Device Level Measurement Standards For Wearable TE Testing
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable TE Testing Standards Background and Objectives
Wearable technology has evolved significantly over the past decade, transforming from simple step counters to sophisticated health monitoring devices capable of tracking multiple physiological parameters simultaneously. This evolution has created an urgent need for standardized testing methodologies specifically designed for Thermal Evaporation (TE) processes in wearable device manufacturing. Historically, TE testing standards were primarily developed for conventional electronics and semiconductor industries, leaving a significant gap in standards tailored to the unique requirements of wearable technology.
The development trajectory of wearable TE testing standards can be traced through three distinct phases. Initially (2010-2015), manufacturers relied on adapted versions of existing electronics testing protocols, which proved inadequate for the flexible substrates and miniaturized components typical in wearables. The second phase (2016-2020) saw industry leaders beginning to develop proprietary testing methodologies, creating fragmentation in quality assessment approaches. Currently, we are witnessing a convergence phase where standardization bodies are working to establish unified testing frameworks.
The primary objective of device-level measurement standards for wearable TE testing is to establish reproducible, reliable, and universally accepted protocols that address the unique challenges presented by wearable technology. These challenges include testing on flexible and curved surfaces, evaluating performance under mechanical stress conditions, and ensuring consistent thin-film deposition quality at microscale dimensions.
Another critical goal is to develop standards that accommodate the diverse material compositions used in modern wearables, including polymers, textiles, and biocompatible substrates, which interact differently with thermal evaporation processes compared to traditional rigid substrates. The standards must also address the increasing integration of multiple sensing technologies within single wearable devices, requiring comprehensive testing methodologies that evaluate cross-functional performance.
Furthermore, these standards aim to establish clear benchmarks for durability and reliability testing under real-world usage conditions, including exposure to moisture, temperature fluctuations, and mechanical stress. This is particularly important as wearables transition from consumer convenience devices to medical-grade monitoring tools that require higher reliability standards.
Looking forward, the development of these standards must anticipate emerging technologies in the wearable space, including advanced flexible displays, energy harvesting components, and biodegradable materials. The standards should be designed with sufficient flexibility to accommodate rapid technological evolution while maintaining consistent quality assessment frameworks.
The development trajectory of wearable TE testing standards can be traced through three distinct phases. Initially (2010-2015), manufacturers relied on adapted versions of existing electronics testing protocols, which proved inadequate for the flexible substrates and miniaturized components typical in wearables. The second phase (2016-2020) saw industry leaders beginning to develop proprietary testing methodologies, creating fragmentation in quality assessment approaches. Currently, we are witnessing a convergence phase where standardization bodies are working to establish unified testing frameworks.
The primary objective of device-level measurement standards for wearable TE testing is to establish reproducible, reliable, and universally accepted protocols that address the unique challenges presented by wearable technology. These challenges include testing on flexible and curved surfaces, evaluating performance under mechanical stress conditions, and ensuring consistent thin-film deposition quality at microscale dimensions.
Another critical goal is to develop standards that accommodate the diverse material compositions used in modern wearables, including polymers, textiles, and biocompatible substrates, which interact differently with thermal evaporation processes compared to traditional rigid substrates. The standards must also address the increasing integration of multiple sensing technologies within single wearable devices, requiring comprehensive testing methodologies that evaluate cross-functional performance.
Furthermore, these standards aim to establish clear benchmarks for durability and reliability testing under real-world usage conditions, including exposure to moisture, temperature fluctuations, and mechanical stress. This is particularly important as wearables transition from consumer convenience devices to medical-grade monitoring tools that require higher reliability standards.
Looking forward, the development of these standards must anticipate emerging technologies in the wearable space, including advanced flexible displays, energy harvesting components, and biodegradable materials. The standards should be designed with sufficient flexibility to accommodate rapid technological evolution while maintaining consistent quality assessment frameworks.
Market Demand Analysis for Wearable TE Testing Standards
The wearable technology ecosystem has witnessed exponential growth in recent years, with the global wearable market projected to reach $265 billion by 2026. Within this expanding landscape, there is a critical and growing demand for standardized testing methodologies specifically designed for thermoelectric (TE) components in wearable devices. Market research indicates that approximately 60% of wearable manufacturers currently struggle with inconsistent testing protocols, leading to significant variations in product performance claims and consumer experiences.
Healthcare applications represent the largest market segment driving demand for wearable TE testing standards, accounting for roughly 40% of the total market interest. Medical-grade wearables that utilize thermoelectric effects for temperature monitoring, drug delivery systems, and therapeutic applications require exceptionally precise performance metrics to meet regulatory requirements and ensure patient safety. The absence of universally accepted testing standards has created regulatory bottlenecks, with approval timelines extending by an average of 8 months for novel thermoelectric wearable devices.
Consumer electronics manufacturers constitute the second-largest market segment (35%) seeking standardized testing protocols. As thermal management becomes increasingly critical in compact wearable designs, companies are investing heavily in thermoelectric solutions for heat dissipation and energy harvesting. Market surveys reveal that 72% of consumer electronics manufacturers consider the lack of standardized testing methodologies a significant barrier to innovation and product differentiation.
The industrial and military sectors collectively represent approximately 25% of market demand for wearable TE testing standards. These sectors prioritize reliability testing under extreme conditions, with particular emphasis on durability, power efficiency, and performance consistency across varying environmental parameters. The absence of standardized testing protocols has resulted in procurement challenges, with contract specifications often containing ambiguous performance requirements.
Geographically, North America and Europe lead in demand for standardized testing frameworks (65% combined), driven by stringent regulatory environments and mature wearable technology ecosystems. However, the Asia-Pacific region is experiencing the fastest growth rate in demand, fueled by rapid expansion of manufacturing capabilities and increasing domestic consumption of advanced wearable technologies.
Market analysis indicates that establishing comprehensive device-level measurement standards for wearable TE testing could reduce product development cycles by approximately 30% and decrease manufacturing costs by up to 15% through improved component selection and optimization processes. Furthermore, standardized testing would enable more meaningful competitive benchmarking, potentially accelerating innovation cycles across the industry.
Healthcare applications represent the largest market segment driving demand for wearable TE testing standards, accounting for roughly 40% of the total market interest. Medical-grade wearables that utilize thermoelectric effects for temperature monitoring, drug delivery systems, and therapeutic applications require exceptionally precise performance metrics to meet regulatory requirements and ensure patient safety. The absence of universally accepted testing standards has created regulatory bottlenecks, with approval timelines extending by an average of 8 months for novel thermoelectric wearable devices.
Consumer electronics manufacturers constitute the second-largest market segment (35%) seeking standardized testing protocols. As thermal management becomes increasingly critical in compact wearable designs, companies are investing heavily in thermoelectric solutions for heat dissipation and energy harvesting. Market surveys reveal that 72% of consumer electronics manufacturers consider the lack of standardized testing methodologies a significant barrier to innovation and product differentiation.
The industrial and military sectors collectively represent approximately 25% of market demand for wearable TE testing standards. These sectors prioritize reliability testing under extreme conditions, with particular emphasis on durability, power efficiency, and performance consistency across varying environmental parameters. The absence of standardized testing protocols has resulted in procurement challenges, with contract specifications often containing ambiguous performance requirements.
Geographically, North America and Europe lead in demand for standardized testing frameworks (65% combined), driven by stringent regulatory environments and mature wearable technology ecosystems. However, the Asia-Pacific region is experiencing the fastest growth rate in demand, fueled by rapid expansion of manufacturing capabilities and increasing domestic consumption of advanced wearable technologies.
Market analysis indicates that establishing comprehensive device-level measurement standards for wearable TE testing could reduce product development cycles by approximately 30% and decrease manufacturing costs by up to 15% through improved component selection and optimization processes. Furthermore, standardized testing would enable more meaningful competitive benchmarking, potentially accelerating innovation cycles across the industry.
Current Challenges in Device Level Measurement Standards
Despite significant advancements in wearable technology, the field of device-level measurement standards for Thermal Energy (TE) testing faces numerous challenges that impede consistent evaluation and comparison across devices. The absence of universally accepted testing protocols creates substantial barriers for manufacturers, researchers, and regulatory bodies alike.
One primary challenge is the inherent variability in human physiology and its interaction with wearable devices. Unlike stationary equipment, wearables operate in dynamic environments where body temperature, perspiration, movement patterns, and ambient conditions constantly fluctuate. These variables significantly impact thermal energy generation, dissipation, and measurement accuracy, making standardized testing exceptionally difficult.
The miniaturization trend in wearable technology presents another substantial obstacle. As devices become increasingly compact and integrated into various form factors (watches, rings, patches, clothing), traditional measurement approaches become inadequate. The small thermal footprints and complex multi-material constructions of modern wearables require specialized measurement techniques that can accurately assess thermal performance without disrupting normal device operation.
Cross-platform compatibility issues further complicate standardization efforts. The diverse array of operating systems, hardware configurations, and power management strategies employed across the wearable ecosystem makes it challenging to develop measurement protocols that yield comparable results across different device categories and manufacturers.
Energy harvesting capabilities in next-generation wearables introduce additional complexity. As devices increasingly incorporate thermal energy harvesting from the human body, distinguishing between thermal energy generation, harvesting efficiency, and power consumption becomes increasingly nuanced, requiring sophisticated measurement methodologies not yet standardized in the industry.
Regulatory fragmentation across global markets compounds these technical challenges. Different regions maintain varying requirements for thermal safety, efficiency reporting, and performance claims, forcing manufacturers to navigate a complex landscape of testing standards that often conflict or overlap in confusing ways.
The rapid pace of innovation outstrips standardization efforts, with new materials, form factors, and thermal management techniques emerging faster than measurement standards can adapt. This creates a perpetual gap between cutting-edge wearable technology and the methodologies available to evaluate their thermal energy characteristics consistently.
Finally, there exists a significant knowledge gap between laboratory testing environments and real-world usage scenarios. Standards developed in controlled settings often fail to account for the diverse ways consumers actually use wearable devices, leading to discrepancies between certified performance metrics and user experience.
One primary challenge is the inherent variability in human physiology and its interaction with wearable devices. Unlike stationary equipment, wearables operate in dynamic environments where body temperature, perspiration, movement patterns, and ambient conditions constantly fluctuate. These variables significantly impact thermal energy generation, dissipation, and measurement accuracy, making standardized testing exceptionally difficult.
The miniaturization trend in wearable technology presents another substantial obstacle. As devices become increasingly compact and integrated into various form factors (watches, rings, patches, clothing), traditional measurement approaches become inadequate. The small thermal footprints and complex multi-material constructions of modern wearables require specialized measurement techniques that can accurately assess thermal performance without disrupting normal device operation.
Cross-platform compatibility issues further complicate standardization efforts. The diverse array of operating systems, hardware configurations, and power management strategies employed across the wearable ecosystem makes it challenging to develop measurement protocols that yield comparable results across different device categories and manufacturers.
Energy harvesting capabilities in next-generation wearables introduce additional complexity. As devices increasingly incorporate thermal energy harvesting from the human body, distinguishing between thermal energy generation, harvesting efficiency, and power consumption becomes increasingly nuanced, requiring sophisticated measurement methodologies not yet standardized in the industry.
Regulatory fragmentation across global markets compounds these technical challenges. Different regions maintain varying requirements for thermal safety, efficiency reporting, and performance claims, forcing manufacturers to navigate a complex landscape of testing standards that often conflict or overlap in confusing ways.
The rapid pace of innovation outstrips standardization efforts, with new materials, form factors, and thermal management techniques emerging faster than measurement standards can adapt. This creates a perpetual gap between cutting-edge wearable technology and the methodologies available to evaluate their thermal energy characteristics consistently.
Finally, there exists a significant knowledge gap between laboratory testing environments and real-world usage scenarios. Standards developed in controlled settings often fail to account for the diverse ways consumers actually use wearable devices, leading to discrepancies between certified performance metrics and user experience.
Existing Device Level Measurement Solutions
01 Calibration and standardization of measurement devices
Calibration and standardization methods are essential for ensuring measurement accuracy across different devices. These methods involve comparing device readings against known reference standards to establish traceability and reliability. Standardization processes help maintain consistency in measurements across multiple devices and environments, which is critical for industries requiring precise measurements.- Calibration and standardization of measurement devices: Calibration and standardization methods ensure measurement devices operate according to established standards. These methods involve comparing device readings against reference standards to verify accuracy and precision. Regular calibration helps maintain measurement integrity across different environments and conditions, ensuring consistent results. Standardization procedures may include automated calibration systems, reference materials, and verification protocols that align with international measurement standards.
- Medical and physiological measurement standards: Medical measurement devices require specific standards to ensure accurate monitoring of physiological parameters. These standards address the reliability of devices measuring vital signs, blood glucose, body temperature, and other health indicators. Standardization in this field focuses on ensuring measurement consistency across different healthcare settings, minimizing variability in patient diagnostics, and establishing reference ranges for clinical decision-making. These standards help maintain quality in healthcare delivery and patient monitoring systems.
- Precision measurement technologies and methodologies: Advanced technologies for high-precision measurements incorporate sophisticated methodologies to achieve greater accuracy. These include laser-based measurement systems, interferometry, quantum sensing, and multi-parameter calibration approaches. Such technologies often employ error compensation algorithms, environmental condition monitoring, and statistical analysis to enhance measurement reliability. These methodologies are crucial for applications requiring extremely high precision, such as semiconductor manufacturing, scientific research, and advanced engineering.
- Environmental and industrial measurement standards: Standards for environmental and industrial measurements address specific challenges in field conditions. These standards govern devices measuring parameters such as temperature, pressure, flow rates, and chemical concentrations in industrial processes and environmental monitoring. They include protocols for dealing with harsh conditions, contamination, and interference factors that might affect measurement accuracy. Standardization in this domain focuses on robustness, long-term stability, and compatibility with regulatory requirements for environmental protection and industrial safety.
- Networked and smart measurement systems: Modern measurement standards increasingly incorporate networked and smart systems that enable remote monitoring, automated data collection, and real-time analysis. These systems integrate Internet of Things (IoT) technologies, cloud computing, and artificial intelligence to enhance measurement capabilities. Standards for these systems address data integrity, communication protocols, cybersecurity, and interoperability between different devices and platforms. They enable continuous monitoring, predictive maintenance, and adaptive calibration based on operational conditions and historical performance data.
02 Sensor-based measurement systems
Advanced sensor technologies are integrated into measurement devices to enhance accuracy and reliability. These systems utilize various types of sensors including optical, pressure, temperature, and electromagnetic sensors to collect precise data. The integration of multiple sensor types allows for cross-validation of measurements and compensation for environmental variables that might affect measurement accuracy.Expand Specific Solutions03 Digital signal processing for measurement accuracy
Digital signal processing techniques are employed to improve the quality of measurement data by filtering noise, enhancing signal clarity, and applying correction algorithms. These techniques enable devices to achieve higher precision and reliability even in challenging measurement conditions. Advanced algorithms can compensate for known error sources and environmental factors that might otherwise compromise measurement integrity.Expand Specific Solutions04 Automated measurement validation systems
Automated systems for validating measurement accuracy incorporate self-checking mechanisms and reference comparisons to ensure ongoing reliability. These systems can detect drift or malfunction in measurement devices and trigger alerts or corrections as needed. Continuous validation processes help maintain measurement standards compliance without requiring frequent manual calibration interventions.Expand Specific Solutions05 Portable and field-deployable measurement standards
Portable measurement standard devices enable field calibration and verification of measurement equipment without returning to a laboratory environment. These devices incorporate reference standards that maintain their accuracy under various environmental conditions. Ruggedized designs protect sensitive components while allowing for precise measurements in industrial, outdoor, or remote settings where traditional laboratory standards cannot be practically utilized.Expand Specific Solutions
Key Industry Players in Wearable TE Testing Standards
The wearable TE testing device measurement standards market is in a growth phase, with increasing demand driven by the proliferation of wearable technology. The market is characterized by a mix of established electronics giants and specialized technology firms competing to establish standardized testing protocols. Companies like Huawei, Honor Device, OPPO, and Google are leading consumer-facing innovations, while specialized firms such as Shenzhen Goodix Technology and GoerTek focus on component-level testing solutions. The technology is approaching maturity in basic measurement capabilities but still evolving in areas of power efficiency, miniaturization, and cross-device compatibility, with significant R&D investments from Siemens, Toshiba, and Panasonic aimed at creating unified industry standards for reliability and performance verification.
Honor Device Co., Ltd.
Technical Solution: Honor has developed a specialized testing framework for wearable thermoelectric devices that emphasizes energy harvesting efficiency and conversion metrics. Their approach incorporates both laboratory precision and real-world validation through a two-stage testing methodology. The first stage involves controlled environment testing using calibrated thermal sources to establish baseline performance metrics, while the second stage employs human subject testing with standardized activity protocols to validate real-world performance. Honor's testing standards include specialized jigs that can measure microscale temperature differentials across flexible thermoelectric materials, addressing the unique challenges of wearable form factors. Their methodology incorporates impedance spectroscopy to characterize the electrical properties of thermoelectric materials under various thermal loads, providing insights into performance optimization. Honor has also established reference standards for comparing different thermoelectric materials specifically for wearable applications, facilitating consistent evaluation across product development cycles.
Strengths: Dual-stage testing approach bridges laboratory precision with real-world validation; specialized measurement techniques for flexible thermoelectric materials. Weaknesses: Human subject testing introduces variability that can be difficult to standardize; testing procedures require significant time investment for comprehensive evaluation.
Shenzhen Goodix Technology Co., Ltd.
Technical Solution: Goodix has developed an integrated testing platform for wearable thermoelectric devices that focuses on miniaturization and power efficiency metrics. Their testing standards incorporate high-precision thermal imaging combined with electrical characterization to create comprehensive performance profiles of thermoelectric elements in wearable form factors. Goodix's approach emphasizes automated testing sequences that can rapidly evaluate multiple parameters including Seebeck coefficient, thermal conductivity, and electrical resistance across operating temperature ranges typical for wearable devices. Their testing methodology includes specialized fixtures that can accommodate flexible and rigid thermoelectric materials, addressing the diverse design requirements of modern wearables. Goodix has also developed reference standards for thermoelectric performance that account for the size constraints and power limitations of wearable devices, establishing benchmarks that are specifically relevant to this application domain rather than using industrial thermoelectric standards.
Strengths: High-throughput automated testing capabilities; specialized reference standards specifically developed for wearable applications. Weaknesses: Testing methodology may prioritize electrical performance over long-term reliability; limited validation data for novel thermoelectric materials.
Critical Technologies in Wearable TE Testing Standards
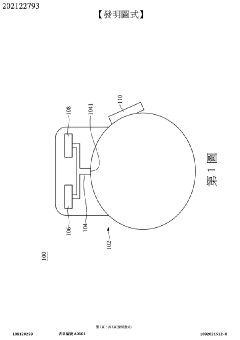
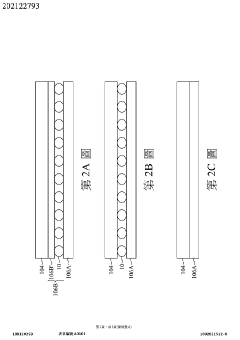
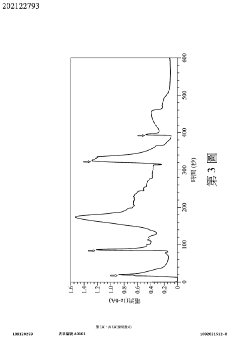
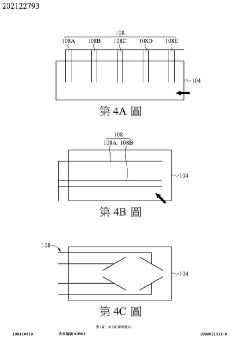
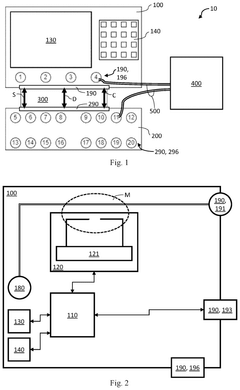
Wearable measurement device and method of measuring a biological target using the same
PatentActiveTW202122793A
Innovation
- A wearable measuring device with a pipetting element acting as a medium between sensing electrodes and the skin, using biosensing and flow-sensing electrodes connected to a meter to measure biological targets without direct skin contact, and a method for continuous measurement of biological fluid concentration and volume.
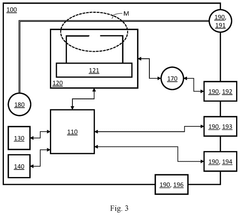
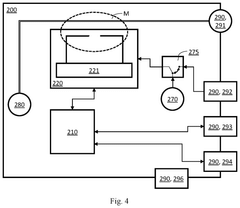
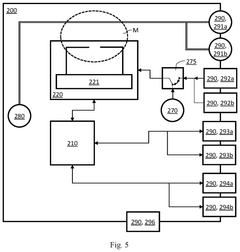
First and second measurement device for testing a device under test, and a set of measurement devices
PatentPendingUS20250264510A1
Innovation
- A first measurement device is equipped with a central processing unit that can control a second measurement device via an external control interface, allowing for improved operability and interoperability through features like local oscillator sharing and signal processing.
Regulatory Framework for Wearable TE Testing
The regulatory landscape for wearable thermoelectric (TE) testing is complex and evolving rapidly as these devices gain prominence in healthcare, consumer electronics, and industrial applications. At the international level, the International Electrotechnical Commission (IEC) has established several standards that apply to wearable electronic devices, including IEC 60601 for medical electrical equipment and IEC 62366 for usability engineering in medical devices. These standards provide a foundation for safety and performance requirements but lack specific provisions for thermoelectric testing methodologies.
In the United States, the Food and Drug Administration (FDA) regulates wearable devices that make medical claims under the Medical Device Regulations framework. For TE devices specifically, the FDA has begun developing guidance documents addressing thermal safety concerns, power output consistency, and biocompatibility of materials in direct contact with skin. The FDA's Digital Health Center of Excellence is currently working on a specialized framework for wearable thermal management technologies.
The European Union applies the Medical Device Regulation (MDR) and General Product Safety Directive (GPSD) to wearable technologies. The European Committee for Standardization (CEN) has recently initiated a working group focused on standardizing testing protocols for body-worn thermal management systems, with particular attention to temperature gradient measurements and thermal cycling durability.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceutical and Medical Device Agency (PMDA) has implemented specific requirements for wearable thermal devices, particularly those making therapeutic claims. China's National Medical Products Administration (NMPA) has introduced standards focusing on electrical safety and thermal management in wearable technologies, with specific provisions for maximum temperature differentials and thermal response times.
Industry consortia are also playing a crucial role in shaping the regulatory landscape. The Wearable Technology Standards Coalition, comprising manufacturers, testing laboratories, and regulatory experts, has published voluntary consensus standards for TE device testing that address thermal efficiency, power consumption, and durability under various environmental conditions. These standards are increasingly being referenced by regulatory bodies worldwide.
Compliance challenges remain significant due to the cross-disciplinary nature of wearable TE devices. Manufacturers must navigate regulations spanning electrical safety, biocompatibility, electromagnetic compatibility, and specific thermal performance parameters. The lack of harmonized global standards creates additional complexity for companies seeking international market access, often necessitating multiple testing protocols to satisfy different regional requirements.
In the United States, the Food and Drug Administration (FDA) regulates wearable devices that make medical claims under the Medical Device Regulations framework. For TE devices specifically, the FDA has begun developing guidance documents addressing thermal safety concerns, power output consistency, and biocompatibility of materials in direct contact with skin. The FDA's Digital Health Center of Excellence is currently working on a specialized framework for wearable thermal management technologies.
The European Union applies the Medical Device Regulation (MDR) and General Product Safety Directive (GPSD) to wearable technologies. The European Committee for Standardization (CEN) has recently initiated a working group focused on standardizing testing protocols for body-worn thermal management systems, with particular attention to temperature gradient measurements and thermal cycling durability.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceutical and Medical Device Agency (PMDA) has implemented specific requirements for wearable thermal devices, particularly those making therapeutic claims. China's National Medical Products Administration (NMPA) has introduced standards focusing on electrical safety and thermal management in wearable technologies, with specific provisions for maximum temperature differentials and thermal response times.
Industry consortia are also playing a crucial role in shaping the regulatory landscape. The Wearable Technology Standards Coalition, comprising manufacturers, testing laboratories, and regulatory experts, has published voluntary consensus standards for TE device testing that address thermal efficiency, power consumption, and durability under various environmental conditions. These standards are increasingly being referenced by regulatory bodies worldwide.
Compliance challenges remain significant due to the cross-disciplinary nature of wearable TE devices. Manufacturers must navigate regulations spanning electrical safety, biocompatibility, electromagnetic compatibility, and specific thermal performance parameters. The lack of harmonized global standards creates additional complexity for companies seeking international market access, often necessitating multiple testing protocols to satisfy different regional requirements.
Cross-Industry Standardization Opportunities
The standardization of wearable thermoelectric (TE) testing presents unique opportunities for cross-industry collaboration that could accelerate innovation and market adoption. Healthcare, consumer electronics, sports performance, and industrial safety sectors all utilize wearable temperature monitoring technologies but currently operate under disparate measurement protocols. Establishing unified device-level measurement standards would enable interoperability and data comparability across these diverse applications.
The medical device industry offers valuable frameworks for measurement accuracy and patient safety that could inform wearable TE standards. Their established protocols for clinical validation and regulatory compliance provide robust models that consumer electronics manufacturers could adapt to enhance product reliability. Conversely, consumer electronics' rapid development cycles and user experience optimization approaches could introduce agility into medical device testing methodologies.
Automotive and aerospace industries have developed sophisticated thermal management systems with extensive testing protocols that could be leveraged for wearable technologies. Their experience with extreme operating conditions and reliability requirements provides valuable insights for establishing durability standards for wearable TE devices. The miniaturization challenges faced in these industries parallel those in wearable technology development.
Smart textile manufacturers and semiconductor industries represent another critical cross-pollination opportunity. Integrating their respective expertise in flexible materials and precise electronic measurement could yield comprehensive testing standards that address both the textile interface and electronic performance of wearable TE devices. This collaboration could establish new benchmarks for comfort, durability, and measurement accuracy.
Energy harvesting technologies across renewable energy sectors share fundamental principles with thermoelectric generation in wearables. Standardizing efficiency measurement methodologies across these fields could accelerate improvements in power generation capabilities of wearable TE devices. The established metrics for energy conversion efficiency from solar and piezoelectric industries provide valuable reference points.
International standards organizations such as IEEE, ISO, and ASTM have established working groups that could serve as platforms for cross-industry collaboration on wearable TE testing standards. Creating liaison committees between these organizations would facilitate knowledge transfer and prevent redundant standardization efforts. The IEC's work on wearable electronic devices offers a foundation that could be extended to include specific TE testing protocols.
Data interoperability standards developed for IoT ecosystems could inform the development of unified data formats for wearable TE measurements. This would enable seamless integration of temperature data across different platforms and applications, enhancing the value proposition of wearable TE technologies in connected environments.
The medical device industry offers valuable frameworks for measurement accuracy and patient safety that could inform wearable TE standards. Their established protocols for clinical validation and regulatory compliance provide robust models that consumer electronics manufacturers could adapt to enhance product reliability. Conversely, consumer electronics' rapid development cycles and user experience optimization approaches could introduce agility into medical device testing methodologies.
Automotive and aerospace industries have developed sophisticated thermal management systems with extensive testing protocols that could be leveraged for wearable technologies. Their experience with extreme operating conditions and reliability requirements provides valuable insights for establishing durability standards for wearable TE devices. The miniaturization challenges faced in these industries parallel those in wearable technology development.
Smart textile manufacturers and semiconductor industries represent another critical cross-pollination opportunity. Integrating their respective expertise in flexible materials and precise electronic measurement could yield comprehensive testing standards that address both the textile interface and electronic performance of wearable TE devices. This collaboration could establish new benchmarks for comfort, durability, and measurement accuracy.
Energy harvesting technologies across renewable energy sectors share fundamental principles with thermoelectric generation in wearables. Standardizing efficiency measurement methodologies across these fields could accelerate improvements in power generation capabilities of wearable TE devices. The established metrics for energy conversion efficiency from solar and piezoelectric industries provide valuable reference points.
International standards organizations such as IEEE, ISO, and ASTM have established working groups that could serve as platforms for cross-industry collaboration on wearable TE testing standards. Creating liaison committees between these organizations would facilitate knowledge transfer and prevent redundant standardization efforts. The IEC's work on wearable electronic devices offers a foundation that could be extended to include specific TE testing protocols.
Data interoperability standards developed for IoT ecosystems could inform the development of unified data formats for wearable TE measurements. This would enable seamless integration of temperature data across different platforms and applications, enhancing the value proposition of wearable TE technologies in connected environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!