Benchmarking Lithium Chloride Production: Synthesis Methods
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Synthesis Background and Objectives
Lithium chloride has emerged as a critical compound in various industrial applications, with its significance growing exponentially over the past decades. The evolution of lithium chloride synthesis methods can be traced back to the early 20th century, when basic chemical reactions involving lithium-containing minerals were first developed. As industrial demands increased, particularly in the fields of battery technology, pharmaceuticals, and metallurgy, the need for more efficient and environmentally sustainable production methods became apparent.
The technological trajectory of lithium chloride synthesis has been characterized by three distinct phases: traditional mineral-based extraction, advanced chemical processing techniques, and the current era of sustainable and high-purity production methodologies. Each phase has contributed significantly to our understanding of the chemical properties and production challenges associated with lithium chloride.
Current global trends indicate a sharp increase in demand for high-purity lithium chloride, driven primarily by the exponential growth in electric vehicle production and energy storage systems. Market projections suggest a compound annual growth rate of approximately 8-10% for lithium compounds over the next decade, highlighting the urgency for technological innovation in synthesis methods.
The primary technical objectives for lithium chloride synthesis research include developing processes that maximize yield while minimizing environmental impact, reducing energy consumption during production, enhancing product purity levels to meet stringent industry standards, and establishing cost-effective methods suitable for large-scale industrial implementation.
Historically, lithium chloride synthesis has faced several persistent challenges, including the efficient separation of lithium from co-occurring elements, managing the environmental impact of extraction processes, and achieving consistent high-purity outputs. These challenges have spurred continuous innovation in separation technologies, catalytic processes, and purification techniques.
Recent breakthroughs in membrane technology, electrochemical processing, and direct lithium extraction (DLE) methods have opened new avenues for lithium chloride production. These advancements aim to address the limitations of conventional methods while meeting the increasing demand for higher purity standards and greater production efficiency.
The benchmarking of various lithium chloride synthesis methods has become essential for industry stakeholders to make informed decisions regarding technology adoption and investment. This technical assessment aims to provide a comprehensive evaluation of existing and emerging synthesis methodologies, considering factors such as energy efficiency, environmental sustainability, scalability, product quality, and economic viability.
The technological trajectory of lithium chloride synthesis has been characterized by three distinct phases: traditional mineral-based extraction, advanced chemical processing techniques, and the current era of sustainable and high-purity production methodologies. Each phase has contributed significantly to our understanding of the chemical properties and production challenges associated with lithium chloride.
Current global trends indicate a sharp increase in demand for high-purity lithium chloride, driven primarily by the exponential growth in electric vehicle production and energy storage systems. Market projections suggest a compound annual growth rate of approximately 8-10% for lithium compounds over the next decade, highlighting the urgency for technological innovation in synthesis methods.
The primary technical objectives for lithium chloride synthesis research include developing processes that maximize yield while minimizing environmental impact, reducing energy consumption during production, enhancing product purity levels to meet stringent industry standards, and establishing cost-effective methods suitable for large-scale industrial implementation.
Historically, lithium chloride synthesis has faced several persistent challenges, including the efficient separation of lithium from co-occurring elements, managing the environmental impact of extraction processes, and achieving consistent high-purity outputs. These challenges have spurred continuous innovation in separation technologies, catalytic processes, and purification techniques.
Recent breakthroughs in membrane technology, electrochemical processing, and direct lithium extraction (DLE) methods have opened new avenues for lithium chloride production. These advancements aim to address the limitations of conventional methods while meeting the increasing demand for higher purity standards and greater production efficiency.
The benchmarking of various lithium chloride synthesis methods has become essential for industry stakeholders to make informed decisions regarding technology adoption and investment. This technical assessment aims to provide a comprehensive evaluation of existing and emerging synthesis methodologies, considering factors such as energy efficiency, environmental sustainability, scalability, product quality, and economic viability.
Market Demand Analysis for Lithium Chloride
The global lithium chloride market has experienced significant growth in recent years, driven primarily by the expanding lithium-ion battery sector. Market research indicates that the global demand for lithium chloride reached approximately 45,000 metric tons in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.2% through 2030. This growth trajectory is substantially higher than the historical average of 4-5% observed during the 2010-2015 period, indicating accelerating market demand.
The battery industry represents the largest consumption segment, accounting for roughly 65% of total lithium chloride demand. This dominance is expected to strengthen further as electric vehicle (EV) adoption continues to accelerate globally. Major automotive markets including China, Europe, and North America have established ambitious electrification targets, with some regions planning to phase out internal combustion engines entirely by 2035-2040.
Beyond batteries, lithium chloride finds significant application in air conditioning systems, where it serves as a desiccant material. This segment constitutes approximately 15% of market demand and is growing steadily at 3-4% annually. The pharmaceutical and chemical synthesis sectors collectively represent another 12% of demand, with applications in organic synthesis reactions and as intermediates for various chemical compounds.
Geographically, Asia-Pacific dominates consumption patterns, accounting for over 70% of global demand. China alone represents nearly 45% of worldwide lithium chloride consumption, driven by its massive battery manufacturing infrastructure. North America and Europe follow with approximately 15% and 10% market shares respectively, though both regions are implementing policies to reduce dependency on Asian supply chains.
Price sensitivity analysis reveals that lithium chloride demand exhibits relatively inelastic behavior in high-tech applications, where performance characteristics outweigh cost considerations. However, in bulk industrial applications, price fluctuations significantly impact consumption patterns. Recent supply constraints have pushed prices upward by nearly 300% between 2020 and 2022, prompting some manufacturers to explore alternative materials or recycling options.
Industry forecasts suggest that emerging applications in energy storage systems, particularly grid-scale solutions, will create substantial new demand streams. Additionally, the pharmaceutical industry's growing interest in lithium-based compounds for mental health treatments represents a small but potentially high-value market segment with premium pricing potential.
The synthesis method employed significantly impacts market dynamics, as production costs vary considerably between different approaches. Traditional brine-based extraction methods remain most economical at scale, while newer direct extraction technologies are gaining traction due to reduced environmental footprint despite higher initial capital requirements.
The battery industry represents the largest consumption segment, accounting for roughly 65% of total lithium chloride demand. This dominance is expected to strengthen further as electric vehicle (EV) adoption continues to accelerate globally. Major automotive markets including China, Europe, and North America have established ambitious electrification targets, with some regions planning to phase out internal combustion engines entirely by 2035-2040.
Beyond batteries, lithium chloride finds significant application in air conditioning systems, where it serves as a desiccant material. This segment constitutes approximately 15% of market demand and is growing steadily at 3-4% annually. The pharmaceutical and chemical synthesis sectors collectively represent another 12% of demand, with applications in organic synthesis reactions and as intermediates for various chemical compounds.
Geographically, Asia-Pacific dominates consumption patterns, accounting for over 70% of global demand. China alone represents nearly 45% of worldwide lithium chloride consumption, driven by its massive battery manufacturing infrastructure. North America and Europe follow with approximately 15% and 10% market shares respectively, though both regions are implementing policies to reduce dependency on Asian supply chains.
Price sensitivity analysis reveals that lithium chloride demand exhibits relatively inelastic behavior in high-tech applications, where performance characteristics outweigh cost considerations. However, in bulk industrial applications, price fluctuations significantly impact consumption patterns. Recent supply constraints have pushed prices upward by nearly 300% between 2020 and 2022, prompting some manufacturers to explore alternative materials or recycling options.
Industry forecasts suggest that emerging applications in energy storage systems, particularly grid-scale solutions, will create substantial new demand streams. Additionally, the pharmaceutical industry's growing interest in lithium-based compounds for mental health treatments represents a small but potentially high-value market segment with premium pricing potential.
The synthesis method employed significantly impacts market dynamics, as production costs vary considerably between different approaches. Traditional brine-based extraction methods remain most economical at scale, while newer direct extraction technologies are gaining traction due to reduced environmental footprint despite higher initial capital requirements.
Current Synthesis Methods and Technical Challenges
The synthesis of lithium chloride (LiCl) currently employs several established methods, each with distinct advantages and limitations. The most prevalent industrial approach involves the reaction of lithium carbonate with hydrochloric acid, yielding lithium chloride, water, and carbon dioxide. This method benefits from relatively straightforward chemistry and moderate reaction conditions, making it economically viable for large-scale production. However, it faces challenges related to the purity of raw materials, as impurities in lithium carbonate can significantly affect the final product quality.
Another significant production route utilizes lithium hydroxide as the starting material, reacting it with hydrochloric acid. This method generally produces higher purity lithium chloride but comes at a higher cost due to the premium price of lithium hydroxide compared to lithium carbonate. The process also requires precise control of reaction parameters to prevent unwanted side reactions and ensure consistent product quality.
Direct extraction from brine represents a growing synthesis approach, particularly in regions with substantial lithium-rich brine resources such as the Salar de Atacama in Chile and the Salar del Hombre Muerto in Argentina. This method involves concentration through solar evaporation followed by chemical treatment to isolate lithium chloride. While potentially more cost-effective than traditional methods, it faces significant technical challenges including variable brine composition, lengthy processing times, and environmental concerns regarding water usage in arid regions.
Electrolysis-based methods have also emerged as promising alternatives, particularly for high-purity applications. These techniques involve the electrolytic processing of lithium-containing solutions to produce lithium chloride with minimal impurities. However, these methods typically require substantial energy input, specialized equipment, and careful process control, limiting their widespread industrial adoption.
A critical technical challenge across all synthesis methods is the removal of magnesium, calcium, and sodium impurities, which can significantly impact the performance of lithium chloride in downstream applications. Current purification techniques often involve multiple precipitation and crystallization steps, adding complexity and cost to the production process.
Energy efficiency represents another significant challenge, particularly for methods requiring high-temperature processing or extensive evaporation. As environmental regulations tighten globally, reducing the carbon footprint of lithium chloride production has become increasingly important, driving research into more sustainable synthesis routes.
Scale-up challenges also persist, especially for newer production methods transitioning from laboratory to industrial scale. These include issues related to reaction kinetics, heat transfer limitations, and equipment design considerations that can significantly impact process economics and product consistency.
Another significant production route utilizes lithium hydroxide as the starting material, reacting it with hydrochloric acid. This method generally produces higher purity lithium chloride but comes at a higher cost due to the premium price of lithium hydroxide compared to lithium carbonate. The process also requires precise control of reaction parameters to prevent unwanted side reactions and ensure consistent product quality.
Direct extraction from brine represents a growing synthesis approach, particularly in regions with substantial lithium-rich brine resources such as the Salar de Atacama in Chile and the Salar del Hombre Muerto in Argentina. This method involves concentration through solar evaporation followed by chemical treatment to isolate lithium chloride. While potentially more cost-effective than traditional methods, it faces significant technical challenges including variable brine composition, lengthy processing times, and environmental concerns regarding water usage in arid regions.
Electrolysis-based methods have also emerged as promising alternatives, particularly for high-purity applications. These techniques involve the electrolytic processing of lithium-containing solutions to produce lithium chloride with minimal impurities. However, these methods typically require substantial energy input, specialized equipment, and careful process control, limiting their widespread industrial adoption.
A critical technical challenge across all synthesis methods is the removal of magnesium, calcium, and sodium impurities, which can significantly impact the performance of lithium chloride in downstream applications. Current purification techniques often involve multiple precipitation and crystallization steps, adding complexity and cost to the production process.
Energy efficiency represents another significant challenge, particularly for methods requiring high-temperature processing or extensive evaporation. As environmental regulations tighten globally, reducing the carbon footprint of lithium chloride production has become increasingly important, driving research into more sustainable synthesis routes.
Scale-up challenges also persist, especially for newer production methods transitioning from laboratory to industrial scale. These include issues related to reaction kinetics, heat transfer limitations, and equipment design considerations that can significantly impact process economics and product consistency.
Benchmarking of Current Synthesis Approaches
01 Extraction from lithium-containing minerals and brines
Lithium chloride can be synthesized by extracting lithium from natural sources such as minerals and brines. This process typically involves leaching lithium-containing materials with acids or other solvents, followed by purification steps to remove impurities. The resulting solution is then processed to obtain lithium chloride crystals. This method is commonly used in industrial production due to the abundance of lithium in natural deposits.- Electrolytic synthesis methods: Lithium chloride can be synthesized through electrolytic processes where lithium-containing materials undergo electrolysis to produce high-purity lithium chloride. These methods often involve the use of specialized electrodes and controlled current conditions to facilitate the conversion of lithium compounds into lithium chloride. Electrolytic methods are advantageous for producing high-purity lithium chloride with controlled particle size and morphology.
- Chemical reaction of lithium compounds with hydrochloric acid: This synthesis method involves the direct reaction of various lithium-containing compounds such as lithium hydroxide, lithium carbonate, or lithium oxide with hydrochloric acid. The reaction produces lithium chloride and water or carbon dioxide as byproducts. The process typically requires controlled reaction conditions including temperature, concentration, and pH to optimize yield and purity of the resulting lithium chloride.
- Extraction from lithium-rich brines and minerals: Lithium chloride can be obtained through extraction processes from natural sources such as lithium-rich brines, salt lakes, or mineral deposits. These methods typically involve concentration of brines through evaporation, followed by selective precipitation or adsorption techniques to isolate lithium chloride from other salts. Advanced extraction technologies may employ ion exchange resins, selective membranes, or solvent extraction to improve efficiency and purity.
- Recycling and recovery from lithium-ion batteries: Methods for synthesizing lithium chloride from spent lithium-ion batteries involve several steps including crushing, separation of components, leaching with acids, and purification processes. These recycling techniques aim to recover valuable lithium compounds which can then be converted to lithium chloride through additional chemical treatments. The process helps reduce environmental impact while providing a sustainable source of lithium compounds for various applications.
- Continuous flow and industrial-scale production methods: Continuous flow synthesis methods for lithium chloride production involve specialized equipment and process designs that allow for uninterrupted production at industrial scale. These methods often incorporate automated control systems, specialized reactors, and optimized process parameters to ensure consistent quality and high yield. Continuous production techniques may include spray drying, crystallization under controlled conditions, or other specialized processes that enable efficient large-scale manufacturing of lithium chloride with specific physical and chemical properties.
02 Chemical reaction of lithium compounds with hydrochloric acid
Lithium chloride can be synthesized by reacting various lithium compounds with hydrochloric acid. This direct chemical reaction approach involves treating lithium-containing materials such as lithium carbonate, lithium hydroxide, or lithium oxide with hydrochloric acid to form lithium chloride. The reaction is followed by concentration and crystallization steps to obtain pure lithium chloride. This method is valued for its simplicity and efficiency in laboratory and industrial settings.Expand Specific Solutions03 Electrochemical processes for lithium chloride production
Electrochemical methods can be employed to synthesize lithium chloride. These processes typically involve electrolysis of lithium-containing solutions or molten salts to produce lithium chloride. Electrochemical approaches offer advantages in terms of purity control and can be integrated with other production steps. These methods are particularly useful when high-purity lithium chloride is required for specialized applications.Expand Specific Solutions04 Recovery from industrial waste and recycling processes
Lithium chloride can be synthesized through recovery from industrial waste streams and recycling processes. This approach involves extracting lithium from spent batteries, industrial byproducts, or other lithium-containing waste materials. The recovered lithium is then converted to lithium chloride through various chemical treatments. This method is becoming increasingly important for sustainable lithium production as demand for lithium compounds continues to grow.Expand Specific Solutions05 Continuous flow and innovative reactor systems
Advanced synthesis methods for lithium chloride employ continuous flow processes and innovative reactor systems. These approaches focus on optimizing reaction conditions, improving yield, and enhancing product purity. Continuous flow systems allow for better control of reaction parameters and can significantly increase production efficiency. These methods often incorporate specialized equipment designed specifically for lithium chloride synthesis at various scales.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The lithium chloride production synthesis methods market is currently in a growth phase, driven by increasing demand for lithium-based products in battery technologies. The global market size is expanding rapidly, with projections indicating substantial growth as electric vehicle adoption accelerates. Technologically, the field shows varying maturity levels across different synthesis approaches. Leading players include Ganfeng Lithium Group and POSCO Holdings, which have developed advanced industrial-scale production methods, while companies like General Lithium Corp and Qinghai Salt Lake Industry focus on resource-based extraction. Research institutions such as RIST and Nankai University are advancing novel synthesis techniques. Emerging players like Deutsche Lithium and Shandong RuiFu Lithium are developing region-specific approaches, while established chemical companies including Sumitomo Metal Mining and LANXESS contribute specialized technical expertise to the evolving competitive landscape.
POSCO Holdings, Inc.
Technical Solution: POSCO Holdings has developed an advanced lithium extraction and conversion technology called "PosLX" specifically for lithium chloride production. Their process employs a selective adsorption technique using proprietary adsorbent materials that can extract lithium ions from various sources including brines, seawater, and geothermal waters with minimal co-extraction of impurities. The technology achieves lithium recovery rates exceeding 80% while reducing processing time from months (traditional evaporation) to just hours. POSCO's system includes a continuous ion exchange process followed by a specialized crystallization method that produces high-purity lithium chloride suitable for battery applications. Their technology has been successfully demonstrated at commercial scale in Argentina's lithium triangle, processing lithium brines with varying chemical compositions. The company has also developed an integrated process that can directly convert the extracted lithium to lithium hydroxide or lithium carbonate depending on market demands, providing production flexibility. POSCO's approach significantly reduces water consumption compared to conventional evaporation methods, with estimates suggesting up to 90% reduction in water usage.
Strengths: Versatile technology applicable to multiple lithium sources; dramatically reduced production timeframe; significantly lower environmental footprint particularly regarding water usage; scalable modular design. Weaknesses: Higher energy requirements compared to traditional methods; specialized adsorbent materials require periodic replacement adding to operational costs; technology still being optimized for maximum efficiency at large scale.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed a proprietary direct lithium extraction (DLE) technology for lithium chloride production from brine resources. Their process employs selective adsorption materials that can efficiently extract lithium ions from various brine sources with minimal impurities. The company has implemented a continuous crystallization process that converts lithium-rich solutions directly to high-purity lithium chloride with 99.5% purity. Their technology reduces production time by approximately 50% compared to traditional evaporation methods and achieves lithium recovery rates of over 80%. Ganfeng has also pioneered a closed-loop system that recycles reagents and minimizes waste discharge, making their process more environmentally sustainable than conventional methods. The company has successfully scaled this technology at their Cauchari-Olaroz project in Argentina, demonstrating commercial viability.
Strengths: Higher recovery rates than traditional evaporation ponds (>80% vs 40-50%); significantly reduced production time; lower environmental footprint with minimal water consumption. Weaknesses: Higher capital expenditure requirements; technology still being optimized for different brine chemistries; energy-intensive compared to some alternative methods.
Critical Patents and Technical Literature Review
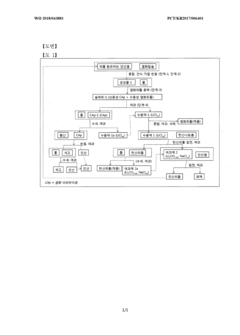
METHOD FOR THE MANUFACTURE OF LITHIUM CHLORIDE AND METHOD FOR THE MANUFACTURE OF LITHIUM CARBONATE
PatentInactiveAR109573A1
Innovation
- Direct conversion of lithium-containing phosphate salt to lithium chloride through high-temperature reaction with calcium chloride, eliminating traditional acid leaching steps.
- Production of high concentration lithium chloride solution (≥10,000 ppm) enabling efficient downstream lithium carbonate production.
- Simple solid-liquid separation process to recover lithium chloride solution while producing chloroapatite as a potentially valuable by-product.
Method for preparing lithium chloride and method for preparing lithium carbonate
PatentWO2018043881A1
Innovation
- A method involving the uniform mixing of lithium-containing phosphate with calcium chloride, followed by high-temperature heating to produce chloroapatite and lithium chloride, and subsequent aqueous solution separation to achieve a high-concentration lithium chloride solution, which is then used to produce lithium carbonate through a simple and environmentally friendly process.
Environmental Impact Assessment of Production Methods
The environmental impact of lithium chloride production methods varies significantly across different synthesis approaches, with each presenting unique ecological challenges and sustainability considerations. Traditional extraction methods, particularly those involving brine evaporation in salt flats, have substantial water consumption implications, requiring approximately 500,000 gallons of water per ton of lithium produced. This intensive water usage creates significant stress on local ecosystems, especially in arid regions where lithium-rich brines are commonly found, such as the "Lithium Triangle" spanning Chile, Argentina, and Bolivia.
Hard-rock mining methods for lithium extraction, while less water-intensive, generate considerable carbon emissions—approximately 15 tons of CO2 per ton of lithium carbonate equivalent (LCE)—and create substantial land disturbance through open-pit mining operations. The processing of spodumene ore requires high-temperature conversion (up to 1100°C), resulting in significant energy consumption and associated greenhouse gas emissions.
Chemical synthesis routes for lithium chloride production introduce different environmental concerns, primarily related to chemical waste management and reagent toxicity. The lithium hydroxide route generates sodium sulfate as a by-product, while the lithium carbonate pathway produces calcium chloride waste streams, both requiring proper disposal protocols to prevent soil and water contamination.
Recent life cycle assessments reveal that direct lithium extraction (DLE) technologies demonstrate promising environmental advantages, reducing water consumption by up to 70% compared to traditional evaporation methods. However, these technologies often require increased energy inputs, creating a sustainability trade-off that must be carefully evaluated in specific implementation contexts.
Waste management represents another critical environmental dimension, with traditional brine operations generating significant quantities of salt waste—approximately 7.5 tons of salt residue per ton of lithium chloride produced. Advanced recycling methods are emerging to address this challenge, with some pilot programs demonstrating up to 90% recovery rates for process chemicals.
Regulatory frameworks governing lithium production environmental impacts vary considerably across jurisdictions, with Chile implementing water usage restrictions in the Atacama region and the European Union developing stringent environmental standards for battery material sourcing through the proposed Battery Regulation framework. These evolving regulatory landscapes are increasingly shaping industry practices toward more sustainable production methods.
Hard-rock mining methods for lithium extraction, while less water-intensive, generate considerable carbon emissions—approximately 15 tons of CO2 per ton of lithium carbonate equivalent (LCE)—and create substantial land disturbance through open-pit mining operations. The processing of spodumene ore requires high-temperature conversion (up to 1100°C), resulting in significant energy consumption and associated greenhouse gas emissions.
Chemical synthesis routes for lithium chloride production introduce different environmental concerns, primarily related to chemical waste management and reagent toxicity. The lithium hydroxide route generates sodium sulfate as a by-product, while the lithium carbonate pathway produces calcium chloride waste streams, both requiring proper disposal protocols to prevent soil and water contamination.
Recent life cycle assessments reveal that direct lithium extraction (DLE) technologies demonstrate promising environmental advantages, reducing water consumption by up to 70% compared to traditional evaporation methods. However, these technologies often require increased energy inputs, creating a sustainability trade-off that must be carefully evaluated in specific implementation contexts.
Waste management represents another critical environmental dimension, with traditional brine operations generating significant quantities of salt waste—approximately 7.5 tons of salt residue per ton of lithium chloride produced. Advanced recycling methods are emerging to address this challenge, with some pilot programs demonstrating up to 90% recovery rates for process chemicals.
Regulatory frameworks governing lithium production environmental impacts vary considerably across jurisdictions, with Chile implementing water usage restrictions in the Atacama region and the European Union developing stringent environmental standards for battery material sourcing through the proposed Battery Regulation framework. These evolving regulatory landscapes are increasingly shaping industry practices toward more sustainable production methods.
Raw Material Supply Chain Analysis
The global lithium supply chain represents a critical component in the production of lithium chloride, with significant implications for cost efficiency, production stability, and environmental impact. Primary lithium sources include brine deposits, hard rock mining (particularly spodumene), and clay deposits, each presenting distinct advantages and challenges within the supply chain framework.
Brine-based lithium extraction, predominantly located in South America's "Lithium Triangle" (Chile, Argentina, and Bolivia), accounts for approximately 58% of global lithium resources. These operations benefit from lower production costs but face challenges related to water consumption in arid regions and lengthy evaporation processes that can extend to 18-24 months. The supply chain from these sources involves extensive transportation networks, with material typically shipped to processing facilities in Asia.
Hard rock mining operations, concentrated in Australia, China, and Zimbabwe, represent approximately 26% of global lithium resources. While offering faster production cycles compared to brine operations, these sources require more energy-intensive processing and generate higher CO2 emissions per ton of lithium produced. The spodumene concentrate must undergo conversion to lithium chemicals, creating additional supply chain complexities.
Recent market analysis indicates significant supply chain vulnerabilities, with China controlling approximately 60% of global lithium processing capacity. This concentration creates potential bottlenecks and geopolitical risks for lithium chloride production. The average lithium chloride production facility requires materials from at least three different countries, highlighting the international interdependence of the supply chain.
Transportation logistics represent a substantial component of lithium chloride production costs, accounting for 8-15% of total expenses depending on production method and facility location. Maritime shipping disruptions, as evidenced during recent global supply chain crises, can significantly impact production schedules and material costs.
Emerging recycling initiatives are beginning to influence the supply chain dynamics, with advanced lithium recovery technologies potentially reducing raw material dependencies by 15-20% by 2030. However, current recycling infrastructure remains insufficient to significantly impact near-term supply considerations for lithium chloride production.
Supply chain sustainability certifications are gaining prominence, with major producers implementing traceability systems to verify responsible sourcing practices. These initiatives respond to increasing regulatory requirements in key markets like the European Union and North America, where upcoming legislation will mandate supply chain due diligence for critical minerals including lithium.
Brine-based lithium extraction, predominantly located in South America's "Lithium Triangle" (Chile, Argentina, and Bolivia), accounts for approximately 58% of global lithium resources. These operations benefit from lower production costs but face challenges related to water consumption in arid regions and lengthy evaporation processes that can extend to 18-24 months. The supply chain from these sources involves extensive transportation networks, with material typically shipped to processing facilities in Asia.
Hard rock mining operations, concentrated in Australia, China, and Zimbabwe, represent approximately 26% of global lithium resources. While offering faster production cycles compared to brine operations, these sources require more energy-intensive processing and generate higher CO2 emissions per ton of lithium produced. The spodumene concentrate must undergo conversion to lithium chemicals, creating additional supply chain complexities.
Recent market analysis indicates significant supply chain vulnerabilities, with China controlling approximately 60% of global lithium processing capacity. This concentration creates potential bottlenecks and geopolitical risks for lithium chloride production. The average lithium chloride production facility requires materials from at least three different countries, highlighting the international interdependence of the supply chain.
Transportation logistics represent a substantial component of lithium chloride production costs, accounting for 8-15% of total expenses depending on production method and facility location. Maritime shipping disruptions, as evidenced during recent global supply chain crises, can significantly impact production schedules and material costs.
Emerging recycling initiatives are beginning to influence the supply chain dynamics, with advanced lithium recovery technologies potentially reducing raw material dependencies by 15-20% by 2030. However, current recycling infrastructure remains insufficient to significantly impact near-term supply considerations for lithium chloride production.
Supply chain sustainability certifications are gaining prominence, with major producers implementing traceability systems to verify responsible sourcing practices. These initiatives respond to increasing regulatory requirements in key markets like the European Union and North America, where upcoming legislation will mandate supply chain due diligence for critical minerals including lithium.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!