drug delivery applications. The global market for EVA-based drug delivery systems is driven by several factors, including the increasing prevalence of chronic diseases, the growing demand for controlled-release medications, and the need for more efficient drug delivery methods.
One of the key market segments for EVA-based drug delivery systems is transdermal patches. These patches offer a non-invasive and convenient method for drug administration, particularly for medications that require sustained release over an extended period. The market for transdermal drug delivery systems is expected to grow substantially, with EVA playing a crucial role in this expansion due to its excellent adhesive properties and compatibility with a wide range of active pharmaceutical ingredients.
Another promising area for EVA in drug delivery is in the development of implantable devices. EVA's biocompatibility and ability to control drug release rates make it an attractive material for long-term implantable drug delivery systems. This segment is particularly relevant for treatments requiring continuous medication over months or even years, such as hormone therapies or certain cancer treatments.
The ophthalmic drug delivery market is also showing increased interest in EVA-based systems. EVA's transparency and ability to form thin films make it suitable for developing ocular inserts and other ophthalmic drug delivery devices. This application is particularly valuable for treating chronic eye conditions that require sustained drug release.
Geographically, North America and Europe currently dominate the market for EVA-based drug delivery systems, primarily due to their advanced healthcare infrastructure and higher adoption rates of innovative drug delivery technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, increasing healthcare expenditure, and a growing awareness of advanced drug delivery methods.
The market is characterized by the presence of both large pharmaceutical companies and specialized drug delivery technology firms. These players are investing heavily in research and development to expand the applications of EVA in drug delivery and to overcome existing limitations. Collaborations between material science companies, drug manufacturers, and research institutions are becoming increasingly common, fostering innovation in this field.
Despite the positive outlook, the market for EVA-based drug delivery systems faces challenges such as regulatory hurdles, the high cost of development, and competition from alternative materials and delivery methods. However, ongoing research into improving EVA's properties and expanding its applications in drug delivery is expected to drive continued market growth and innovation in the coming years.
One of the key market segments for EVA-based drug delivery systems is transdermal patches. These patches offer a non-invasive and convenient method for drug administration, particularly for medications that require sustained release over an extended period. The market for transdermal drug delivery systems is expected to grow substantially, with EVA playing a crucial role in this expansion due to its excellent adhesive properties and compatibility with a wide range of active pharmaceutical ingredients.
Another promising area for EVA in drug delivery is in the development of implantable devices. EVA's biocompatibility and ability to control drug release rates make it an attractive material for long-term implantable drug delivery systems. This segment is particularly relevant for treatments requiring continuous medication over months or even years, such as hormone therapies or certain cancer treatments.
The ophthalmic drug delivery market is also showing increased interest in EVA-based systems. EVA's transparency and ability to form thin films make it suitable for developing ocular inserts and other ophthalmic drug delivery devices. This application is particularly valuable for treating chronic eye conditions that require sustained drug release.
Geographically, North America and Europe currently dominate the market for EVA-based drug delivery systems, primarily due to their advanced healthcare infrastructure and higher adoption rates of innovative drug delivery technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access, increasing healthcare expenditure, and a growing awareness of advanced drug delivery methods.
The market is characterized by the presence of both large pharmaceutical companies and specialized drug delivery technology firms. These players are investing heavily in research and development to expand the applications of EVA in drug delivery and to overcome existing limitations. Collaborations between material science companies, drug manufacturers, and research institutions are becoming increasingly common, fostering innovation in this field.
Despite the positive outlook, the market for EVA-based drug delivery systems faces challenges such as regulatory hurdles, the high cost of development, and competition from alternative materials and delivery methods. However, ongoing research into improving EVA's properties and expanding its applications in drug delivery is expected to drive continued market growth and innovation in the coming years.
Current Challenges in EVA Drug Delivery Technology
Despite the widespread use of Ethylene Vinyl Acetate (EVA) in drug delivery systems, several challenges persist in its application. One of the primary issues is the limited drug loading capacity of EVA matrices. The polymer's hydrophobic nature restricts its ability to incorporate high concentrations of hydrophilic drugs, which constitute a significant portion of pharmaceutical compounds. This limitation often necessitates the use of larger devices or more frequent dosing, potentially impacting patient compliance and treatment efficacy.
Another significant challenge is the control of drug release kinetics from EVA-based systems. While EVA offers sustained release properties, achieving precise control over the release rate remains difficult, especially for long-term delivery applications. The release profile can be affected by various factors, including drug solubility, polymer composition, and environmental conditions, making it challenging to maintain consistent therapeutic levels over extended periods.
The stability of drugs within EVA matrices presents another hurdle. Some active pharmaceutical ingredients may degrade or undergo chemical changes when exposed to the processing conditions required for EVA formulation, such as high temperatures during extrusion or molding. This can lead to reduced drug efficacy or the formation of unwanted by-products, compromising the safety and effectiveness of the delivery system.
Biocompatibility and biodegradability concerns also pose challenges in EVA drug delivery technology. While EVA is generally considered biocompatible, long-term implantation can sometimes lead to localized inflammation or foreign body responses. Additionally, EVA is not biodegradable, which limits its use in applications where the delivery system should be naturally eliminated from the body after drug depletion.
The scalability and reproducibility of EVA-based drug delivery systems in large-scale manufacturing settings remain problematic. Ensuring consistent drug distribution within the polymer matrix and maintaining uniform release characteristics across batches can be challenging, potentially affecting the quality and reliability of the final product.
Regulatory hurdles also present significant challenges in the development and commercialization of EVA drug delivery systems. The complex nature of these combination products often requires extensive safety and efficacy studies, prolonging the approval process and increasing development costs.
Lastly, the environmental impact of non-biodegradable EVA devices is becoming an increasing concern. As sustainability gains importance in healthcare, finding eco-friendly alternatives or developing effective recycling methods for used EVA drug delivery systems is emerging as a critical challenge for the industry.
Another significant challenge is the control of drug release kinetics from EVA-based systems. While EVA offers sustained release properties, achieving precise control over the release rate remains difficult, especially for long-term delivery applications. The release profile can be affected by various factors, including drug solubility, polymer composition, and environmental conditions, making it challenging to maintain consistent therapeutic levels over extended periods.
The stability of drugs within EVA matrices presents another hurdle. Some active pharmaceutical ingredients may degrade or undergo chemical changes when exposed to the processing conditions required for EVA formulation, such as high temperatures during extrusion or molding. This can lead to reduced drug efficacy or the formation of unwanted by-products, compromising the safety and effectiveness of the delivery system.
Biocompatibility and biodegradability concerns also pose challenges in EVA drug delivery technology. While EVA is generally considered biocompatible, long-term implantation can sometimes lead to localized inflammation or foreign body responses. Additionally, EVA is not biodegradable, which limits its use in applications where the delivery system should be naturally eliminated from the body after drug depletion.
The scalability and reproducibility of EVA-based drug delivery systems in large-scale manufacturing settings remain problematic. Ensuring consistent drug distribution within the polymer matrix and maintaining uniform release characteristics across batches can be challenging, potentially affecting the quality and reliability of the final product.
Regulatory hurdles also present significant challenges in the development and commercialization of EVA drug delivery systems. The complex nature of these combination products often requires extensive safety and efficacy studies, prolonging the approval process and increasing development costs.
Lastly, the environmental impact of non-biodegradable EVA devices is becoming an increasing concern. As sustainability gains importance in healthcare, finding eco-friendly alternatives or developing effective recycling methods for used EVA drug delivery systems is emerging as a critical challenge for the industry.
Existing EVA Drug Delivery Mechanisms
01 Composition and synthesis of EVA copolymers
Ethylene Vinyl Acetate (EVA) copolymers are synthesized through the copolymerization of ethylene and vinyl acetate monomers. The composition and properties of EVA can be adjusted by varying the ratio of these monomers, allowing for a wide range of applications. The synthesis process often involves high-pressure polymerization techniques and may include the use of catalysts to control the reaction.- Composition and synthesis of EVA copolymers: Ethylene Vinyl Acetate (EVA) copolymers are synthesized through the copolymerization of ethylene and vinyl acetate monomers. The composition and properties of EVA can be tailored by adjusting the ratio of these monomers and the polymerization conditions. This versatility allows for the production of EVA with varying degrees of flexibility, toughness, and transparency.
- EVA blends and modifications: EVA copolymers can be blended with other polymers or modified with additives to enhance specific properties. These blends and modifications can improve characteristics such as impact resistance, thermal stability, or adhesion. Common blend partners include polyethylene, polypropylene, and various elastomers. Modifications may involve crosslinking or the addition of fillers and stabilizers.
- Applications of EVA in packaging and films: EVA copolymers are widely used in packaging applications and film production. Their flexibility, clarity, and low-temperature toughness make them suitable for food packaging, shrink wrap, and agricultural films. EVA can be processed into multi-layer films, offering improved barrier properties and sealability for various packaging needs.
- EVA in adhesives and sealants: The adhesive properties of EVA make it an excellent choice for hot melt adhesives and sealants. EVA-based adhesives offer good bond strength, flexibility, and resistance to environmental factors. They are used in various industries, including construction, automotive, and consumer goods manufacturing.
- EVA foam and encapsulation applications: EVA can be foamed to create lightweight, flexible materials used in footwear, sports equipment, and automotive components. The closed-cell structure of EVA foam provides excellent shock absorption and insulation properties. Additionally, EVA is used in encapsulation applications for solar panels and electronic components, offering protection from environmental factors.
02 EVA blends and composites
EVA copolymers are often blended with other polymers or materials to create composites with enhanced properties. These blends can improve characteristics such as flexibility, impact resistance, and thermal stability. Common blend components include polyethylene, polypropylene, and various fillers or reinforcing agents. The resulting composites find applications in diverse industries, including packaging, automotive, and construction.Expand Specific Solutions03 Foam and cellular EVA products
EVA can be processed into foam or cellular structures, which are widely used in applications requiring lightweight, cushioning, or insulating properties. The foaming process typically involves the use of blowing agents and specialized extrusion or molding techniques. These foamed EVA products find applications in footwear, sports equipment, and packaging materials.Expand Specific Solutions04 EVA in adhesive and sealant formulations
EVA copolymers are extensively used in the formulation of hot melt adhesives and sealants. The polymer's low melting point, good adhesion properties, and flexibility make it ideal for these applications. EVA-based adhesives are used in packaging, bookbinding, and various assembly processes. The formulations often include additives to enhance specific properties such as tackiness or thermal stability.Expand Specific Solutions05 Modification and functionalization of EVA
EVA copolymers can be chemically modified or functionalized to impart specific properties or improve compatibility with other materials. Common modification techniques include grafting, crosslinking, and the incorporation of functional groups. These modifications can enhance properties such as adhesion, barrier performance, or reactivity, expanding the range of applications for EVA-based materials.Expand Specific Solutions
Key Players in EVA-based Drug Delivery Industry
The exploration of Ethylene Vinyl Acetate (EVA) in drug delivery systems is currently in a growth phase, with increasing market potential and technological advancements. The global drug delivery market is expanding rapidly, driven by the demand for innovative and efficient delivery methods. EVA's versatility and biocompatibility make it an attractive material for various drug delivery applications. Companies like Noven Pharmaceuticals, NOF Corp., and Medtronic are actively involved in developing EVA-based drug delivery systems, leveraging their expertise in transdermal and implantable technologies. Academic institutions such as MIT and Ghent University are contributing to the research and development of EVA-based drug delivery platforms, indicating a growing interest in this field. As the technology matures, we can expect to see more companies entering this space and expanding the applications of EVA in drug delivery systems.
Noven Pharmaceuticals, Inc.
Technical Solution: Noven Pharmaceuticals specializes in transdermal and transmucosal drug delivery systems, with EVA playing a crucial role in their technology. They've developed the DOT Matrix® technology, which uses EVA as a key component in creating multi-polymer adhesive matrices for transdermal patches[10]. This technology allows for the incorporation of both liquid and solid drug components, enabling the delivery of a wide range of active ingredients. Noven has applied this EVA-based technology to develop products like Minivelle®, a estradiol transdermal system for hormone therapy. Their research focuses on optimizing EVA formulations to enhance drug permeation through the skin while maintaining patch adhesion and stability over extended wear times[11].
Strengths: Expertise in transdermal delivery, versatile drug incorporation, non-invasive administration. Weaknesses: Limited to drugs suitable for transdermal delivery, potential for skin irritation, variability in absorption rates between individuals.
Medtronic, Inc.
Technical Solution: Medtronic has utilized EVA in developing drug-eluting medical devices, particularly for cardiovascular applications. Their research has focused on incorporating EVA as a drug reservoir and release-controlling matrix in stents and other implantable devices. One notable application is the use of EVA in drug-eluting coronary stents, where it serves as a polymer carrier for antiproliferative drugs like sirolimus[8]. Medtronic has optimized EVA formulations to achieve controlled drug release profiles that match the healing process of the vessel wall. They've also explored using EVA in combination with other polymers to create multi-layered coatings that can deliver multiple drugs with different release kinetics[9].
Strengths: Integration with medical devices, tailored release profiles, potential for multi-drug delivery. Weaknesses: Limited to device-based delivery, potential for polymer-related inflammation, regulatory complexities for combination products.
Innovative EVA Formulations for Drug Release
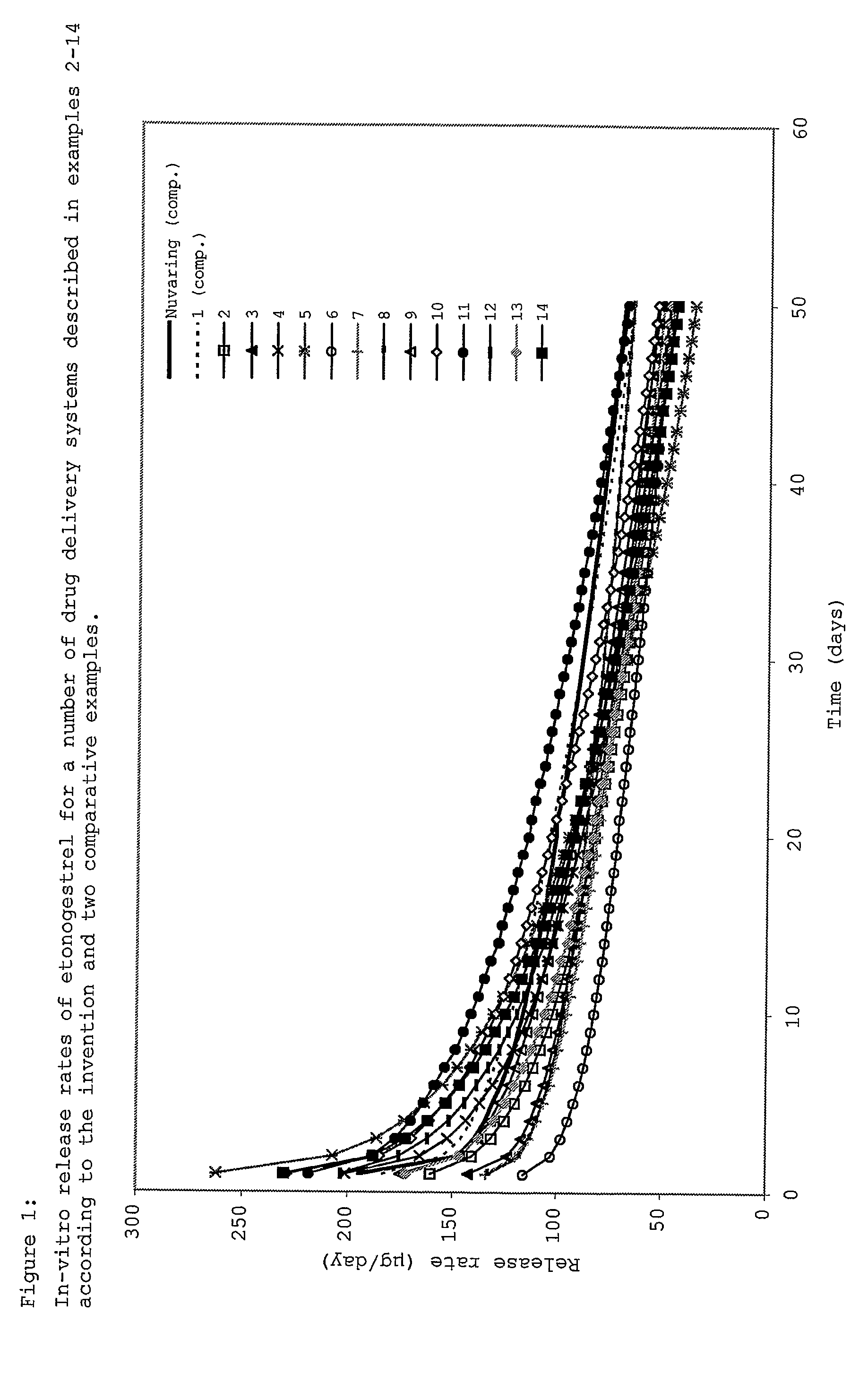
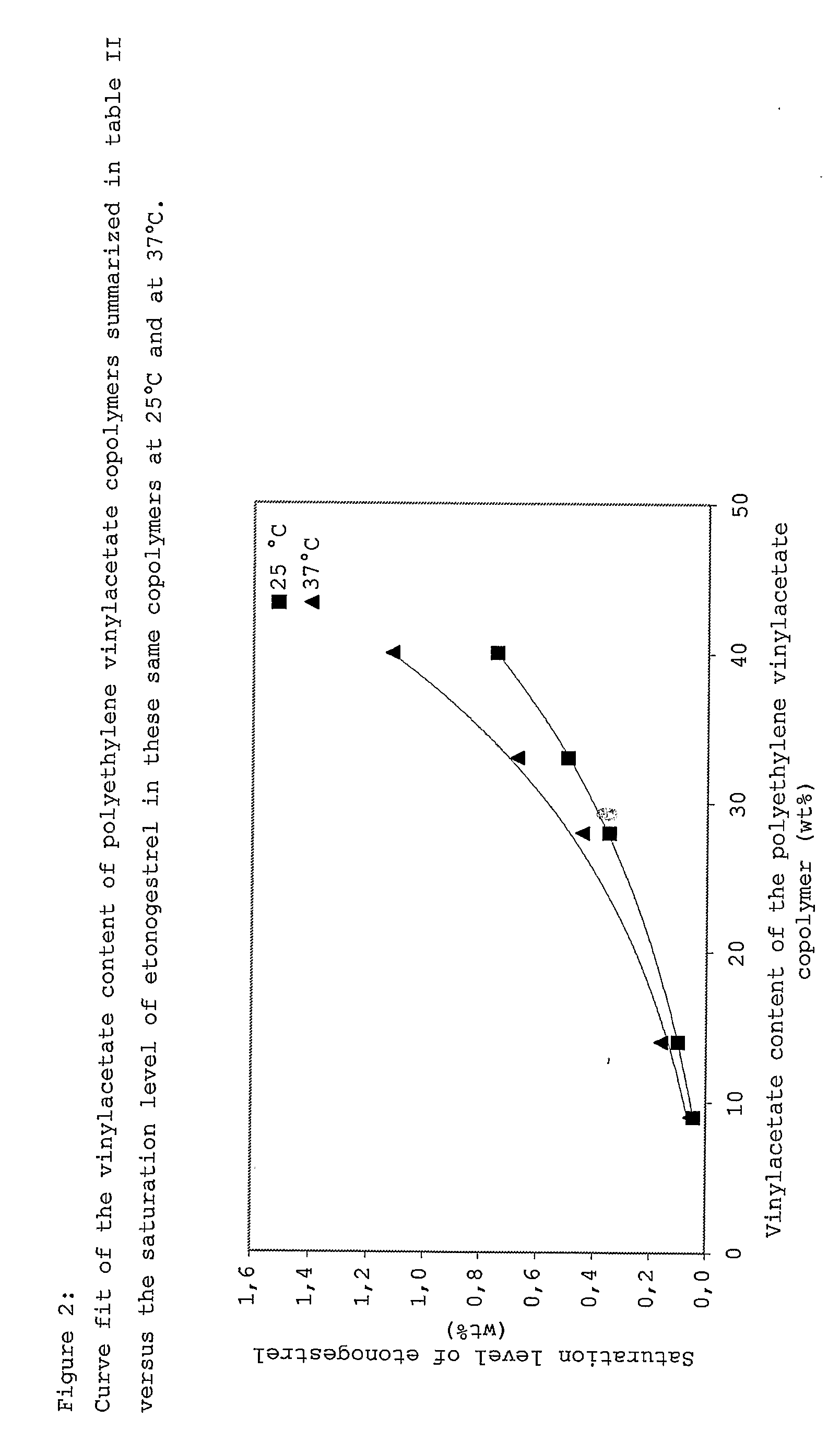
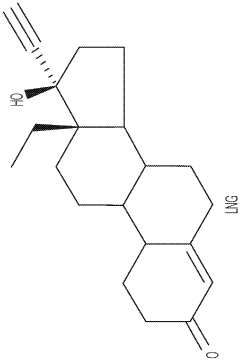
Drug delivery system based on polyethylene vinylacetate copolymers
PatentActiveUS20070141102A1
Innovation
- A drug delivery system comprising a core and skin made of thermoplastic polyethylene vinylacetate copolymer, with the progestogenic compound dissolved below saturation levels, ensuring stability at room temperature and preventing crystallization, while maintaining consistent release rates for contraception and hormone replacement therapy.
Combination therapy intravaginal rings
PatentWO2023141198A1
Innovation
- The development of intravaginal rings using a specific combination of ethylene-vinyl acetate (EVA) polymers in the core and sheath, loaded with dapivirine and levonorgestrel, which provides a controlled and independent release of both drugs over an extended period, improving stability and mechanical properties.
Regulatory Framework for EVA in Pharmaceuticals
The regulatory framework for Ethylene Vinyl Acetate (EVA) in pharmaceuticals is a critical aspect of its application in drug delivery systems. This framework is primarily governed by regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other international health authorities.
In the United States, EVA is regulated under the FDA's guidance for inactive ingredients in drug products. The FDA evaluates the safety and suitability of EVA through its Inactive Ingredient Database (IID), which provides information on the maximum potency per unit dose for various routes of administration. Manufacturers must demonstrate that their use of EVA complies with these guidelines and does not compromise the safety or efficacy of the drug product.
The EMA, responsible for regulating pharmaceuticals in the European Union, has similar requirements for EVA use in drug delivery systems. The agency's guidelines on excipients require manufacturers to provide comprehensive data on the quality, safety, and functionality of EVA in their formulations. This includes information on the material's specifications, manufacturing process, and stability data.
Regulatory bodies also focus on the potential leachables and extractables from EVA-based drug delivery systems. Manufacturers must conduct thorough studies to identify and quantify any substances that may migrate from the EVA matrix into the drug formulation. These studies are essential to ensure patient safety and maintain the integrity of the drug product throughout its shelf life.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that are widely adopted by regulatory agencies worldwide. These guidelines, particularly ICH Q3C on residual solvents, are relevant to EVA manufacturing processes and must be considered when developing EVA-based drug delivery systems.
Regulatory requirements also extend to the manufacturing processes of EVA-based products. Good Manufacturing Practices (GMP) must be followed to ensure consistent quality and safety of the final drug delivery system. This includes validation of the manufacturing process, quality control measures, and documentation of all procedures related to EVA incorporation into the drug product.
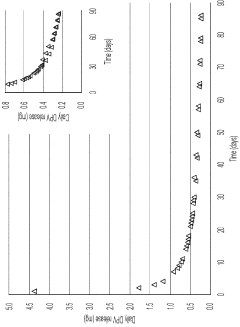
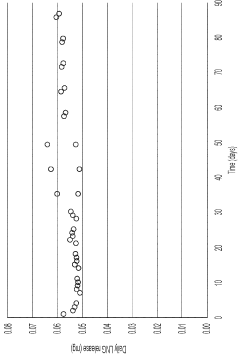
As EVA is often used in controlled release formulations, regulatory bodies pay special attention to the dissolution profiles and release kinetics of drugs from EVA matrices. Manufacturers must provide robust data demonstrating the consistency and predictability of drug release from EVA-based systems under various physiological conditions.
The regulatory landscape for EVA in pharmaceuticals is dynamic, with agencies continually updating their guidelines based on new scientific evidence and technological advancements. Manufacturers and researchers must stay informed about these changes and adapt their development and production processes accordingly to maintain compliance and ensure patient safety.
In the United States, EVA is regulated under the FDA's guidance for inactive ingredients in drug products. The FDA evaluates the safety and suitability of EVA through its Inactive Ingredient Database (IID), which provides information on the maximum potency per unit dose for various routes of administration. Manufacturers must demonstrate that their use of EVA complies with these guidelines and does not compromise the safety or efficacy of the drug product.
The EMA, responsible for regulating pharmaceuticals in the European Union, has similar requirements for EVA use in drug delivery systems. The agency's guidelines on excipients require manufacturers to provide comprehensive data on the quality, safety, and functionality of EVA in their formulations. This includes information on the material's specifications, manufacturing process, and stability data.
Regulatory bodies also focus on the potential leachables and extractables from EVA-based drug delivery systems. Manufacturers must conduct thorough studies to identify and quantify any substances that may migrate from the EVA matrix into the drug formulation. These studies are essential to ensure patient safety and maintain the integrity of the drug product throughout its shelf life.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that are widely adopted by regulatory agencies worldwide. These guidelines, particularly ICH Q3C on residual solvents, are relevant to EVA manufacturing processes and must be considered when developing EVA-based drug delivery systems.
Regulatory requirements also extend to the manufacturing processes of EVA-based products. Good Manufacturing Practices (GMP) must be followed to ensure consistent quality and safety of the final drug delivery system. This includes validation of the manufacturing process, quality control measures, and documentation of all procedures related to EVA incorporation into the drug product.
As EVA is often used in controlled release formulations, regulatory bodies pay special attention to the dissolution profiles and release kinetics of drugs from EVA matrices. Manufacturers must provide robust data demonstrating the consistency and predictability of drug release from EVA-based systems under various physiological conditions.
The regulatory landscape for EVA in pharmaceuticals is dynamic, with agencies continually updating their guidelines based on new scientific evidence and technological advancements. Manufacturers and researchers must stay informed about these changes and adapt their development and production processes accordingly to maintain compliance and ensure patient safety.
Biocompatibility and Safety Considerations of EVA
Ethylene Vinyl Acetate (EVA) has emerged as a promising material in drug delivery systems due to its unique properties and biocompatibility. However, as with any material used in medical applications, thorough evaluation of its safety and biocompatibility is crucial. EVA's biocompatibility is primarily attributed to its non-toxic nature and chemical inertness, making it suitable for various biomedical applications.
One of the key advantages of EVA in drug delivery systems is its low immunogenicity. Studies have shown that EVA does not elicit significant immune responses when implanted or used in contact with biological tissues. This characteristic is particularly important for long-term drug delivery applications, where sustained release over extended periods is desired without triggering adverse immune reactions.
The safety profile of EVA is further enhanced by its resistance to degradation in biological environments. Unlike some biodegradable polymers, EVA maintains its structural integrity over time, reducing the risk of uncontrolled release of degradation products that could potentially cause toxicity or inflammation. This stability also contributes to the predictable and controlled release of drugs from EVA-based delivery systems.
However, it is important to note that the biocompatibility of EVA can be influenced by various factors, including the specific formulation, processing methods, and the presence of additives or residual monomers. Rigorous testing protocols have been developed to assess the biocompatibility of EVA-based drug delivery systems, including in vitro cytotoxicity assays, genotoxicity studies, and in vivo implantation tests.
The long-term effects of EVA implants or devices have also been a subject of extensive research. Studies have demonstrated that EVA exhibits minimal tissue reaction and encapsulation when used in long-term implantable devices. This property is particularly advantageous for sustained-release drug delivery systems, as it allows for consistent drug release without significant interference from the host tissue response.
Despite its favorable biocompatibility profile, ongoing research continues to explore potential improvements and modifications to EVA for enhanced safety and performance in drug delivery applications. Surface modifications, such as plasma treatment or grafting of bioactive molecules, are being investigated to further improve cell adhesion and reduce the risk of foreign body reactions.
In conclusion, the biocompatibility and safety considerations of EVA in drug delivery systems are generally positive, supporting its widespread use in medical applications. However, continuous monitoring and evaluation of new formulations and applications remain essential to ensure the highest standards of patient safety and efficacy in drug delivery systems utilizing EVA.
One of the key advantages of EVA in drug delivery systems is its low immunogenicity. Studies have shown that EVA does not elicit significant immune responses when implanted or used in contact with biological tissues. This characteristic is particularly important for long-term drug delivery applications, where sustained release over extended periods is desired without triggering adverse immune reactions.
The safety profile of EVA is further enhanced by its resistance to degradation in biological environments. Unlike some biodegradable polymers, EVA maintains its structural integrity over time, reducing the risk of uncontrolled release of degradation products that could potentially cause toxicity or inflammation. This stability also contributes to the predictable and controlled release of drugs from EVA-based delivery systems.
However, it is important to note that the biocompatibility of EVA can be influenced by various factors, including the specific formulation, processing methods, and the presence of additives or residual monomers. Rigorous testing protocols have been developed to assess the biocompatibility of EVA-based drug delivery systems, including in vitro cytotoxicity assays, genotoxicity studies, and in vivo implantation tests.
The long-term effects of EVA implants or devices have also been a subject of extensive research. Studies have demonstrated that EVA exhibits minimal tissue reaction and encapsulation when used in long-term implantable devices. This property is particularly advantageous for sustained-release drug delivery systems, as it allows for consistent drug release without significant interference from the host tissue response.
Despite its favorable biocompatibility profile, ongoing research continues to explore potential improvements and modifications to EVA for enhanced safety and performance in drug delivery applications. Surface modifications, such as plasma treatment or grafting of bioactive molecules, are being investigated to further improve cell adhesion and reduce the risk of foreign body reactions.
In conclusion, the biocompatibility and safety considerations of EVA in drug delivery systems are generally positive, supporting its widespread use in medical applications. However, continuous monitoring and evaluation of new formulations and applications remain essential to ensure the highest standards of patient safety and efficacy in drug delivery systems utilizing EVA.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!