Butyrate Applications in Inflammatory Bowel Disease Management
Butyrate in IBD: Background and Objectives
Butyrate, a short-chain fatty acid produced by gut microbiota, has emerged as a promising therapeutic agent in the management of Inflammatory Bowel Disease (IBD). The exploration of butyrate's potential in IBD treatment stems from a growing understanding of the gut microbiome's role in intestinal health and inflammation. This research aims to elucidate the mechanisms by which butyrate may alleviate IBD symptoms and potentially modify disease progression.
The development of butyrate-based therapies for IBD is rooted in decades of research on gut physiology and microbial metabolism. Early studies in the 1980s first identified butyrate as a key energy source for colonic epithelial cells, sparking interest in its potential therapeutic applications. As our understanding of IBD pathogenesis evolved, researchers began to investigate the anti-inflammatory properties of butyrate and its ability to maintain intestinal barrier integrity.
Recent technological advancements in microbiome analysis and metabolomics have accelerated research in this field, allowing for more comprehensive studies on butyrate's effects in IBD. The advent of next-generation sequencing techniques has enabled researchers to correlate butyrate-producing bacterial populations with IBD severity and treatment outcomes, further highlighting the potential of butyrate-based interventions.
The primary objective of this research is to evaluate the efficacy and safety of butyrate applications in IBD management. This includes assessing various delivery methods, such as oral supplements, enemas, and novel formulations designed to target specific regions of the gastrointestinal tract. Additionally, the research aims to identify optimal dosing regimens and potential synergies with existing IBD treatments.
Another crucial goal is to elucidate the molecular mechanisms underlying butyrate's therapeutic effects in IBD. This involves investigating its impact on inflammatory pathways, gut barrier function, and the composition of the intestinal microbiome. Understanding these mechanisms is essential for developing more targeted and effective butyrate-based therapies.
Furthermore, this research seeks to explore the potential of butyrate as a preventive measure in high-risk populations or as a maintenance therapy to prevent IBD relapse. Long-term studies are necessary to evaluate the sustained effects of butyrate supplementation and its impact on disease progression and quality of life for IBD patients.
As we delve deeper into the applications of butyrate in IBD management, it is crucial to consider the heterogeneity of the disease and the potential for personalized treatment approaches. This research aims to identify biomarkers or patient characteristics that may predict responsiveness to butyrate-based therapies, paving the way for more tailored treatment strategies in IBD management.
Market Analysis for IBD Therapeutics
The global market for Inflammatory Bowel Disease (IBD) therapeutics has been experiencing significant growth, driven by the increasing prevalence of IBD and the development of novel treatment options. The market is characterized by a high unmet medical need, as current treatments often fail to provide long-term remission or are associated with severe side effects.
The IBD therapeutics market is segmented into two main categories: Ulcerative Colitis (UC) and Crohn's Disease (CD). Both segments have shown steady growth, with UC slightly outpacing CD in terms of market share. This trend is expected to continue in the coming years, as the incidence of UC is rising more rapidly than CD in many regions.
Biologic therapies, particularly anti-TNF agents, have dominated the IBD market in recent years. However, the landscape is evolving with the introduction of new classes of drugs, including JAK inhibitors, anti-integrins, and IL-23 inhibitors. These novel therapies are expected to reshape the market dynamics and provide more targeted treatment options for patients.
The market for IBD therapeutics is highly competitive, with several pharmaceutical giants vying for market share. Key players include AbbVie, Johnson & Johnson, Takeda, and Pfizer, among others. These companies are investing heavily in research and development to maintain their competitive edge and expand their product portfolios.
Geographically, North America holds the largest share of the IBD therapeutics market, followed by Europe. This dominance is attributed to the high prevalence of IBD in these regions, advanced healthcare infrastructure, and favorable reimbursement policies. However, emerging markets in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare access and rising awareness of IBD.
The IBD therapeutics market is also witnessing a shift towards personalized medicine approaches. This trend is fueled by advancements in biomarker research and diagnostic technologies, allowing for more targeted and effective treatment strategies. As a result, there is growing interest in developing companion diagnostics alongside new therapies to identify patients most likely to respond to specific treatments.
Looking ahead, the IBD therapeutics market is poised for continued growth and innovation. The increasing focus on developing oral formulations of biologics and exploring novel mechanisms of action, such as microbiome-based therapies, presents significant opportunities for market expansion. Additionally, the potential application of butyrate in IBD management represents an exciting avenue for future research and development, potentially offering new treatment options for patients with this challenging condition.
Current Challenges in Butyrate-Based IBD Treatments
Despite the promising potential of butyrate in managing Inflammatory Bowel Disease (IBD), several challenges hinder its widespread application and efficacy. One of the primary obstacles is the rapid absorption and metabolism of butyrate in the upper gastrointestinal tract, which limits its availability in the colon where it is most needed. This necessitates the development of advanced delivery systems to ensure targeted release in the lower intestine.
Another significant challenge is the inconsistent dosing and bioavailability of butyrate across different formulations. The lack of standardization in butyrate-based treatments makes it difficult to establish optimal therapeutic regimens and compare results across clinical studies. This variability also complicates the assessment of butyrate's true efficacy in IBD management.
The palatability of butyrate-based treatments poses another hurdle. Butyrate's strong, unpleasant odor and taste can lead to poor patient compliance, especially in long-term treatment plans. This issue is particularly pronounced in oral formulations, which are often preferred for their convenience but struggle with patient acceptability.
Furthermore, the mechanism of action of butyrate in IBD is not fully elucidated. While its anti-inflammatory and immunomodulatory properties are well-documented, the exact pathways through which butyrate exerts its beneficial effects in IBD remain unclear. This knowledge gap hampers the optimization of butyrate-based therapies and their integration with other treatment modalities.
The production of high-quality, pharmaceutical-grade butyrate at scale is another challenge. Current manufacturing processes are often costly and inefficient, leading to high treatment expenses. This economic barrier limits the accessibility of butyrate-based treatments, particularly in resource-constrained healthcare settings.
Regulatory hurdles also present a significant challenge. The classification of butyrate-based products as drugs, supplements, or medical foods varies across different jurisdictions, leading to inconsistent approval processes and market access. This regulatory ambiguity slows down the development and commercialization of new butyrate-based treatments for IBD.
Lastly, the long-term safety profile of butyrate supplementation in IBD patients requires further investigation. While short-term studies have shown promising results, the potential risks associated with prolonged use, especially in combination with other IBD treatments, need to be thoroughly evaluated to ensure patient safety and treatment efficacy.
Existing Butyrate Delivery Methods for IBD
01 Butyrate as a therapeutic agent
Butyrate is explored as a potential therapeutic agent for various health conditions. It has shown promise in treating inflammatory disorders, metabolic diseases, and gastrointestinal issues. Research indicates that butyrate may have beneficial effects on gut health, immune function, and cellular metabolism.- Butyrate as a therapeutic agent: Butyrate is explored as a potential therapeutic agent for various health conditions. It has shown promise in treating inflammatory disorders, metabolic diseases, and gastrointestinal issues. Research indicates that butyrate may have beneficial effects on gut health, immune function, and cellular metabolism.
- Butyrate production in the gut microbiome: Studies focus on enhancing butyrate production in the gut microbiome. This involves investigating the role of specific bacterial strains, dietary interventions, and prebiotic compounds that promote butyrate-producing bacteria. Increasing butyrate levels in the gut is associated with improved intestinal health and overall well-being.
- Butyrate in animal nutrition: Butyrate is utilized in animal nutrition to improve growth performance, gut health, and overall animal welfare. Research explores the incorporation of butyrate or its precursors in animal feed formulations, particularly for livestock and aquaculture applications. The benefits include enhanced nutrient absorption and improved intestinal barrier function.
- Butyrate derivatives and delivery systems: Development of novel butyrate derivatives and delivery systems aims to enhance its bioavailability and targeted release. This includes encapsulation technologies, prodrug formulations, and controlled-release mechanisms to optimize the therapeutic effects of butyrate while minimizing potential side effects.
- Butyrate in cancer research: Investigations into the potential anti-cancer properties of butyrate are ongoing. Studies explore its role in regulating gene expression, inducing apoptosis in cancer cells, and modulating the tumor microenvironment. Research focuses on understanding the mechanisms of action and developing butyrate-based therapies for various types of cancer.
02 Butyrate production in the gut microbiome
Studies focus on enhancing butyrate production in the gut microbiome. This involves investigating the role of specific bacterial strains, dietary interventions, and prebiotic compounds that promote butyrate-producing bacteria. Increasing butyrate levels in the gut is associated with improved intestinal health and overall well-being.Expand Specific Solutions03 Butyrate derivatives and formulations
Research explores various butyrate derivatives and formulations to enhance its stability, bioavailability, and targeted delivery. This includes developing novel compounds, encapsulation techniques, and controlled-release systems to optimize the therapeutic potential of butyrate and its analogs.Expand Specific Solutions04 Butyrate in animal nutrition and agriculture
Butyrate is investigated for its potential benefits in animal nutrition and agriculture. Studies examine its use as a feed additive to improve growth performance, gut health, and disease resistance in livestock and poultry. Additionally, butyrate-based products are explored for their potential in crop protection and soil health enhancement.Expand Specific Solutions05 Butyrate in industrial applications
Butyrate and its derivatives find applications in various industrial processes. This includes their use as solvents, plasticizers, and intermediates in the production of flavors, fragrances, and pharmaceuticals. Research focuses on developing sustainable and cost-effective methods for butyrate production and its industrial utilization.Expand Specific Solutions
Key Players in Butyrate-IBD Research
The butyrate applications in Inflammatory Bowel Disease (IBD) management market is in a growth phase, with increasing research and clinical interest. The global IBD therapeutics market is projected to reach significant size, driven by rising IBD prevalence. Technologically, butyrate applications are advancing, with companies like Société des Produits Nestlé SA, Baxter International, Inc., and Synlogic Operating Co., Inc. leading innovation. Academic institutions such as The University of Chicago and Ghent University contribute to foundational research. The field sees a mix of established pharmaceutical companies and emerging biotech firms, indicating a competitive landscape with diverse approaches to butyrate-based IBD treatments.
PharmaBiome AG
Clasado Research Services Ltd
Innovative Butyrate Formulations for IBD
- Development of a gastro-resistant butyrate salt formulation for enteral administration that targets the intestine, reducing systemic inflammation by binding to intestinal receptors and modulating cytokine production, thereby treating a broader range of diseases including osteoarthritis, psoriasis, and multiple sclerosis.
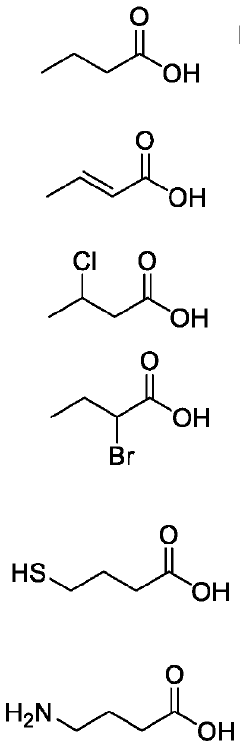
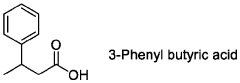
- The use of butyrate analogues, including 4-Mercaptobutyrate, as direct noncompetitive inhibitors of prolyl hydroxylases (PHDs) to stabilize HIF, thereby treating and preventing intestinal diseases by administering compounds like Butyric acid, crotonic acid, 3-Chloro butyric acid, 2-Bromo butyric acid, 4-Mercapto butyric acid, and 3-Phenyl butyric acid, which have a longer biological half-life and enhance HIF stabilization.
Safety and Efficacy Considerations
The safety and efficacy of butyrate applications in inflammatory bowel disease (IBD) management are critical considerations that require thorough evaluation. Butyrate, a short-chain fatty acid produced by gut microbiota, has shown promising results in preclinical and clinical studies for IBD treatment. However, its widespread adoption necessitates a comprehensive assessment of its safety profile and therapeutic efficacy.
Safety considerations for butyrate applications primarily focus on potential adverse effects and long-term consequences of administration. While butyrate is generally well-tolerated, some patients may experience mild gastrointestinal discomfort, including bloating, flatulence, and abdominal pain. These side effects are typically transient and dose-dependent. Long-term safety data is limited, and further research is needed to evaluate the potential risks associated with prolonged butyrate supplementation.
The route of administration plays a crucial role in both safety and efficacy. Oral butyrate supplements may be degraded in the upper gastrointestinal tract, limiting their effectiveness in reaching the colon. To address this, various formulations have been developed, including enteric-coated tablets, microencapsulated forms, and prodrugs. These delivery systems aim to enhance butyrate's bioavailability and targeted release in the colon, potentially improving safety and efficacy profiles.
Efficacy considerations for butyrate in IBD management are multifaceted. Clinical studies have demonstrated its potential to reduce inflammation, improve intestinal barrier function, and modulate the gut microbiome. Butyrate has shown promise in inducing remission and maintaining disease control in ulcerative colitis patients. However, its efficacy in Crohn's disease is less established and requires further investigation.
The optimal dosage and duration of butyrate therapy remain subjects of ongoing research. Dose-response relationships and individual patient variability in response to butyrate treatment need to be carefully evaluated to maximize therapeutic benefits while minimizing potential risks. Additionally, the synergistic effects of butyrate with conventional IBD treatments, such as mesalamine or biologics, warrant exploration to develop more effective combination therapies.
Patient-specific factors, including disease severity, location, and individual genetic and microbiome profiles, may influence the safety and efficacy of butyrate applications. Personalized approaches to butyrate therapy, considering these factors, could potentially enhance treatment outcomes and minimize adverse effects.
In conclusion, while butyrate shows promise in IBD management, rigorous clinical trials and long-term follow-up studies are essential to fully elucidate its safety profile and therapeutic efficacy. Continued research efforts should focus on optimizing delivery methods, determining ideal dosing regimens, and identifying patient subgroups most likely to benefit from butyrate-based interventions.
Regulatory Pathway for Butyrate-Based IBD Therapies
The regulatory pathway for butyrate-based therapies in Inflammatory Bowel Disease (IBD) management involves a complex process of clinical trials, safety assessments, and regulatory approvals. This pathway is primarily overseen by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Initially, preclinical studies are conducted to establish the safety and efficacy of butyrate-based therapies in animal models. These studies evaluate the pharmacokinetics, pharmacodynamics, and potential toxicity of the treatment. Once preclinical data demonstrates promising results, researchers can proceed to human clinical trials.
The clinical trial process typically consists of three phases. Phase I trials focus on safety and dosage in a small group of healthy volunteers or patients. Phase II trials involve a larger group of patients to assess efficacy and further evaluate safety. Phase III trials are large-scale studies that confirm the effectiveness of the treatment, monitor side effects, and compare it to commonly used treatments.
Throughout the clinical trial process, sponsors must adhere to Good Clinical Practice (GCP) guidelines and maintain open communication with regulatory agencies. Regular meetings with these agencies can help address potential concerns and ensure compliance with regulatory requirements.
After successful completion of clinical trials, sponsors submit a New Drug Application (NDA) to the FDA or a Marketing Authorization Application (MAA) to the EMA. These applications include comprehensive data on the drug's safety, efficacy, and manufacturing processes. Regulatory agencies review the submitted data and may request additional information or studies if needed.
If approved, post-marketing surveillance is required to monitor the long-term safety and effectiveness of the therapy in real-world settings. This ongoing process helps identify any rare side effects or long-term complications that may not have been apparent during clinical trials.
It's important to note that the regulatory pathway may vary depending on the specific formulation and delivery method of the butyrate-based therapy. For example, if the therapy is classified as a dietary supplement rather than a drug, it may follow a different regulatory process with less stringent requirements.
Collaboration between researchers, pharmaceutical companies, and regulatory agencies is crucial throughout this process to ensure the development of safe and effective butyrate-based therapies for IBD management. As the field evolves, regulatory pathways may adapt to accommodate new technologies and treatment modalities in this area.