Benchmark Material Choices for Flexible Electronics in Medical Use
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flexible Electronics Evolution and Medical Applications
Flexible electronics represent a revolutionary advancement in electronic technology, evolving from rigid circuit boards to malleable, conformable systems that can maintain functionality while being bent, twisted, or stretched. This evolution began in the 1990s with the development of organic semiconductors and has accelerated dramatically in the past decade with innovations in materials science, manufacturing processes, and system integration.
The trajectory of flexible electronics has been marked by several key milestones. Initially, the focus was on developing flexible displays using organic light-emitting diodes (OLEDs). This expanded to include flexible sensors, batteries, and eventually complete flexible integrated circuits. The miniaturization of components and the development of stretchable interconnects have been crucial enablers for this technology's advancement.
In medical applications, flexible electronics offer unprecedented opportunities for patient monitoring, diagnostics, and treatment. The ability to conform to the human body's complex contours allows for more comfortable, less intrusive medical devices that can provide continuous monitoring without impeding patient movement or comfort. This represents a paradigm shift from traditional rigid medical devices that often restrict patient mobility and can cause discomfort during prolonged use.
The integration of flexible electronics in medical devices has enabled innovations such as smart bandages that monitor wound healing, wearable ECG monitors that provide continuous cardiac assessment, and implantable sensors that can detect biochemical markers in real-time. These applications leverage the unique properties of flexible materials to create devices that are not only functional but also biocompatible and mechanically compatible with human tissue.
Recent advancements have focused on developing materials that can withstand the harsh environment of the human body, including resistance to biofluids, temperature fluctuations, and mechanical stress. Additionally, there has been significant progress in creating flexible electronics that can be powered by the body itself, eliminating the need for external power sources or frequent battery replacements.
The convergence of flexible electronics with other emerging technologies, such as artificial intelligence and 5G connectivity, is opening new frontiers in telemedicine and personalized healthcare. These systems can collect vast amounts of patient data, process it locally or transmit it securely to healthcare providers, and even adapt their functionality based on the patient's condition.
Looking forward, the continued evolution of flexible electronics in medical applications promises to transform healthcare delivery, enabling more preventive, personalized, and patient-centered approaches to medicine. The development of biodegradable flexible electronics also offers the potential for temporary implants that can dissolve harmlessly in the body after serving their purpose, eliminating the need for removal surgeries.
The trajectory of flexible electronics has been marked by several key milestones. Initially, the focus was on developing flexible displays using organic light-emitting diodes (OLEDs). This expanded to include flexible sensors, batteries, and eventually complete flexible integrated circuits. The miniaturization of components and the development of stretchable interconnects have been crucial enablers for this technology's advancement.
In medical applications, flexible electronics offer unprecedented opportunities for patient monitoring, diagnostics, and treatment. The ability to conform to the human body's complex contours allows for more comfortable, less intrusive medical devices that can provide continuous monitoring without impeding patient movement or comfort. This represents a paradigm shift from traditional rigid medical devices that often restrict patient mobility and can cause discomfort during prolonged use.
The integration of flexible electronics in medical devices has enabled innovations such as smart bandages that monitor wound healing, wearable ECG monitors that provide continuous cardiac assessment, and implantable sensors that can detect biochemical markers in real-time. These applications leverage the unique properties of flexible materials to create devices that are not only functional but also biocompatible and mechanically compatible with human tissue.
Recent advancements have focused on developing materials that can withstand the harsh environment of the human body, including resistance to biofluids, temperature fluctuations, and mechanical stress. Additionally, there has been significant progress in creating flexible electronics that can be powered by the body itself, eliminating the need for external power sources or frequent battery replacements.
The convergence of flexible electronics with other emerging technologies, such as artificial intelligence and 5G connectivity, is opening new frontiers in telemedicine and personalized healthcare. These systems can collect vast amounts of patient data, process it locally or transmit it securely to healthcare providers, and even adapt their functionality based on the patient's condition.
Looking forward, the continued evolution of flexible electronics in medical applications promises to transform healthcare delivery, enabling more preventive, personalized, and patient-centered approaches to medicine. The development of biodegradable flexible electronics also offers the potential for temporary implants that can dissolve harmlessly in the body after serving their purpose, eliminating the need for removal surgeries.
Market Analysis for Medical-Grade Flexible Electronics
The global market for medical-grade flexible electronics is experiencing unprecedented growth, driven by increasing demand for wearable health monitoring devices, implantable sensors, and smart medical patches. Current market valuations indicate that the medical flexible electronics sector reached approximately 8.5 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 18.7% through 2028. This remarkable expansion reflects the healthcare industry's shift toward personalized, continuous monitoring solutions and minimally invasive treatment options.
Patient-centric healthcare trends are significantly influencing market dynamics, with hospitals and healthcare providers increasingly adopting flexible electronic solutions to improve patient outcomes while reducing overall healthcare costs. The aging global population, particularly in developed regions like North America, Europe, and parts of Asia, is creating substantial demand for remote monitoring technologies that can help manage chronic conditions while maintaining patient mobility and comfort.
Regional analysis reveals that North America currently dominates the medical-grade flexible electronics market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth rate over the next five years due to increasing healthcare expenditure, growing medical device manufacturing capabilities, and rising adoption of digital health technologies in countries like China, Japan, and South Korea.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of remote patient monitoring and telehealth solutions. This has created new opportunities for flexible electronic devices that can provide continuous health data while minimizing physical contact between healthcare providers and patients. Market research indicates that consumer willingness to adopt wearable health monitoring devices increased by 34% between 2019 and 2022.
Key market segments within medical-grade flexible electronics include biosensors (36% market share), flexible displays (22%), flexible batteries (18%), and flexible integrated circuits (14%). Among these, biosensors represent the fastest-growing segment due to increasing applications in continuous glucose monitoring, cardiac monitoring, and temperature sensing. The disposable medical sensors sub-segment is particularly promising, with an expected CAGR of 22.3% through 2028.
Reimbursement policies and regulatory frameworks significantly impact market adoption rates across different regions. Countries with favorable reimbursement structures for remote patient monitoring technologies are experiencing faster market penetration. Additionally, the establishment of clear regulatory pathways for novel flexible electronic medical devices is critical for market expansion, with the FDA and European Medical Device Regulation playing pivotal roles in shaping market access strategies.
Patient-centric healthcare trends are significantly influencing market dynamics, with hospitals and healthcare providers increasingly adopting flexible electronic solutions to improve patient outcomes while reducing overall healthcare costs. The aging global population, particularly in developed regions like North America, Europe, and parts of Asia, is creating substantial demand for remote monitoring technologies that can help manage chronic conditions while maintaining patient mobility and comfort.
Regional analysis reveals that North America currently dominates the medical-grade flexible electronics market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth rate over the next five years due to increasing healthcare expenditure, growing medical device manufacturing capabilities, and rising adoption of digital health technologies in countries like China, Japan, and South Korea.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of remote patient monitoring and telehealth solutions. This has created new opportunities for flexible electronic devices that can provide continuous health data while minimizing physical contact between healthcare providers and patients. Market research indicates that consumer willingness to adopt wearable health monitoring devices increased by 34% between 2019 and 2022.
Key market segments within medical-grade flexible electronics include biosensors (36% market share), flexible displays (22%), flexible batteries (18%), and flexible integrated circuits (14%). Among these, biosensors represent the fastest-growing segment due to increasing applications in continuous glucose monitoring, cardiac monitoring, and temperature sensing. The disposable medical sensors sub-segment is particularly promising, with an expected CAGR of 22.3% through 2028.
Reimbursement policies and regulatory frameworks significantly impact market adoption rates across different regions. Countries with favorable reimbursement structures for remote patient monitoring technologies are experiencing faster market penetration. Additionally, the establishment of clear regulatory pathways for novel flexible electronic medical devices is critical for market expansion, with the FDA and European Medical Device Regulation playing pivotal roles in shaping market access strategies.
Current Challenges in Biocompatible Flexible Materials
Despite significant advancements in flexible electronics for medical applications, several critical challenges persist in developing truly biocompatible flexible materials. The human body presents a uniquely hostile environment for electronic devices, requiring materials that can withstand biological fluids, enzymatic activity, and immune responses while maintaining functionality over extended periods.
A primary challenge lies in achieving the delicate balance between mechanical flexibility and durability. Current materials often face degradation when subjected to repeated bending, stretching, and compression that occurs naturally within the human body. This mechanical fatigue significantly reduces device lifespan, particularly in applications requiring long-term implantation such as neural interfaces or continuous monitoring systems.
Biocompatibility remains perhaps the most significant hurdle. Many conductive materials and substrates that offer excellent electronic properties trigger inflammatory responses when placed in contact with living tissue. The body's foreign body response can lead to fibrotic encapsulation, effectively isolating the device and rendering it ineffective. Materials must not only avoid toxicity but also prevent protein adsorption and cellular adhesion that can compromise device function.
Moisture permeation presents another substantial challenge. The high humidity environment within the body can cause electronic component failure through oxidation and corrosion. While hermetic packaging solutions exist, they often compromise the flexibility that makes these devices valuable in the first place. Current encapsulation materials like parylene and PDMS offer partial solutions but fall short in providing long-term protection without affecting device performance.
Sterilization compatibility further complicates material selection. Medical devices must withstand standard sterilization procedures such as ethylene oxide treatment, gamma irradiation, or autoclave processing. Many flexible polymers and organic electronic materials degrade significantly during these processes, limiting their practical application in clinical settings.
The interface between rigid electronic components and flexible substrates creates stress concentration points that frequently lead to connection failures. Current interconnect technologies struggle to maintain reliable electrical connections when subjected to the dynamic mechanical stresses present in biological environments. This challenge is particularly evident in applications requiring high-density electrode arrays such as neural interfaces.
Additionally, biodegradation timing presents a complex engineering problem. For temporary implants, materials must maintain functionality for a precise duration before safely degrading. Current biodegradable electronics lack the sophisticated control mechanisms needed to ensure predictable degradation timelines that align with therapeutic windows.
A primary challenge lies in achieving the delicate balance between mechanical flexibility and durability. Current materials often face degradation when subjected to repeated bending, stretching, and compression that occurs naturally within the human body. This mechanical fatigue significantly reduces device lifespan, particularly in applications requiring long-term implantation such as neural interfaces or continuous monitoring systems.
Biocompatibility remains perhaps the most significant hurdle. Many conductive materials and substrates that offer excellent electronic properties trigger inflammatory responses when placed in contact with living tissue. The body's foreign body response can lead to fibrotic encapsulation, effectively isolating the device and rendering it ineffective. Materials must not only avoid toxicity but also prevent protein adsorption and cellular adhesion that can compromise device function.
Moisture permeation presents another substantial challenge. The high humidity environment within the body can cause electronic component failure through oxidation and corrosion. While hermetic packaging solutions exist, they often compromise the flexibility that makes these devices valuable in the first place. Current encapsulation materials like parylene and PDMS offer partial solutions but fall short in providing long-term protection without affecting device performance.
Sterilization compatibility further complicates material selection. Medical devices must withstand standard sterilization procedures such as ethylene oxide treatment, gamma irradiation, or autoclave processing. Many flexible polymers and organic electronic materials degrade significantly during these processes, limiting their practical application in clinical settings.
The interface between rigid electronic components and flexible substrates creates stress concentration points that frequently lead to connection failures. Current interconnect technologies struggle to maintain reliable electrical connections when subjected to the dynamic mechanical stresses present in biological environments. This challenge is particularly evident in applications requiring high-density electrode arrays such as neural interfaces.
Additionally, biodegradation timing presents a complex engineering problem. For temporary implants, materials must maintain functionality for a precise duration before safely degrading. Current biodegradable electronics lack the sophisticated control mechanisms needed to ensure predictable degradation timelines that align with therapeutic windows.
Benchmark Material Solutions for Medical Wearables
01 Flexible substrate materials for electronics
Various substrate materials are used in flexible electronics to provide the necessary mechanical flexibility while maintaining electronic functionality. These materials include polymers, thin films, and composite materials that can bend, fold, or stretch without losing their electronic properties. The flexibility of these substrates enables the development of wearable devices, foldable displays, and other applications where rigid electronics would be impractical.- Flexible substrate materials for electronics: Various substrate materials are used in flexible electronics to provide the necessary mechanical flexibility while maintaining electronic functionality. These materials include polymers, thin films, and composite materials that can bend, fold, or stretch without losing their electronic properties. The flexibility of these substrates enables the development of wearable devices, foldable displays, and other applications where rigid electronics would be impractical.
- Conductive materials for flexible circuits: Specialized conductive materials are essential for creating flexible electronic circuits that can withstand repeated bending and flexing. These include conductive polymers, metal nanowires, carbon-based materials like graphene, and liquid metal alloys. These materials maintain electrical conductivity while accommodating mechanical deformation, allowing for the creation of stretchable interconnects and flexible circuit boards that can conform to non-planar surfaces.
- Flexible display technologies: Flexible display technologies incorporate materials that enable screens to bend, fold, or roll while maintaining image quality. These displays utilize flexible organic light-emitting diodes (OLEDs), electrophoretic materials, or liquid crystal technologies combined with flexible substrates. The materials must balance optical performance with mechanical flexibility, allowing for innovations such as rollable televisions, foldable smartphones, and curved display panels.
- Flexible energy storage and harvesting materials: Materials for flexible energy storage and harvesting components enable power sources that can conform to various shapes and withstand mechanical deformation. These include flexible battery electrodes, solid-state electrolytes, and bendable solar cell materials. Such materials allow for the integration of power sources directly into flexible electronic devices, eliminating the need for rigid battery components and enabling self-powered flexible systems.
- Encapsulation and protection materials for flexible electronics: Specialized encapsulation and protection materials are crucial for ensuring the durability and reliability of flexible electronic devices. These materials protect sensitive components from environmental factors while maintaining flexibility. They include stretchable polymers, thin-film barriers with moisture resistance, and composite materials that can flex without cracking. Effective encapsulation extends the lifespan of flexible electronics by preventing damage from bending, folding, and environmental exposure.
02 Conductive materials for flexible circuits
Specialized conductive materials are essential for creating flexible electronic circuits that can withstand repeated bending and flexing. These include conductive polymers, metal nanowires, carbon-based materials like graphene, and liquid metal alloys. These materials maintain electrical conductivity while accommodating mechanical deformation, allowing for the creation of stretchable interconnects and flexible circuit boards that can conform to non-planar surfaces.Expand Specific Solutions03 Flexible display technologies
Flexible display technologies incorporate materials that enable screens to bend, fold, or roll while maintaining image quality. These displays utilize flexible organic light-emitting diodes (OLEDs), electrophoretic displays, or liquid crystal displays on flexible substrates. The materials used must provide optical clarity, mechanical durability, and electronic stability under deformation, enabling applications such as foldable smartphones, rollable televisions, and curved display panels.Expand Specific Solutions04 Flexible energy storage and harvesting materials
Materials for flexible energy storage and harvesting devices enable power sources that can conform to various shapes and withstand mechanical deformation. These include flexible battery electrodes, solid-state electrolytes, bendable solar cells, and piezoelectric materials for energy harvesting. The flexibility of these materials allows for integration into wearable devices, smart textiles, and other applications where rigid power sources would limit functionality.Expand Specific Solutions05 Encapsulation and protection materials for flexible electronics
Specialized encapsulation and protection materials are crucial for ensuring the durability and reliability of flexible electronic devices. These materials protect sensitive components from environmental factors such as moisture, oxygen, and mechanical damage while maintaining flexibility. They include flexible barrier films, stretchable polymers, and composite materials that can deform without cracking or delaminating, thereby extending the lifespan of flexible electronic devices under real-world usage conditions.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The flexible electronics market for medical applications is currently in a growth phase, characterized by rapid technological advancements and expanding clinical applications. The market size is projected to reach significant value as healthcare systems increasingly adopt wearable and implantable medical devices. In terms of technical maturity, the field shows varying degrees of development across different applications. Leading academic institutions like Tsinghua University, Arizona State University, and University of Tokyo are driving fundamental research, while commercial entities such as Corning, Inc., 3M Innovative Properties, and Imec are focusing on translating research into marketable products. The competition landscape reveals a collaborative ecosystem where research institutions partner with industry players to overcome challenges in material biocompatibility, durability, and manufacturing scalability for medical-grade flexible electronic devices.
Tsinghua University
Technical Solution: Tsinghua University has developed groundbreaking flexible electronic materials specifically optimized for medical applications. Their research team has pioneered a novel nanocomposite approach combining silver nanowires with biocompatible hydrogels to create highly conductive, stretchable materials that maintain electrical performance under deformation conditions typical in medical wearables. These materials demonstrate conductivity exceeding 5000 S/cm while withstanding strains of over 100% without significant performance degradation. For implantable applications, they've engineered biodegradable flexible substrates using modified silk fibroin and poly(lactic-co-glycolic acid) (PLGA) blends that offer controlled degradation profiles ranging from weeks to months depending on clinical requirements. Their recent innovation includes a self-healing conductive composite that can restore up to 98% of its electrical conductivity after mechanical damage, addressing a critical reliability challenge in flexible medical electronics.
Strengths: Exceptional biocompatibility with minimal foreign body response; superior mechanical properties matching biological tissue compliance; innovative self-healing capabilities for enhanced durability. Weaknesses: Limited long-term stability in physiological environments; challenges in mass production and standardization; higher costs compared to conventional rigid electronic materials.
Trustees of the University of Pennsylvania
Technical Solution: The University of Pennsylvania has developed advanced flexible electronic materials specifically engineered for medical applications. Their research focuses on creating biocompatible, mechanically compliant electronic systems that can interface seamlessly with biological tissues. Their flagship technology utilizes carbon nanomaterial-polymer composites that achieve conductivities exceeding 1000 S/cm while maintaining flexibility and stretchability compatible with dynamic biological surfaces. These materials can withstand strains of up to 30% without significant performance degradation, making them ideal for applications like cardiac monitoring patches and neural interfaces. Penn researchers have also pioneered biodegradable flexible electronics using naturally derived polymers like silk fibroin and cellulose derivatives combined with magnesium-based conductors, enabling transient electronic systems with controlled dissolution rates in physiological environments. Their recent innovations include self-healing conductive hydrogels incorporating zwitterionic polymers that maintain stable performance in wet biological environments for extended periods.
Strengths: Exceptional biocompatibility with minimal immune response; excellent mechanical matching to biological tissues; innovative biodegradable options for temporary medical applications. Weaknesses: Lower electronic performance compared to rigid counterparts; challenges in long-term stability under continuous physiological conditions; higher manufacturing complexity and cost compared to conventional electronics.
Key Patents in Biocompatible Flexible Substrates
Stretchable composite conductors for flexible electronics, stretchable plasmonic devices, optical filters, and implantable devices and methods for manufacture thereof
PatentInactiveUS10629324B2
Innovation
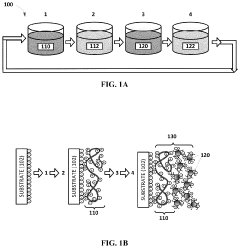
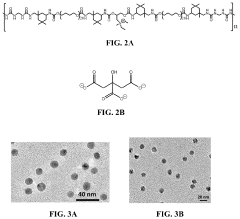
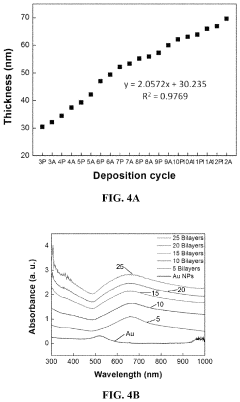
- Development of stretchable electrically conductive composite materials comprising an elastic polymer with a plurality of conductive nanoparticles, such as gold nanoparticles, which exhibit high conductivity even at significant tensile strains through layer-by-layer assembly or vacuum-assisted flocculation processes, allowing for the creation of materials with conductivities exceeding 500 S/cm at strains up to 75%.
Polymer laminated sheet
PatentWO2024257609A1
Innovation
- A polymer laminate sheet composed of a thin polymer film layer and a nonwoven fabric layer, where the polymer thin film layer is 10 nm to 1000 nm thick and the nonwoven fabric layer consists of polymer fibers with diameters between 10 nm and 10 μm, bonded by intermolecular forces, eliminating the need for adhesives and enhancing followability and resistance to damage.
Regulatory Framework for Medical Electronic Materials
The regulatory landscape for medical electronic materials presents a complex framework that manufacturers and developers must navigate to ensure compliance and market access. In the United States, the FDA's Center for Devices and Radiological Health (CDRH) oversees flexible electronic devices through a risk-based classification system. Class I devices face minimal regulation, while Class II requires special controls and Class III demands premarket approval with extensive clinical data. Materials used in flexible electronics must meet biocompatibility standards outlined in ISO 10993, which evaluates cytotoxicity, sensitization, and irritation potential.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for materials in medical applications. These regulations emphasize the need for comprehensive technical documentation, risk management procedures, and post-market surveillance systems. The CE marking process necessitates verification that materials maintain their properties throughout the product lifecycle under normal usage conditions.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a regulatory framework similar to the FDA but with unique requirements for foreign manufacturers. The PMDA places particular emphasis on material stability testing under various environmental conditions relevant to flexible electronics applications.
International standards play a crucial role in harmonizing regulatory approaches. IEC 60601 series standards address electrical safety concerns specific to medical electronic materials, while ISO 13485 establishes quality management systems requirements. For flexible electronics specifically, emerging standards like IEC 62304 for software components and IEC 62366 for usability engineering have become increasingly relevant.
Material documentation requirements vary by jurisdiction but typically include detailed composition information, manufacturing process validation, shelf-life data, and compatibility with sterilization methods. Manufacturers must maintain Device Master Records (DMRs) that document material specifications, processing parameters, and quality control measures.
Regulatory trends indicate movement toward more stringent oversight of novel materials, particularly those incorporating nanomaterials or biologically derived components. The FDA's Digital Health Innovation Action Plan and similar initiatives worldwide are creating pathways for innovative flexible electronic materials while maintaining safety standards. Recent regulatory developments emphasize the importance of cybersecurity considerations for materials used in connected medical devices.
Compliance strategies for flexible electronics manufacturers should include early engagement with regulatory bodies through pre-submission consultations, development of comprehensive biocompatibility testing protocols, and implementation of robust quality management systems that address the unique challenges of flexible material production and integration.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for materials in medical applications. These regulations emphasize the need for comprehensive technical documentation, risk management procedures, and post-market surveillance systems. The CE marking process necessitates verification that materials maintain their properties throughout the product lifecycle under normal usage conditions.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a regulatory framework similar to the FDA but with unique requirements for foreign manufacturers. The PMDA places particular emphasis on material stability testing under various environmental conditions relevant to flexible electronics applications.
International standards play a crucial role in harmonizing regulatory approaches. IEC 60601 series standards address electrical safety concerns specific to medical electronic materials, while ISO 13485 establishes quality management systems requirements. For flexible electronics specifically, emerging standards like IEC 62304 for software components and IEC 62366 for usability engineering have become increasingly relevant.
Material documentation requirements vary by jurisdiction but typically include detailed composition information, manufacturing process validation, shelf-life data, and compatibility with sterilization methods. Manufacturers must maintain Device Master Records (DMRs) that document material specifications, processing parameters, and quality control measures.
Regulatory trends indicate movement toward more stringent oversight of novel materials, particularly those incorporating nanomaterials or biologically derived components. The FDA's Digital Health Innovation Action Plan and similar initiatives worldwide are creating pathways for innovative flexible electronic materials while maintaining safety standards. Recent regulatory developments emphasize the importance of cybersecurity considerations for materials used in connected medical devices.
Compliance strategies for flexible electronics manufacturers should include early engagement with regulatory bodies through pre-submission consultations, development of comprehensive biocompatibility testing protocols, and implementation of robust quality management systems that address the unique challenges of flexible material production and integration.
Durability and Sterilization Considerations
In the medical context, durability and sterilization capabilities represent critical factors for flexible electronic materials. Medical-grade flexible electronics must withstand repeated sterilization cycles without performance degradation, as these devices often require multiple sterilization procedures throughout their operational lifetime. Common sterilization methods include autoclave (high-pressure steam), ethylene oxide (EtO), gamma radiation, and hydrogen peroxide plasma, each imposing distinct stress factors on materials.
Polymer substrates like polyimide (PI) demonstrate exceptional thermal stability, maintaining structural integrity through multiple autoclave cycles at 121°C. However, prolonged exposure to high-temperature steam can eventually lead to hydrolytic degradation. Parylene-C offers superior chemical resistance and maintains barrier properties through EtO sterilization but may experience chain scission during gamma irradiation above 25 kGy. PDMS (polydimethylsiloxane) exhibits excellent flexibility and biocompatibility but requires careful consideration as repeated sterilization can increase surface hardness and reduce elasticity.
Conductive materials present unique challenges in medical applications. Silver nanowire networks, while highly conductive and flexible, may undergo oxidation during steam sterilization, necessitating protective encapsulation. Carbon-based conductors (graphene, carbon nanotubes) demonstrate superior chemical stability across multiple sterilization methods but require optimization of interfacial adhesion to prevent delamination during thermal cycling.
Encapsulation materials serve as critical protective barriers in medical flexible electronics. Multilayer approaches combining inorganic (SiO2, Al2O3) and organic (parylene) materials have demonstrated superior performance, maintaining water vapor transmission rates below 10^-6 g/m²/day after multiple sterilization cycles. Atomic Layer Deposition (ALD) techniques produce exceptionally dense barrier films that maintain integrity through sterilization processes.
Accelerated aging studies reveal that material interfaces represent the most vulnerable points in flexible electronic systems. Thermal expansion mismatches between different material layers can lead to delamination or crack formation during sterilization thermal cycling. Recent innovations include self-healing polymers incorporating dynamic covalent bonds that can repair microcracks formed during sterilization processes, significantly extending device lifetime.
Standardized testing protocols specifically designed for medical flexible electronics remain underdeveloped. Current evaluation methods typically combine ISO 10993 biocompatibility testing with modified versions of IEC 60601 electrical safety standards. The development of specialized testing regimes that accurately simulate repeated sterilization and clinical use conditions represents an important area for future development in this field.
Polymer substrates like polyimide (PI) demonstrate exceptional thermal stability, maintaining structural integrity through multiple autoclave cycles at 121°C. However, prolonged exposure to high-temperature steam can eventually lead to hydrolytic degradation. Parylene-C offers superior chemical resistance and maintains barrier properties through EtO sterilization but may experience chain scission during gamma irradiation above 25 kGy. PDMS (polydimethylsiloxane) exhibits excellent flexibility and biocompatibility but requires careful consideration as repeated sterilization can increase surface hardness and reduce elasticity.
Conductive materials present unique challenges in medical applications. Silver nanowire networks, while highly conductive and flexible, may undergo oxidation during steam sterilization, necessitating protective encapsulation. Carbon-based conductors (graphene, carbon nanotubes) demonstrate superior chemical stability across multiple sterilization methods but require optimization of interfacial adhesion to prevent delamination during thermal cycling.
Encapsulation materials serve as critical protective barriers in medical flexible electronics. Multilayer approaches combining inorganic (SiO2, Al2O3) and organic (parylene) materials have demonstrated superior performance, maintaining water vapor transmission rates below 10^-6 g/m²/day after multiple sterilization cycles. Atomic Layer Deposition (ALD) techniques produce exceptionally dense barrier films that maintain integrity through sterilization processes.
Accelerated aging studies reveal that material interfaces represent the most vulnerable points in flexible electronic systems. Thermal expansion mismatches between different material layers can lead to delamination or crack formation during sterilization thermal cycling. Recent innovations include self-healing polymers incorporating dynamic covalent bonds that can repair microcracks formed during sterilization processes, significantly extending device lifetime.
Standardized testing protocols specifically designed for medical flexible electronics remain underdeveloped. Current evaluation methods typically combine ISO 10993 biocompatibility testing with modified versions of IEC 60601 electrical safety standards. The development of specialized testing regimes that accurately simulate repeated sterilization and clinical use conditions represents an important area for future development in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!