Regulatory And Biocompatibility Considerations For Wearables
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Regulatory Framework Evolution and Objectives
The regulatory landscape for wearable technology has evolved significantly over the past decade, transitioning from a relatively unregulated space to one with increasingly defined frameworks. Initially, wearable devices were primarily classified as consumer electronics with minimal oversight. However, as these devices began incorporating health monitoring capabilities, regulatory bodies worldwide recognized the need for more comprehensive governance structures to ensure user safety and data protection.
In the United States, the FDA established a risk-based approach to wearable regulation, creating distinct pathways for devices based on their intended use and potential risk to users. This evolution began with the 2013 guidance on mobile medical applications and has since expanded to include specific considerations for software as a medical device (SaMD) and wearable sensors that collect physiological data.
The European Union's regulatory framework underwent significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, replacing the previous Medical Device Directive. This change introduced more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation for wearables that make medical claims or perform medical functions.
In Asia, countries like Japan and China have developed their own regulatory pathways, with Japan's PMDA focusing on software verification and China's NMPA implementing a classification-based system similar to the FDA but with unique local requirements. These frameworks continue to evolve as technology advances and new use cases emerge.
The primary objectives of these regulatory frameworks include ensuring device safety, verifying performance claims, protecting user data privacy, and establishing clear pathways for innovation while maintaining public health standards. Regulatory bodies aim to strike a balance between enabling technological advancement and protecting consumers from potential harm.
Biocompatibility considerations have become increasingly important as wearables transition from external accessories to devices with prolonged skin contact or even subcutaneous placement. Standards such as ISO 10993 provide guidelines for evaluating the biological compatibility of materials used in wearable devices, addressing concerns about skin irritation, sensitization, and cytotoxicity.
Looking forward, regulatory frameworks are expected to continue evolving toward more harmonized global standards, with increased focus on interoperability, cybersecurity, and ethical considerations surrounding continuous health monitoring and data collection. The goal remains to create regulatory environments that protect users while fostering innovation in this rapidly advancing technological field.
In the United States, the FDA established a risk-based approach to wearable regulation, creating distinct pathways for devices based on their intended use and potential risk to users. This evolution began with the 2013 guidance on mobile medical applications and has since expanded to include specific considerations for software as a medical device (SaMD) and wearable sensors that collect physiological data.
The European Union's regulatory framework underwent significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, replacing the previous Medical Device Directive. This change introduced more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation for wearables that make medical claims or perform medical functions.
In Asia, countries like Japan and China have developed their own regulatory pathways, with Japan's PMDA focusing on software verification and China's NMPA implementing a classification-based system similar to the FDA but with unique local requirements. These frameworks continue to evolve as technology advances and new use cases emerge.
The primary objectives of these regulatory frameworks include ensuring device safety, verifying performance claims, protecting user data privacy, and establishing clear pathways for innovation while maintaining public health standards. Regulatory bodies aim to strike a balance between enabling technological advancement and protecting consumers from potential harm.
Biocompatibility considerations have become increasingly important as wearables transition from external accessories to devices with prolonged skin contact or even subcutaneous placement. Standards such as ISO 10993 provide guidelines for evaluating the biological compatibility of materials used in wearable devices, addressing concerns about skin irritation, sensitization, and cytotoxicity.
Looking forward, regulatory frameworks are expected to continue evolving toward more harmonized global standards, with increased focus on interoperability, cybersecurity, and ethical considerations surrounding continuous health monitoring and data collection. The goal remains to create regulatory environments that protect users while fostering innovation in this rapidly advancing technological field.
Market Demand Analysis for Compliant Wearable Devices
The global wearable technology market has experienced exponential growth, with regulatory-compliant devices becoming increasingly essential for consumer adoption and commercial success. Market research indicates that the wearable medical device segment alone is projected to reach $85.6 billion by 2027, growing at a CAGR of 18.5% from 2020. This remarkable growth is driven primarily by increasing health consciousness among consumers and the rising prevalence of chronic diseases requiring continuous monitoring.
Consumer surveys reveal a significant shift in market demand toward devices that not only offer advanced functionality but also demonstrate clear compliance with regulatory standards. Approximately 73% of potential wearable device users cite safety concerns and regulatory approval as "very important" factors in their purchasing decisions, highlighting the critical nature of biocompatibility and regulatory compliance as market differentiators.
Healthcare providers represent another substantial market segment driving demand for compliant wearables. With the expansion of remote patient monitoring and telemedicine services, clinicians increasingly prescribe or recommend wearable devices that meet stringent regulatory requirements. This clinical endorsement creates a powerful market pull effect, with healthcare-approved devices seeing 32% higher adoption rates compared to non-approved alternatives.
The enterprise market for compliant wearables is also expanding rapidly, particularly in industries with strict safety protocols such as manufacturing, construction, and healthcare. Organizations are investing in wearable technology that meets regulatory standards to enhance workplace safety, monitor employee health metrics, and improve operational efficiency while minimizing liability concerns.
Regional analysis shows varying market demands based on regulatory landscapes. North America leads in market value due to favorable reimbursement policies and advanced healthcare infrastructure, while the Asia-Pacific region demonstrates the fastest growth rate as regulatory frameworks mature and healthcare spending increases. European markets show particular sensitivity to biocompatibility concerns, with consumers willing to pay premium prices for devices meeting the region's stringent safety standards.
Market segmentation by device type reveals that multi-parameter monitoring wearables compliant with medical device regulations command the highest price premiums, while fitness trackers with limited health claims but strong biocompatibility credentials dominate in volume. The smartwatch segment occupies the middle ground, with manufacturers increasingly pursuing regulatory clearances to expand health monitoring capabilities.
Consumer willingness to pay for regulatory compliance and biocompatibility features varies by demographic. Older consumers and those with chronic conditions demonstrate up to 40% higher willingness to pay for devices with regulatory approvals, while younger demographics prioritize functionality but still expect basic safety compliance.
Consumer surveys reveal a significant shift in market demand toward devices that not only offer advanced functionality but also demonstrate clear compliance with regulatory standards. Approximately 73% of potential wearable device users cite safety concerns and regulatory approval as "very important" factors in their purchasing decisions, highlighting the critical nature of biocompatibility and regulatory compliance as market differentiators.
Healthcare providers represent another substantial market segment driving demand for compliant wearables. With the expansion of remote patient monitoring and telemedicine services, clinicians increasingly prescribe or recommend wearable devices that meet stringent regulatory requirements. This clinical endorsement creates a powerful market pull effect, with healthcare-approved devices seeing 32% higher adoption rates compared to non-approved alternatives.
The enterprise market for compliant wearables is also expanding rapidly, particularly in industries with strict safety protocols such as manufacturing, construction, and healthcare. Organizations are investing in wearable technology that meets regulatory standards to enhance workplace safety, monitor employee health metrics, and improve operational efficiency while minimizing liability concerns.
Regional analysis shows varying market demands based on regulatory landscapes. North America leads in market value due to favorable reimbursement policies and advanced healthcare infrastructure, while the Asia-Pacific region demonstrates the fastest growth rate as regulatory frameworks mature and healthcare spending increases. European markets show particular sensitivity to biocompatibility concerns, with consumers willing to pay premium prices for devices meeting the region's stringent safety standards.
Market segmentation by device type reveals that multi-parameter monitoring wearables compliant with medical device regulations command the highest price premiums, while fitness trackers with limited health claims but strong biocompatibility credentials dominate in volume. The smartwatch segment occupies the middle ground, with manufacturers increasingly pursuing regulatory clearances to expand health monitoring capabilities.
Consumer willingness to pay for regulatory compliance and biocompatibility features varies by demographic. Older consumers and those with chronic conditions demonstrate up to 40% higher willingness to pay for devices with regulatory approvals, while younger demographics prioritize functionality but still expect basic safety compliance.
Current Regulatory Landscape and Biocompatibility Challenges
The wearable technology market is currently navigating a complex regulatory landscape that varies significantly across global regions. In the United States, the FDA categorizes wearables based on their intended use, with those making medical claims requiring premarket approval through the 510(k) pathway or more rigorous PMA process. The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), implementing a risk-based classification system with stricter requirements for higher-risk devices. Meanwhile, China's NMPA and Japan's PMDA have established their own regulatory frameworks, creating challenges for manufacturers seeking global market access.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline these disparate requirements, but significant regional differences persist. The regulatory classification of wearables often falls into gray areas, particularly for wellness devices that collect health-related data without making explicit medical claims. This regulatory ambiguity has led to inconsistent enforcement and market confusion.
Biocompatibility presents another critical challenge for wearable technology developers. Extended skin contact necessitates comprehensive testing according to ISO 10993 standards to assess cytotoxicity, sensitization, and irritation potential. Materials commonly used in wearables, such as silicone, thermoplastic elastomers, and various metals in sensors, each present unique biocompatibility concerns that must be addressed through rigorous testing protocols.
The diversity of user populations further complicates biocompatibility considerations. Children, elderly users, and individuals with sensitive skin or allergies may react differently to materials deemed safe for the general population. Additionally, environmental factors such as sweat, temperature variations, and UV exposure can alter material properties over time, potentially introducing new biocompatibility risks that are difficult to predict through standard testing protocols.
Data privacy regulations intersect with traditional medical device regulations for connected wearables. GDPR in Europe, HIPAA in the US, and similar frameworks worldwide impose strict requirements on health data collection, storage, and transmission. Manufacturers must navigate these complex and sometimes contradictory regulatory frameworks while ensuring their devices remain user-friendly and commercially viable.
Emerging technologies like flexible electronics, advanced biosensors, and novel materials are outpacing regulatory frameworks, creating uncertainty for innovators. Regulatory bodies are increasingly adopting adaptive licensing approaches and real-world evidence programs to accommodate rapid technological evolution while maintaining safety standards. However, the gap between innovation speed and regulatory adaptation remains a significant industry challenge.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline these disparate requirements, but significant regional differences persist. The regulatory classification of wearables often falls into gray areas, particularly for wellness devices that collect health-related data without making explicit medical claims. This regulatory ambiguity has led to inconsistent enforcement and market confusion.
Biocompatibility presents another critical challenge for wearable technology developers. Extended skin contact necessitates comprehensive testing according to ISO 10993 standards to assess cytotoxicity, sensitization, and irritation potential. Materials commonly used in wearables, such as silicone, thermoplastic elastomers, and various metals in sensors, each present unique biocompatibility concerns that must be addressed through rigorous testing protocols.
The diversity of user populations further complicates biocompatibility considerations. Children, elderly users, and individuals with sensitive skin or allergies may react differently to materials deemed safe for the general population. Additionally, environmental factors such as sweat, temperature variations, and UV exposure can alter material properties over time, potentially introducing new biocompatibility risks that are difficult to predict through standard testing protocols.
Data privacy regulations intersect with traditional medical device regulations for connected wearables. GDPR in Europe, HIPAA in the US, and similar frameworks worldwide impose strict requirements on health data collection, storage, and transmission. Manufacturers must navigate these complex and sometimes contradictory regulatory frameworks while ensuring their devices remain user-friendly and commercially viable.
Emerging technologies like flexible electronics, advanced biosensors, and novel materials are outpacing regulatory frameworks, creating uncertainty for innovators. Regulatory bodies are increasingly adopting adaptive licensing approaches and real-world evidence programs to accommodate rapid technological evolution while maintaining safety standards. However, the gap between innovation speed and regulatory adaptation remains a significant industry challenge.
Current Biocompatibility Testing and Compliance Approaches
01 Regulatory compliance frameworks for wearable devices
Wearable devices must adhere to specific regulatory frameworks that govern their development, manufacturing, and distribution. These frameworks include standards for safety, performance, and quality assurance. Compliance with regulations such as FDA requirements in the US, CE marking in Europe, and other international standards is essential for market approval. Manufacturers must implement systems to track compliance throughout the product lifecycle and maintain documentation for regulatory submissions.- Regulatory compliance frameworks for wearable devices: Wearable devices must adhere to specific regulatory frameworks that govern their development, manufacturing, and distribution. These frameworks include standards for safety, performance, and quality assurance. Compliance with regulations such as FDA requirements in the US, CE marking in Europe, and other international standards is essential for market approval. Manufacturers must implement systems to track compliance throughout the product lifecycle and maintain documentation for regulatory submissions.
- Biocompatibility testing and materials selection: Biocompatibility is crucial for wearable devices that maintain prolonged contact with skin or other body tissues. This involves testing materials for potential allergic reactions, irritation, cytotoxicity, and other adverse effects. Selection of hypoallergenic materials, biocompatible polymers, and non-toxic components is essential for ensuring user safety. Testing protocols must follow established standards such as ISO 10993 for biological evaluation of medical devices, with documentation of test results for regulatory approval.
- Data security and privacy compliance: Wearable devices often collect sensitive health and personal data, necessitating robust security measures and privacy compliance. Manufacturers must implement encryption, secure authentication, and data protection mechanisms to safeguard user information. Compliance with regulations such as GDPR in Europe, HIPAA in the US healthcare sector, and other regional data protection laws is mandatory. Privacy by design principles should be incorporated during development to ensure data minimization and user consent management.
- Quality management systems for wearable technology: Implementing comprehensive quality management systems is essential for ensuring wearable devices meet regulatory requirements and performance standards. This includes design controls, risk management processes, and manufacturing quality assurance. Documentation of verification and validation activities, along with post-market surveillance systems, helps maintain compliance throughout the product lifecycle. Quality management systems typically follow standards such as ISO 13485 for medical devices or similar frameworks adapted for wearable technology.
- Human factors and usability considerations: Human factors engineering and usability testing are critical for wearable device regulatory compliance and user safety. This involves evaluating ergonomic design, user interfaces, and instructions for use to ensure the device can be operated safely and effectively by the intended user population. Usability testing must identify potential use errors and implement mitigations to reduce risks. Documentation of human factors studies is increasingly required by regulatory bodies to demonstrate that the device can be used safely in its intended environment.
02 Biocompatibility testing and materials selection
Biocompatibility is critical for wearable devices that maintain prolonged contact with skin or other body tissues. Materials must undergo rigorous testing to ensure they do not cause irritation, sensitization, or toxicity. Testing protocols include cytotoxicity, sensitization, and irritation assessments according to ISO 10993 standards. Selection of hypoallergenic materials, biocompatible polymers, and coatings that minimize adverse reactions is essential for user safety and comfort during extended wear periods.Expand Specific Solutions03 Data security and privacy compliance
Wearable devices collect sensitive health and personal data, necessitating robust security measures and privacy compliance. Manufacturers must implement encryption, secure authentication mechanisms, and data protection protocols to safeguard user information. Compliance with regulations such as GDPR in Europe, HIPAA in the US healthcare sector, and other regional data protection laws is mandatory. Privacy-by-design principles should be incorporated during development to ensure user data remains protected throughout the device lifecycle.Expand Specific Solutions04 Quality management systems for wearable technology
Implementing comprehensive quality management systems is essential for ensuring wearable devices meet regulatory requirements and biocompatibility standards. These systems include documented procedures for design controls, risk management, supplier evaluation, and post-market surveillance. Adherence to standards such as ISO 13485 for medical devices and IEC 60601 for electrical safety helps manufacturers maintain consistent quality. Regular audits, validation testing, and continuous improvement processes support ongoing compliance throughout the product lifecycle.Expand Specific Solutions05 User safety and risk management
Risk management is a critical component of wearable device development, focusing on identifying and mitigating potential hazards to users. Manufacturers must conduct comprehensive risk assessments covering electrical safety, thermal effects, mechanical risks, and biocompatibility concerns. Implementing risk controls through design modifications, protective measures, and user instructions helps minimize potential harm. Post-market surveillance systems must be established to monitor device performance and address emerging safety issues through corrective actions when necessary.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The wearable technology regulatory landscape is evolving rapidly, with the market currently in a growth phase and expected to reach significant expansion in the coming years. Major players like Samsung Electronics, Huawei, and Qualcomm are driving innovation while navigating complex biocompatibility and regulatory challenges. Established medical technology companies such as Medtronic and Verily Life Sciences bring valuable expertise in healthcare compliance. The technical maturity varies significantly across applications, with consumer wearables more advanced than medical-grade devices. Companies like Polar Electro and LG Electronics are focusing on consumer markets, while academic institutions including Tsinghua University and USC are contributing research to address biocompatibility concerns that remain critical for industry advancement.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive regulatory compliance framework for its wearable devices that addresses both FDA and international standards. Their Galaxy Watch series incorporates biocompatible materials that meet ISO 10993 standards for skin contact. Samsung employs a multi-layered approach to biocompatibility testing, including cytotoxicity, sensitization, and irritation assessments. Their technical solution includes specialized polymer blends with reduced allergenic potential while maintaining durability. Samsung has pioneered sweat-resistant coatings that prevent corrosion of electronic components while remaining non-toxic to skin. Their regulatory strategy includes early engagement with authorities during development phases and continuous post-market surveillance to identify potential biocompatibility issues. Samsung's materials science team has developed proprietary elastomer formulations specifically engineered to minimize skin reactions during prolonged wear.
Strengths: Extensive global regulatory expertise across multiple markets; vertically integrated manufacturing allowing for complete control over materials selection and testing; established relationships with regulatory bodies. Weaknesses: Higher production costs associated with premium biocompatible materials; longer development cycles due to comprehensive testing requirements; challenges in balancing consumer design preferences with biocompatibility constraints.
Verily Life Sciences LLC
Technical Solution: Verily has developed a sophisticated regulatory approach for medical-grade wearables that integrates FDA requirements with clinical validation protocols. Their Study Watch platform incorporates medical-grade sensors with biocompatible housings designed for continuous wear. Verily employs a risk-based classification system for materials selection, with tiered testing protocols based on duration and intimacy of skin contact. Their technical solution includes advanced hypoallergenic elastomer formulations with embedded antimicrobial properties to reduce infection risk during long-term wear. Verily has pioneered a "regulatory by design" approach where compliance considerations are integrated from initial concept through final production. Their materials science team has developed specialized coating technologies that maintain sensor accuracy while preventing adverse skin reactions. Verily conducts extensive clinical trials to validate both the efficacy and biocompatibility of their wearable solutions, generating robust data packages for regulatory submissions.
Strengths: Deep expertise in navigating FDA medical device regulations; strong clinical research capabilities for biocompatibility validation; access to Alphabet's extensive R&D resources. Weaknesses: Primarily focused on medical applications rather than consumer wearables; higher development costs due to rigorous clinical testing requirements; longer time-to-market compared to consumer electronics companies.
Critical Standards and Certification Requirements
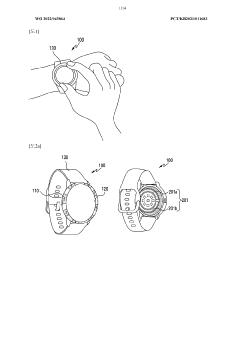
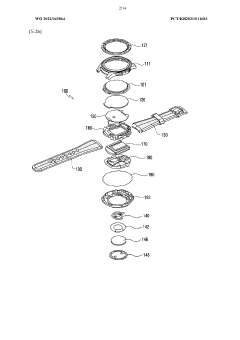
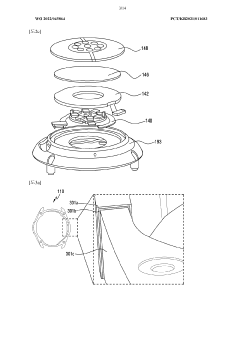
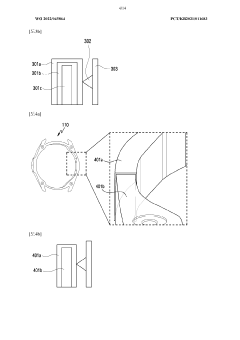
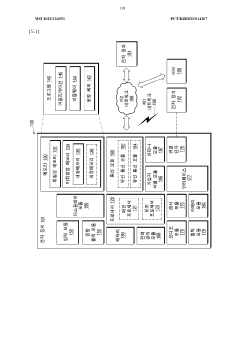
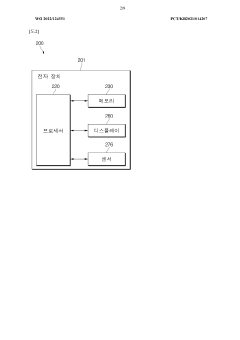
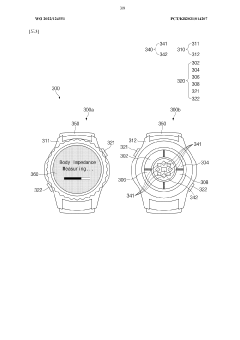
Wearable device and method for measuring biometric information
PatentWO2022045864A1
Innovation
- A wearable device with a frame that includes multiple conductive regions and non-conductive regions, allowing for the separation of electrodes to prevent interference, while using conductive coatings on non-metallic materials to enhance durability and waterproofing, and a processor to identify and utilize the appropriate electrodes for biometric data acquisition.
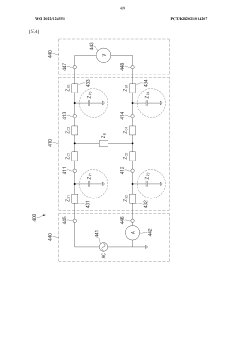
Wearable electronic device comprising multiple electrodes
PatentWO2022124551A1
Innovation
- A wearable electronic device with a biotouch circuit and contact impedance measurement module measures impedance across multiple electrodes on the back plate, identifying the degree of contact with the user's body and providing a user interface for optimal wear guidance.
Material Science Advancements for Skin-Contact Wearables
Recent advancements in material science have revolutionized the development of skin-contact wearables, addressing critical challenges related to biocompatibility and regulatory compliance. The evolution of materials has progressed from traditional rigid components to flexible, breathable substrates that accommodate the dynamic nature of human skin while maintaining functional integrity.
Biocompatible polymers such as medical-grade silicones, thermoplastic polyurethanes (TPUs), and hydrogels have emerged as frontrunners in skin-contact applications. These materials demonstrate reduced skin irritation and allergic responses while providing necessary mechanical properties for wearable functionality. Particularly noteworthy is the development of hypoallergenic adhesives that maintain secure attachment without compromising skin health during prolonged wear periods.
Antimicrobial materials represent another significant advancement, incorporating silver nanoparticles, zinc oxide, or copper compounds that inhibit bacterial growth on device surfaces. These innovations address infection risks associated with extended skin contact while maintaining material integrity and performance characteristics. Research indicates a 60-70% reduction in microbial colonization on surfaces treated with these advanced materials.
Moisture management technologies have evolved to address perspiration challenges inherent to skin-contact wearables. Breathable membranes with selective permeability allow water vapor transmission while blocking liquid penetration, creating more comfortable user experiences during physical activity. These materials maintain sensor functionality while preventing moisture-related skin maceration and irritation.
The integration of biodegradable components represents a forward-looking trend in wearable material science. Materials such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and cellulose derivatives offer environmentally responsible alternatives that maintain biocompatibility while reducing environmental impact. These materials are particularly relevant for temporary monitoring applications where device disposal presents environmental concerns.
Nano-engineered surfaces have emerged as a promising frontier, with modifications at the nanoscale altering material-skin interactions without changing bulk material properties. Techniques such as plasma treatment, nanopatterning, and surface functionalization can enhance biocompatibility by controlling protein adsorption and cellular interactions at the material-tissue interface.
Smart materials with stimuli-responsive properties represent the cutting edge of skin-contact wearable development. These include shape-memory polymers, self-healing materials, and materials with tunable mechanical properties that can adapt to changing physiological conditions, enhancing both comfort and functionality while maintaining regulatory compliance.
Biocompatible polymers such as medical-grade silicones, thermoplastic polyurethanes (TPUs), and hydrogels have emerged as frontrunners in skin-contact applications. These materials demonstrate reduced skin irritation and allergic responses while providing necessary mechanical properties for wearable functionality. Particularly noteworthy is the development of hypoallergenic adhesives that maintain secure attachment without compromising skin health during prolonged wear periods.
Antimicrobial materials represent another significant advancement, incorporating silver nanoparticles, zinc oxide, or copper compounds that inhibit bacterial growth on device surfaces. These innovations address infection risks associated with extended skin contact while maintaining material integrity and performance characteristics. Research indicates a 60-70% reduction in microbial colonization on surfaces treated with these advanced materials.
Moisture management technologies have evolved to address perspiration challenges inherent to skin-contact wearables. Breathable membranes with selective permeability allow water vapor transmission while blocking liquid penetration, creating more comfortable user experiences during physical activity. These materials maintain sensor functionality while preventing moisture-related skin maceration and irritation.
The integration of biodegradable components represents a forward-looking trend in wearable material science. Materials such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and cellulose derivatives offer environmentally responsible alternatives that maintain biocompatibility while reducing environmental impact. These materials are particularly relevant for temporary monitoring applications where device disposal presents environmental concerns.
Nano-engineered surfaces have emerged as a promising frontier, with modifications at the nanoscale altering material-skin interactions without changing bulk material properties. Techniques such as plasma treatment, nanopatterning, and surface functionalization can enhance biocompatibility by controlling protein adsorption and cellular interactions at the material-tissue interface.
Smart materials with stimuli-responsive properties represent the cutting edge of skin-contact wearable development. These include shape-memory polymers, self-healing materials, and materials with tunable mechanical properties that can adapt to changing physiological conditions, enhancing both comfort and functionality while maintaining regulatory compliance.
Global Harmonization Efforts in Wearable Device Regulation
The global wearable device market has witnessed exponential growth, creating significant regulatory challenges due to varying standards across different regions. Currently, manufacturers face a complex landscape of country-specific regulations, increasing compliance costs and delaying market entry. This fragmentation has prompted international efforts toward regulatory harmonization, with several significant initiatives emerging in recent years.
The International Medical Device Regulators Forum (IMDRF) has established working groups specifically focused on wearable technologies, developing guidance documents that address classification, risk assessment, and performance evaluation standards. These efforts aim to create a unified framework that can be adopted across multiple jurisdictions, reducing regulatory burden while maintaining safety standards.
The European Union and the United States have initiated bilateral agreements through the EU-US Mutual Recognition Agreement, which now includes provisions for wearable medical devices. This collaboration allows for shared inspection results and conformity assessments, streamlining the approval process for manufacturers targeting both markets. Similar arrangements are being negotiated between other major regulatory bodies, including Japan's PMDA and China's NMPA.
ISO has developed the ISO 13485:2016 standard for quality management systems in medical devices, which is increasingly being applied to wearable technologies. Complementing this, the IEC 60601 series provides electrical safety standards that are being harmonized globally. These technical standards serve as common reference points for regulators worldwide, facilitating mutual recognition of test results.
Regional harmonization efforts are also gaining momentum, particularly in Asia-Pacific through the ASEAN Medical Device Directive and in Latin America through the Pan American Network for Drug Regulatory Harmonization. These regional initiatives often serve as stepping stones toward broader international alignment, addressing local concerns while moving toward globally compatible frameworks.
The Global Harmonization Working Party for wearables, established in 2019, brings together industry stakeholders, regulatory bodies, and technical experts to develop consensus-based approaches. Their work focuses on creating standardized biocompatibility testing protocols and common terminology for wearable device classification, addressing a critical gap in current regulatory frameworks.
Despite progress, challenges remain in harmonizing regulations for novel wearable technologies that blur traditional device categories. Emerging technologies like smart textiles and temporary electronic tattoos often fall between regulatory definitions, highlighting the need for more flexible, technology-neutral frameworks. The International Coalition for Wearable Standards is currently developing adaptive regulatory models that can accommodate rapid technological evolution while maintaining appropriate oversight.
The International Medical Device Regulators Forum (IMDRF) has established working groups specifically focused on wearable technologies, developing guidance documents that address classification, risk assessment, and performance evaluation standards. These efforts aim to create a unified framework that can be adopted across multiple jurisdictions, reducing regulatory burden while maintaining safety standards.
The European Union and the United States have initiated bilateral agreements through the EU-US Mutual Recognition Agreement, which now includes provisions for wearable medical devices. This collaboration allows for shared inspection results and conformity assessments, streamlining the approval process for manufacturers targeting both markets. Similar arrangements are being negotiated between other major regulatory bodies, including Japan's PMDA and China's NMPA.
ISO has developed the ISO 13485:2016 standard for quality management systems in medical devices, which is increasingly being applied to wearable technologies. Complementing this, the IEC 60601 series provides electrical safety standards that are being harmonized globally. These technical standards serve as common reference points for regulators worldwide, facilitating mutual recognition of test results.
Regional harmonization efforts are also gaining momentum, particularly in Asia-Pacific through the ASEAN Medical Device Directive and in Latin America through the Pan American Network for Drug Regulatory Harmonization. These regional initiatives often serve as stepping stones toward broader international alignment, addressing local concerns while moving toward globally compatible frameworks.
The Global Harmonization Working Party for wearables, established in 2019, brings together industry stakeholders, regulatory bodies, and technical experts to develop consensus-based approaches. Their work focuses on creating standardized biocompatibility testing protocols and common terminology for wearable device classification, addressing a critical gap in current regulatory frameworks.
Despite progress, challenges remain in harmonizing regulations for novel wearable technologies that blur traditional device categories. Emerging technologies like smart textiles and temporary electronic tattoos often fall between regulatory definitions, highlighting the need for more flexible, technology-neutral frameworks. The International Coalition for Wearable Standards is currently developing adaptive regulatory models that can accommodate rapid technological evolution while maintaining appropriate oversight.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!