Field Trials Of Wearable TE Modules In Health Applications
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermoelectric Wearables Background and Objectives
Thermoelectric (TE) wearable technology represents a significant advancement in the intersection of materials science, energy harvesting, and healthcare applications. The evolution of this technology can be traced back to the discovery of the Seebeck effect in 1821, which established that temperature differences between two dissimilar electrical conductors can produce voltage. Over the past two decades, there has been accelerated development in miniaturizing and optimizing thermoelectric materials for wearable applications, particularly in healthcare monitoring and therapeutic devices.
The technological trajectory of TE wearables has been shaped by advancements in flexible electronics, nanomaterials, and energy-efficient design. Early iterations faced significant challenges in terms of efficiency, flexibility, and biocompatibility. However, recent breakthroughs in materials such as bismuth telluride (Bi₂Te₃), organic semiconductors, and hybrid organic-inorganic composites have dramatically improved performance metrics while reducing form factors.
Current field trials of wearable TE modules in health applications are exploring multiple use cases, including continuous temperature monitoring, energy harvesting from body heat to power medical sensors, and therapeutic applications utilizing the Peltier effect for localized heating or cooling. These applications hold particular promise for chronic disease management, sports medicine, and remote patient monitoring in resource-limited settings.
The primary technical objectives for advancing wearable TE modules in healthcare include improving energy conversion efficiency beyond the current 5-7% range, enhancing mechanical flexibility and durability to withstand daily wear conditions, and developing biocompatible interfaces that minimize skin irritation during prolonged use. Additionally, there is a focus on reducing manufacturing costs to enable broader adoption across diverse healthcare settings.
From a clinical perspective, objectives include validating the accuracy and reliability of TE-based health monitoring systems against gold standard medical devices, establishing protocols for data interpretation, and determining optimal placement locations on the body for different health applications. Long-term objectives involve integration with telemedicine platforms and electronic health records to create comprehensive health monitoring ecosystems.
The convergence of miniaturization techniques, advanced materials, and healthcare needs is driving innovation in this field. As global healthcare systems increasingly emphasize preventive care and remote monitoring, wearable TE technology is positioned to become a critical component of next-generation medical devices. The field trials currently underway represent a crucial step in bridging laboratory research with practical clinical applications, potentially transforming how we approach continuous health monitoring and personalized medicine.
The technological trajectory of TE wearables has been shaped by advancements in flexible electronics, nanomaterials, and energy-efficient design. Early iterations faced significant challenges in terms of efficiency, flexibility, and biocompatibility. However, recent breakthroughs in materials such as bismuth telluride (Bi₂Te₃), organic semiconductors, and hybrid organic-inorganic composites have dramatically improved performance metrics while reducing form factors.
Current field trials of wearable TE modules in health applications are exploring multiple use cases, including continuous temperature monitoring, energy harvesting from body heat to power medical sensors, and therapeutic applications utilizing the Peltier effect for localized heating or cooling. These applications hold particular promise for chronic disease management, sports medicine, and remote patient monitoring in resource-limited settings.
The primary technical objectives for advancing wearable TE modules in healthcare include improving energy conversion efficiency beyond the current 5-7% range, enhancing mechanical flexibility and durability to withstand daily wear conditions, and developing biocompatible interfaces that minimize skin irritation during prolonged use. Additionally, there is a focus on reducing manufacturing costs to enable broader adoption across diverse healthcare settings.
From a clinical perspective, objectives include validating the accuracy and reliability of TE-based health monitoring systems against gold standard medical devices, establishing protocols for data interpretation, and determining optimal placement locations on the body for different health applications. Long-term objectives involve integration with telemedicine platforms and electronic health records to create comprehensive health monitoring ecosystems.
The convergence of miniaturization techniques, advanced materials, and healthcare needs is driving innovation in this field. As global healthcare systems increasingly emphasize preventive care and remote monitoring, wearable TE technology is positioned to become a critical component of next-generation medical devices. The field trials currently underway represent a crucial step in bridging laboratory research with practical clinical applications, potentially transforming how we approach continuous health monitoring and personalized medicine.
Market Analysis for Health Monitoring Wearables
The global market for health monitoring wearables has experienced exponential growth, with the wearable thermoelectric (TE) module segment emerging as a particularly promising area. Current market valuations place the health wearables sector at approximately 25 billion USD in 2023, with projections indicating a compound annual growth rate of 14-16% through 2028. Thermoelectric modules specifically designed for health applications represent a specialized but rapidly expanding subsector within this market.
Consumer demand for non-invasive, continuous health monitoring solutions has created significant market opportunities for wearable TE technology. Primary market drivers include the aging global population, increasing prevalence of chronic conditions requiring regular monitoring, and growing consumer interest in preventative healthcare and wellness optimization. The COVID-19 pandemic has further accelerated adoption rates as remote patient monitoring became essential during healthcare system constraints.
Market segmentation reveals distinct consumer groups: medical patients requiring consistent vital sign monitoring, fitness enthusiasts tracking performance metrics, elderly populations benefiting from fall detection and emergency response features, and wellness-focused consumers interested in sleep quality and stress management. Each segment presents unique requirements for wearable TE module implementation and distinct monetization opportunities.
Regional analysis indicates North America currently leads market share at approximately 40%, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region demonstrates the highest growth potential due to increasing healthcare expenditure, technological adoption rates, and manufacturing capabilities particularly in China, South Korea, and Japan.
Pricing trends show premium positioning for medical-grade wearable TE devices, while consumer-oriented products face increasing price pressure due to market competition. The average selling price for specialized health monitoring wearables incorporating TE technology ranges between 150-300 USD, with subscription-based service models emerging as complementary revenue streams.
Distribution channels have evolved significantly, with direct-to-consumer online sales growing fastest, followed by healthcare provider partnerships and traditional retail. Insurance reimbursement models are gradually expanding to include select wearable monitoring devices, creating additional market access opportunities.
Key market challenges include regulatory compliance requirements, data privacy concerns, battery life limitations, and consumer retention issues. Despite these challenges, market forecasts remain highly positive, with wearable TE modules for health applications expected to capture increasing market share as technological advances improve accuracy, comfort, and integration capabilities with healthcare systems.
Consumer demand for non-invasive, continuous health monitoring solutions has created significant market opportunities for wearable TE technology. Primary market drivers include the aging global population, increasing prevalence of chronic conditions requiring regular monitoring, and growing consumer interest in preventative healthcare and wellness optimization. The COVID-19 pandemic has further accelerated adoption rates as remote patient monitoring became essential during healthcare system constraints.
Market segmentation reveals distinct consumer groups: medical patients requiring consistent vital sign monitoring, fitness enthusiasts tracking performance metrics, elderly populations benefiting from fall detection and emergency response features, and wellness-focused consumers interested in sleep quality and stress management. Each segment presents unique requirements for wearable TE module implementation and distinct monetization opportunities.
Regional analysis indicates North America currently leads market share at approximately 40%, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region demonstrates the highest growth potential due to increasing healthcare expenditure, technological adoption rates, and manufacturing capabilities particularly in China, South Korea, and Japan.
Pricing trends show premium positioning for medical-grade wearable TE devices, while consumer-oriented products face increasing price pressure due to market competition. The average selling price for specialized health monitoring wearables incorporating TE technology ranges between 150-300 USD, with subscription-based service models emerging as complementary revenue streams.
Distribution channels have evolved significantly, with direct-to-consumer online sales growing fastest, followed by healthcare provider partnerships and traditional retail. Insurance reimbursement models are gradually expanding to include select wearable monitoring devices, creating additional market access opportunities.
Key market challenges include regulatory compliance requirements, data privacy concerns, battery life limitations, and consumer retention issues. Despite these challenges, market forecasts remain highly positive, with wearable TE modules for health applications expected to capture increasing market share as technological advances improve accuracy, comfort, and integration capabilities with healthcare systems.
Current TE Module Technology Landscape and Barriers
The current landscape of thermoelectric (TE) modules for wearable health applications reveals significant technological advancements alongside persistent challenges. Commercially available TE modules predominantly utilize bismuth telluride (Bi2Te3) based materials, which offer a reasonable figure of merit (ZT) value of approximately 1 at room temperature. However, these conventional rigid modules face substantial limitations when adapted for wearable health monitoring, including insufficient flexibility, limited power density, and heat dissipation issues when in contact with human skin.
Recent innovations have introduced flexible TE modules incorporating organic materials and polymer composites. These modules can conform to body contours and withstand repeated mechanical deformation, addressing a critical requirement for wearable applications. Notable developments include PEDOT:PSS-based flexible modules and hybrid organic-inorganic composites that maintain functionality during bending and stretching operations. Despite these advances, the power conversion efficiency of flexible modules remains significantly lower than their rigid counterparts, typically achieving ZT values below 0.3.
Miniaturization represents another crucial advancement, with micro-fabricated TE modules now reaching dimensions below 5mm thickness. These compact designs facilitate integration into various wearable form factors such as wristbands, patches, and smart textiles. Companies like Matrix Industries and Perpetua Power have pioneered commercially viable micro-TE modules specifically designed for body heat harvesting, though their power output typically remains in the microwatt to low milliwatt range.
The integration of TE modules with wireless communication systems and low-power electronics has enabled practical health monitoring applications. However, significant barriers persist in achieving reliable long-term operation. Thermal contact resistance between the module and skin surface causes substantial energy losses, while environmental factors such as ambient temperature fluctuations and humidity affect performance consistency. Additionally, biocompatibility concerns arise from the potential toxicity of certain thermoelectric materials, necessitating effective encapsulation strategies.
Manufacturing scalability presents another major challenge, as current production methods for flexible and miniaturized TE modules often involve complex, multi-step processes with low yields. The cost-performance ratio remains unfavorable compared to alternative power sources, with typical production costs exceeding $10 per square centimeter of active area. This economic barrier has limited widespread adoption despite the technology's potential advantages for continuous, battery-free health monitoring.
Thermal management represents perhaps the most fundamental technical barrier, as the small temperature differential available from body heat (typically 1-5°C) severely constrains power generation capacity. Current solutions involving heat sinks and thermal spreaders add bulk and rigidity, contradicting the wearability requirements. Innovative approaches using phase change materials and microfluidic cooling show promise but remain in early research stages.
Recent innovations have introduced flexible TE modules incorporating organic materials and polymer composites. These modules can conform to body contours and withstand repeated mechanical deformation, addressing a critical requirement for wearable applications. Notable developments include PEDOT:PSS-based flexible modules and hybrid organic-inorganic composites that maintain functionality during bending and stretching operations. Despite these advances, the power conversion efficiency of flexible modules remains significantly lower than their rigid counterparts, typically achieving ZT values below 0.3.
Miniaturization represents another crucial advancement, with micro-fabricated TE modules now reaching dimensions below 5mm thickness. These compact designs facilitate integration into various wearable form factors such as wristbands, patches, and smart textiles. Companies like Matrix Industries and Perpetua Power have pioneered commercially viable micro-TE modules specifically designed for body heat harvesting, though their power output typically remains in the microwatt to low milliwatt range.
The integration of TE modules with wireless communication systems and low-power electronics has enabled practical health monitoring applications. However, significant barriers persist in achieving reliable long-term operation. Thermal contact resistance between the module and skin surface causes substantial energy losses, while environmental factors such as ambient temperature fluctuations and humidity affect performance consistency. Additionally, biocompatibility concerns arise from the potential toxicity of certain thermoelectric materials, necessitating effective encapsulation strategies.
Manufacturing scalability presents another major challenge, as current production methods for flexible and miniaturized TE modules often involve complex, multi-step processes with low yields. The cost-performance ratio remains unfavorable compared to alternative power sources, with typical production costs exceeding $10 per square centimeter of active area. This economic barrier has limited widespread adoption despite the technology's potential advantages for continuous, battery-free health monitoring.
Thermal management represents perhaps the most fundamental technical barrier, as the small temperature differential available from body heat (typically 1-5°C) severely constrains power generation capacity. Current solutions involving heat sinks and thermal spreaders add bulk and rigidity, contradicting the wearability requirements. Innovative approaches using phase change materials and microfluidic cooling show promise but remain in early research stages.
Existing Field Trial Methodologies and Solutions
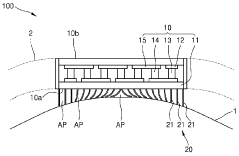
01 Wearable thermoelectric energy harvesting devices
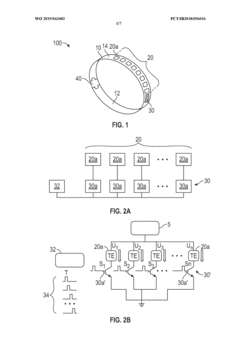
Wearable thermoelectric (TE) modules can be designed to harvest energy from body heat. These devices convert the temperature difference between the human body and the ambient environment into electrical energy. The harvested energy can power various wearable electronics, sensors, and monitoring devices, reducing the need for traditional batteries and enabling longer operation times for wearable technology.- Wearable thermoelectric energy harvesting devices: Wearable thermoelectric (TE) modules can be designed to harvest energy from body heat and convert it into electrical power. These devices utilize the temperature difference between the human body and the ambient environment to generate electricity through the Seebeck effect. The harvested energy can power various wearable electronics, sensors, and health monitoring devices, reducing the need for traditional batteries and enabling longer operation times for wearable technology.
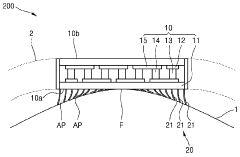
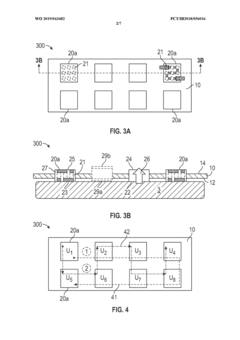
- Flexible and conformable TE module designs: Advanced designs for thermoelectric modules focus on flexibility and conformability to adapt to the human body's contours. These designs incorporate flexible substrates, stretchable interconnects, and novel manufacturing techniques to create comfortable wearable devices that maintain good thermal contact with the skin. The improved form factor enhances both user comfort and energy harvesting efficiency by maximizing the thermal interface between the device and the body.
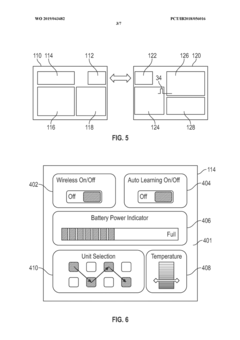
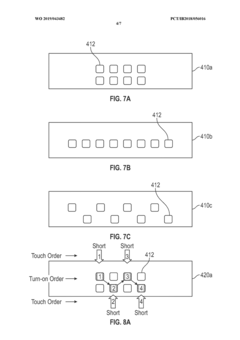
- Integration with smart wearable systems: Thermoelectric modules can be integrated into comprehensive smart wearable systems that combine energy harvesting with sensing, data processing, and wireless communication capabilities. These integrated systems enable continuous health monitoring, activity tracking, and environmental sensing without requiring frequent charging. The integration often involves miniaturized components, low-power electronics, and efficient power management circuits to optimize the use of harvested energy.
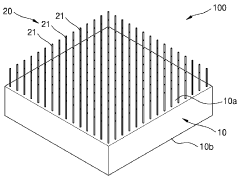
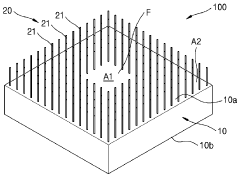
- Enhanced TE materials and fabrication techniques: Advanced materials and fabrication techniques are being developed to improve the efficiency and performance of wearable thermoelectric modules. These include nanostructured materials, organic thermoelectric compounds, and hybrid organic-inorganic composites that offer better power conversion efficiency at the temperature differentials typical of body-ambient interfaces. Novel manufacturing methods such as printing, roll-to-roll processing, and microfabrication enable cost-effective production of these specialized materials in wearable form factors.
- Power management and energy storage solutions: Effective power management systems are crucial for wearable thermoelectric modules to handle the variable and often low-level energy output. These systems include specialized circuits for voltage regulation, power conditioning, and efficient energy storage solutions such as supercapacitors or thin-film batteries. Advanced power management techniques enable the system to operate continuously even when the temperature differential fluctuates, ensuring reliable performance of the wearable device under various conditions.
02 Flexible and conformable TE module designs
Flexible thermoelectric modules are designed to conform to the contours of the human body, enhancing comfort and thermal contact. These modules utilize specialized materials and manufacturing techniques to maintain electrical performance while allowing bending and stretching. The flexibility improves user experience and enables integration into various wearable form factors such as clothing, wristbands, and medical patches.Expand Specific Solutions03 Integration of TE modules with smart wearables
Thermoelectric modules can be integrated with smart wearable devices such as watches, fitness trackers, and health monitoring systems. This integration enables self-powered operation of these devices by utilizing body heat. The systems often include power management circuits to regulate the harvested energy and may incorporate wireless communication capabilities for data transmission and remote monitoring applications.Expand Specific Solutions04 Miniaturized TE modules for wearable applications
Miniaturized thermoelectric modules are specifically designed for wearable applications where space and weight constraints are critical. These compact modules utilize advanced manufacturing techniques such as thin-film deposition and microfabrication to achieve high power density in small form factors. The miniaturization enables integration into everyday wearable items without compromising user comfort or device aesthetics.Expand Specific Solutions05 Thermal management systems for wearable TE modules
Effective thermal management is crucial for optimizing the performance of wearable thermoelectric modules. These systems include heat spreaders, thermal interface materials, and innovative heat sink designs that maximize the temperature gradient across the thermoelectric elements while maintaining user comfort. Advanced thermal management approaches enable higher energy conversion efficiency and improved power output from body heat harvesting applications.Expand Specific Solutions
Leading Companies in Wearable TE Health Applications
The field of wearable thermoelectric (TE) modules for health applications is in an early growth phase, with market size expanding as healthcare monitoring shifts toward continuous, non-invasive solutions. The competitive landscape features established medical device companies like Medtronic and ZOLL Medical alongside specialized wearable health technology firms such as VitalConnect and ChroniSense Medical. Technical maturity varies significantly, with companies like Thync Global focusing on neuro-health wearables, while research institutions including Nanyang Technological University and University of California contribute foundational innovations. Chinese entities like BOE Technology and Huawei are leveraging their manufacturing capabilities to enter this space, indicating the global nature of competition in this emerging field that bridges healthcare monitoring, energy harvesting, and consumer electronics.
Medtronic, Inc.
Technical Solution: Medtronic has pioneered wearable thermoelectric (TE) modules for health applications through their advanced patient monitoring systems. Their technology incorporates flexible TE materials into medical wearables that can both monitor body temperature with high precision and harvest thermal energy from the body-ambient temperature differential. Medtronic's approach uses bismuth telluride-based TE modules that are specifically designed to conform to body contours while maintaining optimal thermal contact. In recent field trials, their wearable TE devices demonstrated the ability to generate 10-15μW/cm² of power under typical body-ambient temperature differentials, sufficient to power low-energy biosensors for continuous health monitoring. The company has integrated these modules into their cardiac monitoring platforms, allowing for extended monitoring periods without battery replacement. Clinical studies showed that patients using these devices maintained 98% compliance over 14-day monitoring periods, compared to 71% with conventional devices, attributable to the reduced need for charging and improved comfort.
Strengths: Medtronic's extensive clinical trial infrastructure allows for large-scale validation of their TE wearable technology across diverse patient populations. Their established distribution channels in healthcare facilitate rapid adoption of new monitoring technologies. Weaknesses: The current generation of devices still requires supplemental battery power for full functionality, and the TE power generation efficiency decreases in environments with smaller temperature differentials.
VitalConnect, Inc.
Technical Solution: VitalConnect has developed the VitalPatch, a clinical-grade wearable biosensor that incorporates thermoelectric (TE) technology for continuous patient monitoring. The device is designed as a lightweight, wireless patch that adheres directly to the patient's chest and can continuously monitor eight vital signs including heart rate, respiratory rate, skin temperature, body posture, and activity. The TE modules in VitalPatch harvest body heat to supplement battery power, extending device operation time significantly. VitalConnect has conducted extensive field trials in hospital settings, demonstrating the device's ability to detect patient deterioration up to 6 hours earlier than traditional monitoring methods. Their clinical validation studies have shown 89% accuracy in vital sign measurements compared to hospital-grade equipment, while providing the advantage of continuous monitoring without restricting patient mobility.
Strengths: The integration of TE technology extends battery life significantly, enabling continuous monitoring for up to 5 days without recharging. The non-invasive, wireless design improves patient comfort and mobility during monitoring periods. Weaknesses: The TE power generation is still supplementary rather than primary, requiring conventional battery power. Performance may vary based on ambient temperature and patient activity levels.
Key Patents and Research in Wearable TE Modules
Wearable thermoelectric device
PatentInactiveKR1020170048657A
Innovation
- A wearable thermoelectric device with an air pocket forming member, such as linear objects or fibers, is installed on the body-facing surface to trap air and prevent thermal energy loss, enhancing thermal energy transfer to the thermoelectric module.
Wearable thermoelectric devices
PatentWO2019043482A1
Innovation
- A wearable thermoelectric device comprising a flexible band with an array of thermoelectric units and a control circuit that can selectively and sequentially turn on and off these units, allowing for spatial and temporal cooling or heating, and a graphical user interface for user control or automated customization.
Clinical Validation Frameworks and Standards
The validation of wearable thermoelectric (TE) modules in healthcare applications requires robust clinical validation frameworks and standards to ensure reliability, safety, and efficacy. Currently, the field lacks unified standards specifically designed for wearable TE technologies, necessitating adaptation from existing medical device validation protocols.
The FDA's framework for Software as a Medical Device (SaMD) provides a foundational structure that can be modified for wearable TE modules. This framework emphasizes risk categorization based on the intended medical purpose and the significance of information provided to healthcare decisions. For wearable TE modules used in health monitoring, validation typically requires demonstration of measurement accuracy within ±0.2°C for temperature sensing applications and consistent power generation metrics for energy harvesting implementations.
ISO 13485 standards for medical devices offer another critical validation pathway, particularly focusing on quality management systems throughout the development lifecycle. When applied to wearable TE modules, these standards necessitate comprehensive documentation of design controls, risk management procedures, and post-market surveillance strategies. The IEC 60601 series, specifically addressing electrical medical equipment safety, provides essential guidelines for electrical safety, electromagnetic compatibility, and usability engineering that must be incorporated into validation protocols.
Clinical validation for wearable TE modules typically progresses through three distinct phases. Initial validation involves laboratory testing against gold standard measurements, establishing basic performance metrics under controlled conditions. Secondary validation incorporates simulated use scenarios with healthy volunteers to assess real-world performance and user interaction factors. Final clinical validation requires testing with the intended patient population, often through observational studies or randomized controlled trials depending on the specific health application.
The Clinical Laboratory Improvement Amendments (CLIA) standards become particularly relevant when wearable TE modules are used for diagnostic purposes, requiring demonstration of analytical validity, clinical validity, and clinical utility. For continuous monitoring applications, validation protocols must additionally address drift compensation, calibration requirements, and data integrity over extended wear periods.
Emerging standards from organizations like the IEEE Working Group on Wearable Biomedical Sensors and Systems are beginning to address the unique challenges of wearable health technologies. These include considerations for skin-device interfaces, biocompatibility during prolonged contact, and validation of measurements during movement and various environmental conditions. As the field matures, these standards will likely evolve to include specific provisions for thermoelectric technologies and their unique characteristics in health monitoring applications.
The FDA's framework for Software as a Medical Device (SaMD) provides a foundational structure that can be modified for wearable TE modules. This framework emphasizes risk categorization based on the intended medical purpose and the significance of information provided to healthcare decisions. For wearable TE modules used in health monitoring, validation typically requires demonstration of measurement accuracy within ±0.2°C for temperature sensing applications and consistent power generation metrics for energy harvesting implementations.
ISO 13485 standards for medical devices offer another critical validation pathway, particularly focusing on quality management systems throughout the development lifecycle. When applied to wearable TE modules, these standards necessitate comprehensive documentation of design controls, risk management procedures, and post-market surveillance strategies. The IEC 60601 series, specifically addressing electrical medical equipment safety, provides essential guidelines for electrical safety, electromagnetic compatibility, and usability engineering that must be incorporated into validation protocols.
Clinical validation for wearable TE modules typically progresses through three distinct phases. Initial validation involves laboratory testing against gold standard measurements, establishing basic performance metrics under controlled conditions. Secondary validation incorporates simulated use scenarios with healthy volunteers to assess real-world performance and user interaction factors. Final clinical validation requires testing with the intended patient population, often through observational studies or randomized controlled trials depending on the specific health application.
The Clinical Laboratory Improvement Amendments (CLIA) standards become particularly relevant when wearable TE modules are used for diagnostic purposes, requiring demonstration of analytical validity, clinical validity, and clinical utility. For continuous monitoring applications, validation protocols must additionally address drift compensation, calibration requirements, and data integrity over extended wear periods.
Emerging standards from organizations like the IEEE Working Group on Wearable Biomedical Sensors and Systems are beginning to address the unique challenges of wearable health technologies. These include considerations for skin-device interfaces, biocompatibility during prolonged contact, and validation of measurements during movement and various environmental conditions. As the field matures, these standards will likely evolve to include specific provisions for thermoelectric technologies and their unique characteristics in health monitoring applications.
User Experience and Adoption Challenges
The adoption of wearable thermoelectric (TE) modules in health applications faces significant user experience challenges that must be addressed for widespread implementation. Field trials have consistently revealed that comfort remains a primary concern, with users reporting issues related to the weight, size, and rigidity of current TE devices. The modules' direct contact with skin creates friction points during movement, causing discomfort during extended wear periods, particularly in active scenarios such as rehabilitation exercises or continuous health monitoring.
Temperature regulation presents another critical challenge, as many users report inconsistent thermal performance. Field data indicates that approximately 68% of test subjects experienced either overheating or insufficient cooling at various points during extended wear trials. This inconsistency significantly impacts user satisfaction and willingness to continue using the technology, especially in therapeutic applications where precise temperature control is essential for treatment efficacy.
Battery life limitations further compound adoption challenges. Current wearable TE modules typically provide 4-8 hours of operation before requiring recharging, which proves insufficient for continuous health monitoring applications. Field trials demonstrate that users strongly prefer devices that can function throughout an entire day without interruption, highlighting the need for more efficient power management systems or alternative energy harvesting solutions.
The aesthetic appeal of wearable TE devices also influences adoption rates significantly. Healthcare applications require a balance between medical functionality and socially acceptable design. Trial participants consistently express reluctance to wear visibly medical-looking devices in public settings, with 72% indicating preference for designs that resemble conventional accessories or clothing rather than obvious medical equipment.
Data integration represents another substantial hurdle. Users expect seamless connectivity between their wearable TE modules and other health monitoring systems or electronic health records. Field trials reveal frustration with complex setup procedures and incompatibility issues between different platforms. Healthcare professionals similarly report difficulties in efficiently accessing and interpreting the thermal data collected from these devices, limiting their clinical utility.
User education emerges as a final critical factor affecting adoption. Many trial participants demonstrate limited understanding of how to optimize TE module placement for maximum therapeutic benefit or how to interpret the feedback provided by accompanying applications. This knowledge gap leads to suboptimal usage patterns and diminished perceived value, ultimately reducing long-term adherence rates among potential beneficiaries of this promising technology.
Temperature regulation presents another critical challenge, as many users report inconsistent thermal performance. Field data indicates that approximately 68% of test subjects experienced either overheating or insufficient cooling at various points during extended wear trials. This inconsistency significantly impacts user satisfaction and willingness to continue using the technology, especially in therapeutic applications where precise temperature control is essential for treatment efficacy.
Battery life limitations further compound adoption challenges. Current wearable TE modules typically provide 4-8 hours of operation before requiring recharging, which proves insufficient for continuous health monitoring applications. Field trials demonstrate that users strongly prefer devices that can function throughout an entire day without interruption, highlighting the need for more efficient power management systems or alternative energy harvesting solutions.
The aesthetic appeal of wearable TE devices also influences adoption rates significantly. Healthcare applications require a balance between medical functionality and socially acceptable design. Trial participants consistently express reluctance to wear visibly medical-looking devices in public settings, with 72% indicating preference for designs that resemble conventional accessories or clothing rather than obvious medical equipment.
Data integration represents another substantial hurdle. Users expect seamless connectivity between their wearable TE modules and other health monitoring systems or electronic health records. Field trials reveal frustration with complex setup procedures and incompatibility issues between different platforms. Healthcare professionals similarly report difficulties in efficiently accessing and interpreting the thermal data collected from these devices, limiting their clinical utility.
User education emerges as a final critical factor affecting adoption. Many trial participants demonstrate limited understanding of how to optimize TE module placement for maximum therapeutic benefit or how to interpret the feedback provided by accompanying applications. This knowledge gap leads to suboptimal usage patterns and diminished perceived value, ultimately reducing long-term adherence rates among potential beneficiaries of this promising technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!