Breakthroughs in Zirconia Catalysts for Chemical Reactions
Zirconia Catalysis Evolution and Objectives
Zirconia catalysts have emerged as a significant area of research and development in the field of chemical reactions over the past few decades. The evolution of zirconia catalysis can be traced back to the 1970s when researchers first recognized the potential of zirconium oxide as a catalyst support. Since then, the field has witnessed remarkable progress, driven by the unique properties of zirconia, including its high thermal stability, amphoteric nature, and ability to exist in multiple crystalline phases.
The technological trajectory of zirconia catalysts has been characterized by continuous improvements in synthesis methods, surface modifications, and the development of novel composite materials. Early research focused primarily on utilizing zirconia as a support for other active catalytic species. However, as understanding of its intrinsic catalytic properties grew, efforts shifted towards developing zirconia-based catalysts where zirconia itself serves as the active component.
A significant milestone in the evolution of zirconia catalysis was the discovery of its sulfated form in the 1980s. Sulfated zirconia exhibited exceptional acidity and catalytic activity, particularly in hydrocarbon isomerization reactions. This breakthrough opened up new avenues for the application of zirconia catalysts in petrochemical processes and fine chemical synthesis.
In recent years, the focus has shifted towards developing more complex zirconia-based materials, such as mixed metal oxides and nanostructured zirconia catalysts. These advanced materials aim to combine the inherent advantages of zirconia with enhanced catalytic performance, selectivity, and stability. The advent of nanotechnology has further accelerated progress in this field, enabling precise control over catalyst morphology and surface properties.
The primary objectives of current research in zirconia catalysis are multifaceted. Firstly, there is a strong emphasis on improving the catalytic efficiency and selectivity of zirconia-based materials for a wide range of chemical reactions, including oxidation, reduction, acid-base reactions, and C-C bond formation. Secondly, researchers are striving to enhance the stability and longevity of zirconia catalysts under harsh reaction conditions, addressing issues such as thermal degradation and catalyst poisoning.
Another key objective is the development of environmentally friendly and sustainable catalytic processes using zirconia. This includes the design of catalysts for biomass conversion, CO2 utilization, and the production of renewable fuels and chemicals. Additionally, there is a growing interest in exploring the potential of zirconia catalysts in emerging fields such as photocatalysis and electrocatalysis, which could lead to breakthroughs in energy conversion and storage technologies.
As the field progresses, a crucial goal is to bridge the gap between fundamental research and industrial applications. This involves scaling up synthesis methods, optimizing catalyst formulations for specific industrial processes, and addressing economic considerations to ensure the commercial viability of zirconia-based catalytic technologies.
Market Demand Analysis for Zirconia Catalysts
The market demand for zirconia catalysts has been experiencing significant growth in recent years, driven by the increasing need for efficient and sustainable chemical processes across various industries. The global zirconia catalyst market is projected to expand at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2026, reaching a market value of $2.3 billion by the end of the forecast period.
One of the primary factors fueling this growth is the rising demand for clean energy solutions and environmental sustainability. Zirconia catalysts play a crucial role in reducing emissions and improving the efficiency of various chemical reactions, making them highly sought after in the automotive, petrochemical, and renewable energy sectors. The automotive industry, in particular, has been a major driver of demand, as zirconia catalysts are essential components in catalytic converters for reducing harmful emissions from vehicles.
The petrochemical industry is another significant consumer of zirconia catalysts, utilizing them in various processes such as fluid catalytic cracking, hydrocracking, and isomerization. As the global demand for petrochemical products continues to rise, the need for efficient catalysts to optimize these processes has also increased. Zirconia catalysts offer superior performance and stability compared to traditional catalysts, making them an attractive option for refineries and chemical plants looking to improve their operational efficiency and product quality.
In the renewable energy sector, zirconia catalysts are gaining traction in the production of biofuels and the conversion of biomass to valuable chemicals. As governments worldwide implement stricter regulations on carbon emissions and promote the use of renewable energy sources, the demand for zirconia catalysts in these applications is expected to grow substantially in the coming years.
The pharmaceutical industry is also emerging as a promising market for zirconia catalysts, particularly in the synthesis of complex organic compounds and active pharmaceutical ingredients (APIs). The ability of zirconia catalysts to facilitate selective and efficient chemical transformations makes them valuable tools in drug discovery and manufacturing processes.
Geographically, Asia-Pacific is expected to be the fastest-growing market for zirconia catalysts, driven by rapid industrialization, increasing automotive production, and stringent environmental regulations in countries like China and India. North America and Europe are also significant markets, with a focus on sustainable technologies and advanced chemical processes driving demand for high-performance catalysts.
As the global focus on sustainability and environmental protection intensifies, the market demand for zirconia catalysts is expected to continue its upward trajectory. Manufacturers are investing in research and development to enhance the performance and versatility of zirconia catalysts, further expanding their potential applications across various industries.
Current Challenges in Zirconia Catalyst Technology
Despite significant advancements in zirconia catalyst technology, several challenges persist in the field, hindering its widespread adoption and optimal performance in various chemical reactions. One of the primary obstacles is the limited thermal stability of zirconia catalysts at high temperatures. While zirconia exhibits excellent catalytic properties, prolonged exposure to elevated temperatures can lead to sintering and phase transformations, resulting in a loss of active surface area and catalytic efficiency.
Another significant challenge is the control of surface acidity and basicity of zirconia catalysts. The acid-base properties of zirconia play a crucial role in determining its catalytic activity and selectivity. However, achieving precise control over these properties remains difficult, particularly when tailoring catalysts for specific reactions or industrial processes.
The development of highly dispersed and stable zirconia nanoparticles presents another hurdle. Nanostructured zirconia catalysts offer enhanced surface area and improved catalytic performance, but maintaining their dispersion and preventing agglomeration during reactions is challenging. This issue is particularly pronounced in liquid-phase reactions or when dealing with high-pressure conditions.
Furthermore, the synthesis of zirconia catalysts with controlled porosity and hierarchical structures remains a complex task. While porous structures can significantly enhance catalytic activity by providing increased accessibility to active sites, achieving uniform and stable pore structures in zirconia catalysts is often difficult.
The incorporation of dopants and the creation of mixed oxide systems with zirconia also present challenges. While doping can enhance catalytic properties and stability, finding the optimal dopant concentrations and achieving homogeneous distribution within the zirconia matrix is not straightforward. Similarly, creating well-dispersed mixed oxide systems that maintain synergistic effects between zirconia and other components over extended periods is challenging.
Lastly, the scalability of zirconia catalyst production for industrial applications remains a significant hurdle. Many synthesis methods that yield high-performance zirconia catalysts at the laboratory scale face difficulties in scaling up while maintaining consistent quality and performance. This challenge is particularly evident in the production of advanced nanostructured or hierarchical zirconia catalysts.
Addressing these challenges requires interdisciplinary approaches, combining expertise in materials science, chemical engineering, and catalysis. Overcoming these obstacles will be crucial for unlocking the full potential of zirconia catalysts in various chemical reactions and industrial processes.
State-of-the-Art Zirconia Catalyst Solutions
01 Zirconia-based catalyst composition
Zirconia-based catalysts are developed with specific compositions to enhance catalytic activity. These catalysts often include additional components such as other metal oxides or rare earth elements to modify the properties and performance of zirconia. The composition can be tailored to suit specific reactions and improve overall catalytic efficiency.- Zirconia-based catalyst composition: Zirconia-based catalysts are developed with specific compositions to enhance catalytic activity. These catalysts often include additional components such as rare earth elements, transition metals, or other oxides to modify the properties and performance of the zirconia catalyst. The composition is tailored to optimize the catalytic activity for specific reactions or processes.
- Surface area and porosity optimization: The catalytic activity of zirconia catalysts is significantly influenced by their surface area and porosity. Techniques are employed to increase the surface area and control the pore structure of zirconia catalysts. This optimization enhances the accessibility of active sites and improves the overall catalytic performance.
- Doping and modification of zirconia catalysts: Doping zirconia catalysts with various elements or compounds can significantly alter their catalytic properties. This modification can enhance the catalyst's stability, selectivity, and activity. Common dopants include sulfates, phosphates, and various metal ions, which can create new active sites or modify the electronic structure of the catalyst.
- Preparation methods for high-activity zirconia catalysts: The method of preparing zirconia catalysts greatly affects their catalytic activity. Various synthesis techniques, such as sol-gel methods, hydrothermal synthesis, and precipitation methods, are employed to control the crystalline structure, particle size, and surface properties of zirconia catalysts. These preparation methods are optimized to produce catalysts with enhanced activity and selectivity.
- Application-specific zirconia catalyst design: Zirconia catalysts are tailored for specific applications to maximize their catalytic activity. This involves designing catalysts with particular properties suited for reactions such as hydrogenation, dehydrogenation, isomerization, or oxidation. The catalyst design considers factors like reaction conditions, feedstock composition, and desired product selectivity to optimize the catalytic performance for the intended application.
02 Surface area and porosity optimization
The catalytic activity of zirconia catalysts is significantly influenced by their surface area and porosity. Techniques are employed to increase the surface area and create an optimal pore structure, enhancing the accessibility of active sites. This optimization improves the catalyst's performance in various reactions.Expand Specific Solutions03 Doping and modification of zirconia catalysts
Doping zirconia catalysts with other elements or compounds can greatly enhance their catalytic activity. This modification can stabilize specific crystal phases, increase oxygen mobility, or introduce new active sites. Common dopants include rare earth elements, transition metals, and other metal oxides.Expand Specific Solutions04 Thermal stability and phase control
Controlling the crystal phase and improving the thermal stability of zirconia catalysts are crucial for maintaining high catalytic activity. Methods are developed to stabilize the desired crystal structure (such as tetragonal or cubic) at high temperatures, preventing phase transitions that could lead to loss of activity.Expand Specific Solutions05 Application-specific zirconia catalyst design
Zirconia catalysts are tailored for specific applications, such as hydrocarbon processing, emissions control, or fine chemical synthesis. The catalyst design considers factors like reaction conditions, selectivity requirements, and resistance to poisoning. This application-specific approach optimizes the catalytic activity for the intended use.Expand Specific Solutions
Key Players in Zirconia Catalyst Industry
The field of zirconia catalysts for chemical reactions is in a growth phase, with increasing market size and technological advancements. The global market for zirconia-based products is expanding, driven by demand in various industries. Technologically, zirconia catalysts are progressing from established applications to more innovative uses. Companies like ExxonMobil Chemical Patents, Inc., BASF Corp., and China Petroleum & Chemical Corp. are at the forefront, investing in R&D to enhance catalyst performance and efficiency. Emerging players such as Clariant International AG and Celanese International Corp. are also making significant contributions, focusing on specialized applications and novel synthesis methods. The involvement of research institutions like Centre National de la Recherche Scientifique indicates ongoing fundamental research, suggesting potential for further breakthroughs in this field.
China Petroleum & Chemical Corp.
Clariant International AG
Breakthrough Innovations in Zirconia Catalysis
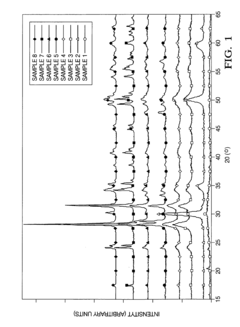
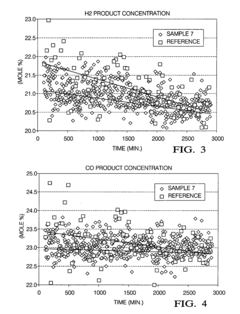
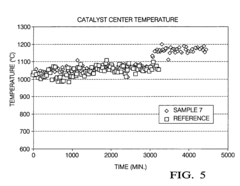
- A manganese-promoted zirconia support material is developed, which is hydrothermally stable and can be easily extruded without binders or stabilizing agents, using ZrO2 and MnOx with MnOx content between 1 wt% to 50 wt%, providing improved mechanical strength and catalytic performance in aqueous phase hydrogenolysis and hydrogenation reactions.
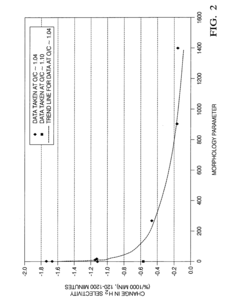
- A zirconia-supported catalyst with a morphology parameter of greater than or equal to about 800, comprising a higher monoclinic to tetragonal phase ratio and increased surface area, is used, which is more stable and retains hydrogen selectivity over time, even at high temperatures.
Environmental Impact of Zirconia Catalysts
The environmental impact of zirconia catalysts in chemical reactions is a crucial aspect to consider as these materials gain prominence in various industrial processes. Zirconia catalysts have shown significant potential in reducing the environmental footprint of chemical manufacturing by enabling more efficient and selective reactions.
One of the primary environmental benefits of zirconia catalysts is their ability to operate at lower temperatures compared to traditional catalysts. This characteristic leads to reduced energy consumption in chemical processes, thereby decreasing greenhouse gas emissions associated with energy production. The lower operating temperatures also contribute to extended catalyst lifetimes, reducing the frequency of catalyst replacement and the associated waste generation.
Zirconia catalysts have demonstrated exceptional performance in emissions control applications, particularly in the automotive industry. Their use in three-way catalytic converters has proven effective in simultaneously reducing harmful emissions such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons. This capability directly contributes to improved air quality and reduced environmental pollution from vehicular sources.
In the realm of sustainable chemistry, zirconia catalysts play a vital role in promoting green chemical processes. They facilitate the conversion of biomass-derived feedstocks into valuable chemicals and fuels, supporting the transition towards renewable resources and reducing dependence on fossil fuels. This shift not only mitigates carbon emissions but also addresses concerns related to resource depletion.
The recyclability and reusability of zirconia catalysts further enhance their environmental credentials. Unlike some traditional catalysts that degrade rapidly, zirconia-based materials often maintain their catalytic activity over multiple reaction cycles. This durability reduces the need for frequent catalyst replacement, minimizing waste generation and the environmental impact associated with catalyst production and disposal.
Water treatment is another area where zirconia catalysts contribute to environmental protection. Their application in advanced oxidation processes for wastewater treatment has shown promise in degrading persistent organic pollutants and emerging contaminants. This capability addresses growing concerns about water pollution and supports efforts to preserve water quality and ecosystems.
However, it is important to note that the production of zirconia catalysts itself has environmental implications. The mining and processing of zirconium ores, as well as the synthesis of catalysts, require energy and resources. Ongoing research aims to develop more sustainable production methods and to optimize the use of zirconia in catalytic systems to maximize environmental benefits while minimizing the ecological footprint of catalyst manufacturing.
In conclusion, while zirconia catalysts offer significant environmental advantages in various chemical processes, a holistic approach is necessary to fully assess their net environmental impact. Continued research and development in this field are essential to further enhance the sustainability profile of zirconia catalysts and to address any potential environmental concerns associated with their production and use.
Scalability and Industrial Applications
The scalability and industrial applications of zirconia catalysts represent a crucial aspect of their potential for widespread adoption in chemical processes. As research progresses, the focus on translating laboratory-scale breakthroughs to industrial-scale production becomes increasingly important.
One of the primary challenges in scaling up zirconia catalysts is maintaining their high surface area and catalytic activity during large-scale synthesis. Researchers have made significant strides in developing methods to produce zirconia catalysts with consistent properties in larger quantities. These methods include sol-gel processes, hydrothermal synthesis, and spray pyrolysis, which can be adapted for industrial-scale production while preserving the desired catalyst characteristics.
The industrial applications of zirconia catalysts span a wide range of chemical processes. In the petrochemical industry, zirconia-based catalysts have shown promise in hydrocracking and isomerization reactions, offering improved selectivity and stability compared to traditional catalysts. The automotive sector has also benefited from zirconia catalysts in emission control systems, where they demonstrate excellent thermal stability and resistance to sulfur poisoning.
Another area of industrial interest is the use of zirconia catalysts in the production of fine chemicals and pharmaceuticals. Their ability to catalyze selective oxidation and reduction reactions makes them valuable in synthesizing complex organic compounds. Additionally, the food industry has explored zirconia catalysts for the production of high-fructose corn syrup and other sweeteners, taking advantage of their efficiency in isomerization reactions.
The scalability of zirconia catalysts also extends to their potential in green chemistry applications. As industries seek more sustainable processes, zirconia catalysts offer opportunities for cleaner production methods. For instance, they have shown promise in the conversion of biomass to valuable chemicals, potentially replacing petroleum-based feedstocks in various industrial processes.
To further enhance the industrial viability of zirconia catalysts, researchers are focusing on improving their longevity and regeneration capabilities. Developing methods to efficiently recover and reuse these catalysts is crucial for their economic feasibility in large-scale operations. This includes exploring novel reactor designs and process intensification techniques that maximize catalyst performance while minimizing material usage.
As the field advances, the integration of zirconia catalysts into existing industrial infrastructure presents both challenges and opportunities. Adapting current production facilities to accommodate these new catalysts requires careful engineering and economic considerations. However, the potential benefits in terms of improved efficiency, selectivity, and environmental performance make this transition an attractive prospect for many industries.