Biocompatibility And Safety Testing For Wearable OTEs
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
OTE Biocompatibility Background and Objectives
Optical Tissue Engineering (OTE) represents a convergence of biomedical engineering, materials science, and optics, focusing on the development of wearable devices that interface directly with biological tissues. The evolution of this technology has progressed from basic implantable materials to sophisticated wearable systems capable of real-time monitoring and therapeutic intervention. Current trends indicate a shift toward minimally invasive, biocompatible devices that seamlessly integrate with the human body while maintaining optical functionality.
The primary objective of biocompatibility testing for wearable OTEs is to ensure these devices can maintain prolonged contact with biological tissues without causing adverse reactions or compromising their optical performance. This dual requirement presents unique challenges not encountered in traditional medical devices or optical systems alone. Historical approaches to biocompatibility have often focused on either optical materials or biomedical implants separately, creating a knowledge gap in the integrated field.
Recent advancements in biomaterials have expanded the potential applications of OTEs from simple monitoring devices to complex therapeutic systems capable of drug delivery, tissue regeneration, and neural interfacing. These developments have heightened the importance of comprehensive biocompatibility assessment protocols specifically designed for optically active materials in biological environments.
The regulatory landscape surrounding wearable OTEs continues to evolve, with organizations such as the FDA and ISO developing specialized guidelines for these hybrid technologies. Current standards like ISO 10993 provide a foundation but require adaptation to address the unique challenges posed by devices that must maintain optical clarity while ensuring tissue compatibility.
Key technological milestones in this field include the development of hydrogel-based optical waveguides in the early 2000s, followed by flexible photonic crystals in the 2010s, and more recently, biodegradable optical fibers capable of controlled dissolution after their functional period. Each advancement has introduced new biocompatibility considerations that must be systematically addressed.
The ultimate goal of biocompatibility testing for wearable OTEs extends beyond mere safety to encompass long-term functionality, stability, and integration with biological systems. This includes understanding how protein adsorption affects optical properties, how tissue encapsulation impacts light transmission, and how material degradation might release potentially harmful byproducts or alter device performance.
Moving forward, the field aims to establish standardized testing protocols that specifically address the unique interface between optical functionality and biological compatibility, enabling faster development cycles and more reliable performance predictions for next-generation wearable optical tissue engineering devices.
The primary objective of biocompatibility testing for wearable OTEs is to ensure these devices can maintain prolonged contact with biological tissues without causing adverse reactions or compromising their optical performance. This dual requirement presents unique challenges not encountered in traditional medical devices or optical systems alone. Historical approaches to biocompatibility have often focused on either optical materials or biomedical implants separately, creating a knowledge gap in the integrated field.
Recent advancements in biomaterials have expanded the potential applications of OTEs from simple monitoring devices to complex therapeutic systems capable of drug delivery, tissue regeneration, and neural interfacing. These developments have heightened the importance of comprehensive biocompatibility assessment protocols specifically designed for optically active materials in biological environments.
The regulatory landscape surrounding wearable OTEs continues to evolve, with organizations such as the FDA and ISO developing specialized guidelines for these hybrid technologies. Current standards like ISO 10993 provide a foundation but require adaptation to address the unique challenges posed by devices that must maintain optical clarity while ensuring tissue compatibility.
Key technological milestones in this field include the development of hydrogel-based optical waveguides in the early 2000s, followed by flexible photonic crystals in the 2010s, and more recently, biodegradable optical fibers capable of controlled dissolution after their functional period. Each advancement has introduced new biocompatibility considerations that must be systematically addressed.
The ultimate goal of biocompatibility testing for wearable OTEs extends beyond mere safety to encompass long-term functionality, stability, and integration with biological systems. This includes understanding how protein adsorption affects optical properties, how tissue encapsulation impacts light transmission, and how material degradation might release potentially harmful byproducts or alter device performance.
Moving forward, the field aims to establish standardized testing protocols that specifically address the unique interface between optical functionality and biological compatibility, enabling faster development cycles and more reliable performance predictions for next-generation wearable optical tissue engineering devices.
Market Analysis for Biocompatible Wearable OTEs
The global market for biocompatible wearable Organic Thin-Film Electronics (OTEs) is experiencing significant growth, driven by increasing consumer demand for health monitoring devices and advancements in flexible electronics technology. Current market valuations indicate that the biocompatible wearable electronics sector reached approximately 12 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 15-18% through 2028.
Consumer healthcare represents the largest market segment, accounting for nearly 40% of the total market share. This segment is primarily fueled by the rising adoption of continuous health monitoring devices for chronic disease management, particularly diabetes, cardiovascular conditions, and sleep disorders. The integration of biocompatible materials in these devices has substantially improved user comfort and compliance rates.
The athletic and fitness sector follows closely, representing about 30% of the market. Premium sports brands are increasingly incorporating biocompatible OTE sensors into performance monitoring equipment, creating significant market pull for advanced materials that can withstand perspiration and physical stress while maintaining skin compatibility.
Medical applications constitute approximately 25% of the market, with hospitals and healthcare providers adopting wearable OTEs for patient monitoring and drug delivery systems. This segment shows the highest growth potential due to increasing healthcare digitization and remote patient monitoring trends.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 20% annually, primarily driven by expanding healthcare infrastructure in China and India, coupled with strong electronics manufacturing capabilities.
Consumer surveys indicate that biocompatibility concerns significantly influence purchasing decisions, with 72% of consumers rating skin compatibility as "very important" or "extremely important" when selecting wearable devices. This represents a substantial shift from 2018 data, when only 45% of consumers prioritized this feature.
Market barriers include high development costs for biocompatible materials, stringent regulatory requirements, and technical challenges in maintaining electronic performance while ensuring biocompatibility. The average time-to-market for biocompatible wearable OTEs is 18-24 months, approximately 30% longer than conventional electronic wearables due to extended testing requirements.
Emerging opportunities exist in specialized markets such as pediatric monitoring, geriatric care, and occupational safety, where traditional wearables have shown limitations due to comfort and biocompatibility issues. These niche segments collectively represent a potential market of 3 billion USD by 2025.
Consumer healthcare represents the largest market segment, accounting for nearly 40% of the total market share. This segment is primarily fueled by the rising adoption of continuous health monitoring devices for chronic disease management, particularly diabetes, cardiovascular conditions, and sleep disorders. The integration of biocompatible materials in these devices has substantially improved user comfort and compliance rates.
The athletic and fitness sector follows closely, representing about 30% of the market. Premium sports brands are increasingly incorporating biocompatible OTE sensors into performance monitoring equipment, creating significant market pull for advanced materials that can withstand perspiration and physical stress while maintaining skin compatibility.
Medical applications constitute approximately 25% of the market, with hospitals and healthcare providers adopting wearable OTEs for patient monitoring and drug delivery systems. This segment shows the highest growth potential due to increasing healthcare digitization and remote patient monitoring trends.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 20% annually, primarily driven by expanding healthcare infrastructure in China and India, coupled with strong electronics manufacturing capabilities.
Consumer surveys indicate that biocompatibility concerns significantly influence purchasing decisions, with 72% of consumers rating skin compatibility as "very important" or "extremely important" when selecting wearable devices. This represents a substantial shift from 2018 data, when only 45% of consumers prioritized this feature.
Market barriers include high development costs for biocompatible materials, stringent regulatory requirements, and technical challenges in maintaining electronic performance while ensuring biocompatibility. The average time-to-market for biocompatible wearable OTEs is 18-24 months, approximately 30% longer than conventional electronic wearables due to extended testing requirements.
Emerging opportunities exist in specialized markets such as pediatric monitoring, geriatric care, and occupational safety, where traditional wearables have shown limitations due to comfort and biocompatibility issues. These niche segments collectively represent a potential market of 3 billion USD by 2025.
Current Biocompatibility Testing Challenges
Despite significant advancements in wearable Organic Thermoelectric Elements (OTEs), biocompatibility testing faces substantial challenges that impede market entry and widespread adoption. Current testing protocols were primarily designed for traditional medical devices and implants, creating a regulatory gap for wearable OTEs that maintain prolonged skin contact without invasive characteristics.
The lack of standardized testing frameworks specifically tailored for organic thermoelectric materials represents a critical obstacle. Existing ISO 10993 standards provide general guidance but fail to address the unique properties of OTEs, including their composite nature, variable material degradation patterns, and potential for novel interaction mechanisms with biological systems.
Material leaching presents a particular concern, as organic thermoelectric compounds may release components during extended wear periods under various environmental conditions. Current testing methodologies struggle to simulate real-world usage scenarios that include exposure to sweat, varying pH levels, mechanical stress, and temperature fluctuations that could accelerate material degradation or alter biocompatibility profiles.
Long-term exposure assessment remains inadequately addressed by conventional testing approaches. While acute toxicity testing is well-established, protocols for evaluating chronic low-level exposure effects from wearable OTEs are underdeveloped. This gap is especially problematic given that these devices are intended for continuous wear over months or years, potentially creating cumulative biological effects that current testing fails to capture.
Regulatory inconsistencies across global markets further complicate biocompatibility assessment. Different regions maintain varying requirements and interpretations of safety standards, creating a fragmented compliance landscape that increases development costs and time-to-market for manufacturers attempting global distribution of wearable OTE products.
The integration of multiple materials in modern OTE designs introduces complex testing challenges. These devices typically combine organic semiconductors, flexible substrates, conductive elements, and protective coatings—each with distinct biocompatibility profiles that may interact synergistically. Current testing approaches often evaluate components individually rather than assessing the final assembled product under realistic usage conditions.
Additionally, there exists a significant knowledge gap regarding potential immunological responses to novel organic thermoelectric materials. Traditional cytotoxicity and sensitization tests may not adequately predict unique immune reactions that could develop with extended exposure to these materials, particularly as they undergo environmental degradation or structural changes during normal use.
The lack of standardized testing frameworks specifically tailored for organic thermoelectric materials represents a critical obstacle. Existing ISO 10993 standards provide general guidance but fail to address the unique properties of OTEs, including their composite nature, variable material degradation patterns, and potential for novel interaction mechanisms with biological systems.
Material leaching presents a particular concern, as organic thermoelectric compounds may release components during extended wear periods under various environmental conditions. Current testing methodologies struggle to simulate real-world usage scenarios that include exposure to sweat, varying pH levels, mechanical stress, and temperature fluctuations that could accelerate material degradation or alter biocompatibility profiles.
Long-term exposure assessment remains inadequately addressed by conventional testing approaches. While acute toxicity testing is well-established, protocols for evaluating chronic low-level exposure effects from wearable OTEs are underdeveloped. This gap is especially problematic given that these devices are intended for continuous wear over months or years, potentially creating cumulative biological effects that current testing fails to capture.
Regulatory inconsistencies across global markets further complicate biocompatibility assessment. Different regions maintain varying requirements and interpretations of safety standards, creating a fragmented compliance landscape that increases development costs and time-to-market for manufacturers attempting global distribution of wearable OTE products.
The integration of multiple materials in modern OTE designs introduces complex testing challenges. These devices typically combine organic semiconductors, flexible substrates, conductive elements, and protective coatings—each with distinct biocompatibility profiles that may interact synergistically. Current testing approaches often evaluate components individually rather than assessing the final assembled product under realistic usage conditions.
Additionally, there exists a significant knowledge gap regarding potential immunological responses to novel organic thermoelectric materials. Traditional cytotoxicity and sensitization tests may not adequately predict unique immune reactions that could develop with extended exposure to these materials, particularly as they undergo environmental degradation or structural changes during normal use.
Current Biocompatibility Assessment Protocols
01 Biocompatible materials for wearable OTEs
Wearable organic thermoelectric elements require biocompatible materials that can safely interface with human skin. These materials include specific polymers and organic compounds that minimize allergic reactions and skin irritation while maintaining thermoelectric efficiency. The biocompatible materials are designed to be flexible, breathable, and non-toxic, allowing for extended wear without adverse health effects. These materials undergo rigorous testing to ensure they meet safety standards for direct skin contact applications.- Biocompatible materials for wearable OTEs: Wearable organic thermoelectric elements require biocompatible materials that can safely interface with human skin. These materials include specific polymers and organic compounds that minimize allergic reactions and skin irritation. The biocompatibility of these materials is essential for ensuring user safety during prolonged contact with the skin, while still maintaining efficient thermoelectric performance. These materials are designed to be flexible, breathable, and non-toxic to accommodate the dynamic nature of human movement and skin physiology.
- Safety testing protocols for wearable OTE devices: Comprehensive safety testing protocols have been developed for wearable organic thermoelectric elements to ensure they meet biocompatibility standards. These protocols include cytotoxicity testing, skin sensitization assessments, and long-term wear studies. The testing evaluates potential risks such as thermal effects, electrical safety, and chemical leaching. Regulatory compliance with medical device standards is also considered to ensure that these wearable energy harvesting technologies can be safely used in direct contact with human skin for extended periods without adverse effects.
- Encapsulation techniques for OTEs in wearable applications: Encapsulation methods play a crucial role in ensuring the biocompatibility and safety of wearable organic thermoelectric elements. These techniques involve coating or sealing the active thermoelectric materials with biocompatible polymers or other barrier materials to prevent direct contact between potentially harmful substances and the skin. Advanced encapsulation approaches also protect the OTE components from sweat, moisture, and mechanical stress while maintaining flexibility and comfort. Proper encapsulation extends device lifespan and prevents degradation that could lead to safety concerns.
- Integration of OTEs with flexible substrates for skin-friendly wearables: The integration of organic thermoelectric elements with flexible, skin-friendly substrates enables the development of comfortable and safe wearable energy harvesting devices. These substrates are designed to match the mechanical properties of human skin, reducing irritation and discomfort during movement. Techniques for bonding OTEs to textiles, silicones, and other flexible materials have been developed to create breathable interfaces that minimize heat accumulation and moisture buildup. This integration approach balances thermoelectric performance with user comfort and safety considerations.
- Thermal management systems for safe OTE operation on skin: Thermal management systems are essential for ensuring the safe operation of wearable organic thermoelectric elements in direct contact with skin. These systems control heat dissipation and prevent excessive temperature gradients that could cause discomfort or burns. Advanced designs incorporate heat-spreading materials, thermal insulators, and passive cooling mechanisms to maintain safe operating temperatures. Some approaches also include temperature-sensing components that can adjust performance or provide alerts when thermal conditions approach unsafe levels, ensuring user safety while maximizing energy harvesting efficiency.
02 Safety testing protocols for wearable thermoelectric devices
Comprehensive safety testing protocols have been developed specifically for wearable organic thermoelectric elements. These protocols include cytotoxicity assessments, skin sensitization tests, and long-term biocompatibility evaluations. The testing regimens examine potential electrical hazards, thermal risks, and chemical leaching under various conditions including perspiration and mechanical stress. These standardized testing methods ensure that wearable OTEs meet international safety regulations and standards before they can be approved for consumer use.Expand Specific Solutions03 Encapsulation techniques for OTE protection
Advanced encapsulation techniques have been developed to isolate potentially harmful thermoelectric materials from direct skin contact. These encapsulation methods use biocompatible polymers and barrier materials that prevent leaching of compounds while maintaining thermal conductivity necessary for device function. The encapsulation layers are designed to be breathable, flexible, and durable, withstanding the mechanical stresses of daily wear while providing a protective barrier between the active thermoelectric materials and human tissue.Expand Specific Solutions04 Flexible substrate integration for wearable comfort
Flexible substrates are crucial for integrating organic thermoelectric elements into comfortable wearable devices. These substrates allow the thermoelectric components to conform to body contours while maintaining electrical performance. The materials used include specialized polymers and textiles that combine flexibility with durability, ensuring the device can withstand repeated bending and stretching during normal wear. The integration of OTEs with flexible substrates improves user comfort while maintaining the biocompatibility and safety profile of the overall device.Expand Specific Solutions05 Heat management systems for skin-safe operation
Heat management systems are essential for ensuring wearable organic thermoelectric elements operate within skin-safe temperature ranges. These systems incorporate thermal regulators, heat dissipation structures, and temperature sensors to prevent overheating. Advanced designs include passive cooling mechanisms and thermal cutoff features that activate when temperatures approach unsafe levels. These heat management solutions ensure that the device maintains optimal performance while preventing thermal discomfort or potential burns, which is critical for the safety of wearable thermoelectric technology.Expand Specific Solutions
Leading OTE Manufacturers and Testing Labs
The biocompatibility and safety testing market for wearable OTEs (On-The-Ear devices) is currently in a growth phase, with increasing demand driven by consumer electronics expansion. The market is projected to reach significant scale as wearable technology adoption accelerates globally. From a technological maturity perspective, established players like Samsung Electronics, Philips, and IBM are leading with comprehensive testing protocols and advanced materials research, while academic institutions such as USC and Nanyang Technological University contribute fundamental research. Emerging companies like Notus Labs and CorNova are introducing innovative testing methodologies. Meta Platforms Technologies is advancing integration capabilities, while research organizations like Fraunhofer-Gesellschaft are developing standardized testing frameworks to address the evolving regulatory landscape.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive biocompatibility testing framework for their wearable OTE devices that integrates both standard regulatory compliance and enhanced user comfort assessments. Their approach begins with material selection focusing on medical-grade polymers and elastomers specifically formulated to minimize skin irritation during prolonged contact. Samsung's testing protocol includes standard ISO 10993 series compliance testing supplemented with proprietary extended wear studies that simulate real-world usage patterns. Their methodology incorporates specialized sweat resistance testing using artificial perspiration solutions with varying pH levels and salt concentrations to assess material degradation and potential leaching of compounds during extended wear[4]. Samsung has implemented advanced surface characterization techniques including AFM (Atomic Force Microscopy) and contact angle measurements to optimize the interface between device materials and skin. Their safety assessment includes comprehensive allergen screening against common sensitizers found in consumer products, and they've developed a specialized ear canal simulation system that replicates the microenvironment conditions including temperature, humidity, and natural oils to evaluate material performance under realistic conditions. Additionally, Samsung conducts extensive human factors testing with diverse user populations to assess subjective comfort and identify potential issues that might not be apparent in laboratory testing.
Strengths: Extensive experience with consumer electronics materials and manufacturing processes; robust global testing infrastructure; strong integration between material science and human factors engineering. Weaknesses: Primary focus on consumer applications rather than medical-grade standards; potential trade-offs between aesthetic design requirements and optimal biocompatible materials; challenges in balancing durability with skin-friendly material properties.
Koninklijke Philips NV
Technical Solution: Philips has developed a comprehensive biocompatibility testing framework specifically for wearable OTEs (On-The-Ear devices) that integrates both in vitro and in vivo testing methodologies. Their approach includes cytotoxicity assessment using ISO 10993-5 standards with specialized protocols for ear-contact materials, sensitization testing with modified LLNA (Local Lymph Node Assay) techniques, and long-term wear studies monitoring skin conditions over extended periods. Philips employs advanced biocompatible materials including medical-grade silicones and hypoallergenic polymers specifically formulated to minimize irritation during prolonged ear contact. Their testing protocol incorporates real-world usage scenarios with sweat simulation chambers that replicate various environmental conditions and physical activities to assess material degradation and potential leaching of compounds[1]. Additionally, they've pioneered non-invasive monitoring techniques to evaluate skin response during clinical trials, using specialized imaging and biomarker analysis to detect subclinical inflammatory responses before visible symptoms appear.
Strengths: Comprehensive end-to-end testing methodology that exceeds regulatory requirements; extensive experience with medical-grade materials suitable for long-term skin contact; advanced simulation capabilities for real-world usage scenarios. Weaknesses: Higher development costs compared to consumer-grade approaches; longer time-to-market due to rigorous testing protocols; materials optimized for biocompatibility may have limitations in durability or aesthetic options.
Key Innovations in OTE Safety Testing
Method for preparing thermoelectric material by inducing polymer crystallization through liquid crystal micromolecules and application
PatentPendingCN117659632A
Innovation
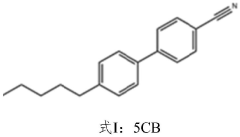
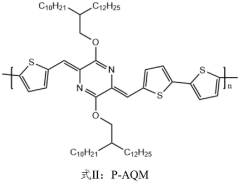
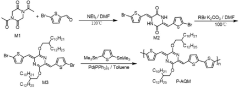
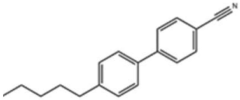
- High-efficiency thermoelectric materials are prepared by using liquid crystal small molecules to induce polymer crystallization. By blending liquid crystal small molecules with biphenyl main bodies and cyano and alkyl side chains with polymers, organic thermoelectric thin film devices are prepared using solution processing to achieve high mobility and Highly crystalline material.
Regulatory Framework for Wearable OTEs
The regulatory landscape for wearable Organic Thermoelectric Elements (OTEs) is complex and evolving, with frameworks varying significantly across global markets. In the United States, the FDA classifies wearable OTEs based on their intended use and risk profile, with most falling under Class II medical devices requiring 510(k) clearance when making therapeutic claims. The FDA's guidance on biocompatibility testing follows ISO 10993 standards, with particular emphasis on skin sensitization, irritation, and cytotoxicity for devices with prolonged skin contact.
The European Union regulates these devices under the Medical Device Regulation (MDR) when health claims are made, requiring CE marking and compliance with Essential Requirements. The MDR places stronger emphasis on post-market surveillance and clinical evidence than previous frameworks, with specific requirements for devices containing nanomaterials—a common component in advanced OTEs.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for wearable technology, with increasing harmonization with international standards but maintaining distinct national requirements for market entry.
For consumer-grade wearable OTEs without medical claims, regulations focus primarily on electrical safety (IEC 60601), electromagnetic compatibility (IEC 60601-1-2), and general product safety standards. However, the regulatory distinction between wellness and medical devices remains blurry in many jurisdictions, creating compliance challenges for manufacturers.
International standards organizations play a crucial role in establishing testing protocols for biocompatibility. ISO 10993-1:2018 provides the framework for biological evaluation of medical devices, with specific parts addressing various aspects of biocompatibility testing. For wearable OTEs, ISO 10993-5 (cytotoxicity), ISO 10993-10 (irritation and skin sensitization), and ISO 10993-23 (irritation tests) are particularly relevant.
Emerging regulatory considerations include the environmental impact of OTE materials and disposal protocols, with the EU's Restriction of Hazardous Substances (RoHS) and Waste Electrical and Electronic Equipment (WEEE) directives setting global benchmarks. Additionally, data privacy regulations such as GDPR in Europe and CCPA in California impact wearable OTEs that collect physiological or location data, requiring manufacturers to implement privacy-by-design principles.
Regulatory compliance strategies for wearable OTE developers must include early engagement with regulatory bodies through pre-submission consultations, careful classification determination, and comprehensive testing documentation that addresses both established and emerging concerns around novel materials used in thermoelectric applications.
The European Union regulates these devices under the Medical Device Regulation (MDR) when health claims are made, requiring CE marking and compliance with Essential Requirements. The MDR places stronger emphasis on post-market surveillance and clinical evidence than previous frameworks, with specific requirements for devices containing nanomaterials—a common component in advanced OTEs.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for wearable technology, with increasing harmonization with international standards but maintaining distinct national requirements for market entry.
For consumer-grade wearable OTEs without medical claims, regulations focus primarily on electrical safety (IEC 60601), electromagnetic compatibility (IEC 60601-1-2), and general product safety standards. However, the regulatory distinction between wellness and medical devices remains blurry in many jurisdictions, creating compliance challenges for manufacturers.
International standards organizations play a crucial role in establishing testing protocols for biocompatibility. ISO 10993-1:2018 provides the framework for biological evaluation of medical devices, with specific parts addressing various aspects of biocompatibility testing. For wearable OTEs, ISO 10993-5 (cytotoxicity), ISO 10993-10 (irritation and skin sensitization), and ISO 10993-23 (irritation tests) are particularly relevant.
Emerging regulatory considerations include the environmental impact of OTE materials and disposal protocols, with the EU's Restriction of Hazardous Substances (RoHS) and Waste Electrical and Electronic Equipment (WEEE) directives setting global benchmarks. Additionally, data privacy regulations such as GDPR in Europe and CCPA in California impact wearable OTEs that collect physiological or location data, requiring manufacturers to implement privacy-by-design principles.
Regulatory compliance strategies for wearable OTE developers must include early engagement with regulatory bodies through pre-submission consultations, careful classification determination, and comprehensive testing documentation that addresses both established and emerging concerns around novel materials used in thermoelectric applications.
Long-term User Safety Monitoring Strategies
Long-term safety monitoring for wearable Organic Thermoelectric Elements (OTEs) requires comprehensive strategies that extend beyond initial testing phases. Establishing continuous monitoring protocols is essential for identifying delayed biocompatibility issues that may not manifest during preliminary testing periods. These protocols should include regular assessment of skin conditions, immune responses, and potential systemic effects through periodic medical evaluations.
Data collection systems represent a critical component of long-term safety surveillance. Implementing automated reporting mechanisms through the wearable devices themselves enables real-time monitoring of key parameters such as temperature fluctuations, electrical output consistency, and material degradation indicators. This telemetry data can be supplemented with user-reported symptoms through dedicated mobile applications, creating a comprehensive safety profile for each device.
Post-market surveillance programs must be structured to capture rare adverse events that may occur in diverse populations. This requires establishing centralized databases where clinicians and users can report unexpected reactions, with standardized classification systems to facilitate trend analysis. Statistical methods should be employed to distinguish between coincidental health issues and those potentially attributable to OTE exposure.
Material degradation monitoring represents a particular challenge for long-term safety assessment. Periodic non-invasive testing protocols should be developed to evaluate the structural integrity of organic components, potential leaching of compounds, and changes in electrical characteristics over time. Techniques such as spectroscopic analysis of device surfaces and performance benchmarking can provide early warnings of material failure before safety is compromised.
Population-specific monitoring considerations must be incorporated into safety strategies. Certain demographic groups, including those with sensitive skin conditions, compromised immune systems, or specific genetic predispositions, may require enhanced surveillance protocols. Stratified analysis of safety data across different user populations can identify vulnerable groups requiring additional protective measures or modified device specifications.
Regulatory frameworks for long-term monitoring should establish clear thresholds for intervention, including criteria for device recalls, mandatory design modifications, or usage restrictions. These frameworks must balance the need for comprehensive safety data against the practical limitations of monitoring programs and user compliance. International harmonization of these monitoring standards would facilitate global data sharing and more rapid identification of safety signals across diverse populations.
Data collection systems represent a critical component of long-term safety surveillance. Implementing automated reporting mechanisms through the wearable devices themselves enables real-time monitoring of key parameters such as temperature fluctuations, electrical output consistency, and material degradation indicators. This telemetry data can be supplemented with user-reported symptoms through dedicated mobile applications, creating a comprehensive safety profile for each device.
Post-market surveillance programs must be structured to capture rare adverse events that may occur in diverse populations. This requires establishing centralized databases where clinicians and users can report unexpected reactions, with standardized classification systems to facilitate trend analysis. Statistical methods should be employed to distinguish between coincidental health issues and those potentially attributable to OTE exposure.
Material degradation monitoring represents a particular challenge for long-term safety assessment. Periodic non-invasive testing protocols should be developed to evaluate the structural integrity of organic components, potential leaching of compounds, and changes in electrical characteristics over time. Techniques such as spectroscopic analysis of device surfaces and performance benchmarking can provide early warnings of material failure before safety is compromised.
Population-specific monitoring considerations must be incorporated into safety strategies. Certain demographic groups, including those with sensitive skin conditions, compromised immune systems, or specific genetic predispositions, may require enhanced surveillance protocols. Stratified analysis of safety data across different user populations can identify vulnerable groups requiring additional protective measures or modified device specifications.
Regulatory frameworks for long-term monitoring should establish clear thresholds for intervention, including criteria for device recalls, mandatory design modifications, or usage restrictions. These frameworks must balance the need for comprehensive safety data against the practical limitations of monitoring programs and user compliance. International harmonization of these monitoring standards would facilitate global data sharing and more rapid identification of safety signals across diverse populations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!