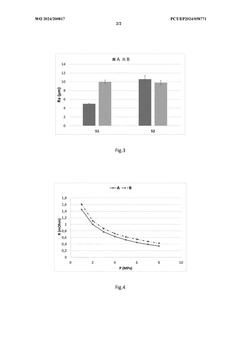
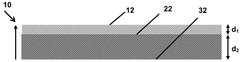
Catalyst Layer Architecture: Impact on Mass Transport and Electrolyser Performance
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst Layer Evolution
The evolution of catalyst layer architecture in electrolysers has been a critical factor in advancing the performance and efficiency of these systems. Initially, catalyst layers were simple, consisting of a uniform distribution of catalyst particles on a conductive substrate. However, as research progressed, the importance of optimizing the catalyst layer structure became increasingly apparent.
In the early stages, researchers focused on increasing catalyst loading to improve performance. This approach, while effective to some extent, led to diminishing returns and high material costs. The next phase of development saw a shift towards enhancing the intrinsic activity of catalysts through the use of advanced materials and nanostructures.
A significant breakthrough came with the recognition of the three-phase boundary concept. This understanding led to the development of catalyst layers that maximized the interface between the electrolyte, catalyst, and reactant gas. Researchers began to design porous structures that allowed for efficient mass transport while maintaining high catalytic activity.
The introduction of ionomer binders marked another crucial step in catalyst layer evolution. These materials improved the ionic conductivity within the layer, facilitating more efficient proton transport. This innovation led to the development of catalyst-coated membranes, which became a standard in PEM electrolysers.
Recent advancements have focused on creating hierarchical structures within the catalyst layer. These designs incorporate both macro and micropores, optimizing mass transport at different scales. The use of support materials, such as carbon nanotubes and graphene, has further enhanced the structural integrity and conductivity of catalyst layers.
The latest trends in catalyst layer evolution involve the integration of advanced manufacturing techniques. 3D printing and electrospraying methods are being explored to create precisely controlled catalyst layer architectures. These techniques allow for the fine-tuning of porosity, thickness, and catalyst distribution, leading to unprecedented levels of performance optimization.
Furthermore, the development of gradient catalyst layers has shown promise in balancing the conflicting requirements of reactant transport and catalyst utilization. By varying the composition and structure across the thickness of the layer, researchers have achieved improved overall electrolyser performance.
As the field continues to advance, there is a growing focus on developing catalyst layers that are not only high-performing but also durable and cost-effective. This has led to research into novel materials and architectures that can withstand the harsh operating conditions of electrolysers while minimizing the use of precious metals.
In the early stages, researchers focused on increasing catalyst loading to improve performance. This approach, while effective to some extent, led to diminishing returns and high material costs. The next phase of development saw a shift towards enhancing the intrinsic activity of catalysts through the use of advanced materials and nanostructures.
A significant breakthrough came with the recognition of the three-phase boundary concept. This understanding led to the development of catalyst layers that maximized the interface between the electrolyte, catalyst, and reactant gas. Researchers began to design porous structures that allowed for efficient mass transport while maintaining high catalytic activity.
The introduction of ionomer binders marked another crucial step in catalyst layer evolution. These materials improved the ionic conductivity within the layer, facilitating more efficient proton transport. This innovation led to the development of catalyst-coated membranes, which became a standard in PEM electrolysers.
Recent advancements have focused on creating hierarchical structures within the catalyst layer. These designs incorporate both macro and micropores, optimizing mass transport at different scales. The use of support materials, such as carbon nanotubes and graphene, has further enhanced the structural integrity and conductivity of catalyst layers.
The latest trends in catalyst layer evolution involve the integration of advanced manufacturing techniques. 3D printing and electrospraying methods are being explored to create precisely controlled catalyst layer architectures. These techniques allow for the fine-tuning of porosity, thickness, and catalyst distribution, leading to unprecedented levels of performance optimization.
Furthermore, the development of gradient catalyst layers has shown promise in balancing the conflicting requirements of reactant transport and catalyst utilization. By varying the composition and structure across the thickness of the layer, researchers have achieved improved overall electrolyser performance.
As the field continues to advance, there is a growing focus on developing catalyst layers that are not only high-performing but also durable and cost-effective. This has led to research into novel materials and architectures that can withstand the harsh operating conditions of electrolysers while minimizing the use of precious metals.
Market Demand Analysis
The market demand for advanced catalyst layer architectures in electrolysers has been growing rapidly, driven by the increasing focus on green hydrogen production and the need for more efficient and cost-effective water electrolysis technologies. As countries and industries worldwide commit to decarbonization goals, the demand for electrolysers with improved performance and reduced costs has surged.
The global electrolyser market is experiencing significant expansion, with projections indicating substantial growth in the coming years. This growth is primarily fueled by the rising adoption of hydrogen as a clean energy carrier and the push for renewable energy integration. Governments and private sector investments in hydrogen infrastructure projects are further stimulating market demand for high-performance electrolysers.
In the context of catalyst layer architecture, there is a strong market pull for innovations that can address the key challenges in mass transport and overall electrolyser performance. End-users across various industries, including energy, transportation, and industrial processes, are seeking electrolysers with higher efficiency, increased durability, and reduced capital costs.
The market demand is particularly focused on catalyst layer designs that can optimize the triple-phase boundary, enhance reactant distribution, and mitigate mass transport limitations. These improvements are crucial for achieving higher current densities, lower overpotentials, and extended operational lifetimes of electrolysers.
Furthermore, there is a growing interest in scalable and cost-effective manufacturing processes for advanced catalyst layer architectures. The market is looking for solutions that can be readily integrated into existing production lines and offer a balance between performance enhancement and economic viability.
Geographically, the demand for improved catalyst layer architectures is strong in regions with ambitious hydrogen strategies, such as the European Union, Japan, South Korea, and parts of North America. These markets are driving innovation through supportive policies, research funding, and industrial collaborations.
The automotive sector, in particular, is emerging as a significant driver of demand for high-performance electrolysers. As fuel cell electric vehicles gain traction, there is an increasing need for efficient and large-scale hydrogen production, which directly translates to demand for advanced electrolyser technologies.
In summary, the market demand for catalyst layer architectures that can positively impact mass transport and electrolyser performance is robust and growing. This demand is underpinned by the global shift towards sustainable energy solutions, the expansion of the hydrogen economy, and the need for more efficient and cost-effective electrolysis technologies across multiple industries.
The global electrolyser market is experiencing significant expansion, with projections indicating substantial growth in the coming years. This growth is primarily fueled by the rising adoption of hydrogen as a clean energy carrier and the push for renewable energy integration. Governments and private sector investments in hydrogen infrastructure projects are further stimulating market demand for high-performance electrolysers.
In the context of catalyst layer architecture, there is a strong market pull for innovations that can address the key challenges in mass transport and overall electrolyser performance. End-users across various industries, including energy, transportation, and industrial processes, are seeking electrolysers with higher efficiency, increased durability, and reduced capital costs.
The market demand is particularly focused on catalyst layer designs that can optimize the triple-phase boundary, enhance reactant distribution, and mitigate mass transport limitations. These improvements are crucial for achieving higher current densities, lower overpotentials, and extended operational lifetimes of electrolysers.
Furthermore, there is a growing interest in scalable and cost-effective manufacturing processes for advanced catalyst layer architectures. The market is looking for solutions that can be readily integrated into existing production lines and offer a balance between performance enhancement and economic viability.
Geographically, the demand for improved catalyst layer architectures is strong in regions with ambitious hydrogen strategies, such as the European Union, Japan, South Korea, and parts of North America. These markets are driving innovation through supportive policies, research funding, and industrial collaborations.
The automotive sector, in particular, is emerging as a significant driver of demand for high-performance electrolysers. As fuel cell electric vehicles gain traction, there is an increasing need for efficient and large-scale hydrogen production, which directly translates to demand for advanced electrolyser technologies.
In summary, the market demand for catalyst layer architectures that can positively impact mass transport and electrolyser performance is robust and growing. This demand is underpinned by the global shift towards sustainable energy solutions, the expansion of the hydrogen economy, and the need for more efficient and cost-effective electrolysis technologies across multiple industries.
Technical Challenges
The catalyst layer architecture in electrolysers faces several significant technical challenges that impact mass transport and overall performance. One of the primary issues is the trade-off between catalytic activity and mass transport efficiency. As the catalyst layer becomes thicker to accommodate more active sites, it simultaneously impedes the transport of reactants and products, leading to concentration gradients and reduced efficiency.
Another challenge lies in optimizing the three-phase boundary where the catalyst, electrolyte, and gas phases meet. Achieving an ideal balance between these components is crucial for maximizing reaction rates and minimizing transport limitations. However, maintaining this delicate equilibrium across the entire catalyst layer remains a complex task, especially under varying operating conditions.
The durability of the catalyst layer presents an ongoing challenge. Degradation mechanisms such as catalyst particle agglomeration, dissolution, and detachment can lead to a loss of active surface area over time. This degradation is often exacerbated by the harsh operating environment of electrolysers, including high potentials, temperature fluctuations, and the presence of reactive species.
Mass transport limitations within the catalyst layer significantly impact electrolyser performance. Inefficient removal of product gases can lead to bubble formation, which blocks active sites and increases ohmic resistance. Similarly, inadequate supply of reactant water to the catalyst surface can result in localized dry spots, reducing the effective reaction area and potentially causing irreversible damage to the catalyst layer.
The heterogeneous nature of catalyst layers poses challenges in achieving uniform current distribution and reaction rates. Variations in thickness, porosity, and composition across the layer can lead to uneven utilization of the catalyst and localized hotspots, potentially compromising both performance and longevity of the electrolyser.
Integrating advanced materials, such as support structures or ionomer binders, into the catalyst layer architecture introduces additional complexities. While these components can enhance conductivity and stability, they may also introduce new mass transport barriers or alter the electrochemical environment at the catalyst surface.
Scaling up catalyst layer fabrication techniques from laboratory to industrial scale presents significant challenges in maintaining consistency and performance. Ensuring uniform deposition, controlled porosity, and optimal catalyst distribution across large-area electrodes remains a critical hurdle in the commercialization of high-performance electrolysers.
Another challenge lies in optimizing the three-phase boundary where the catalyst, electrolyte, and gas phases meet. Achieving an ideal balance between these components is crucial for maximizing reaction rates and minimizing transport limitations. However, maintaining this delicate equilibrium across the entire catalyst layer remains a complex task, especially under varying operating conditions.
The durability of the catalyst layer presents an ongoing challenge. Degradation mechanisms such as catalyst particle agglomeration, dissolution, and detachment can lead to a loss of active surface area over time. This degradation is often exacerbated by the harsh operating environment of electrolysers, including high potentials, temperature fluctuations, and the presence of reactive species.
Mass transport limitations within the catalyst layer significantly impact electrolyser performance. Inefficient removal of product gases can lead to bubble formation, which blocks active sites and increases ohmic resistance. Similarly, inadequate supply of reactant water to the catalyst surface can result in localized dry spots, reducing the effective reaction area and potentially causing irreversible damage to the catalyst layer.
The heterogeneous nature of catalyst layers poses challenges in achieving uniform current distribution and reaction rates. Variations in thickness, porosity, and composition across the layer can lead to uneven utilization of the catalyst and localized hotspots, potentially compromising both performance and longevity of the electrolyser.
Integrating advanced materials, such as support structures or ionomer binders, into the catalyst layer architecture introduces additional complexities. While these components can enhance conductivity and stability, they may also introduce new mass transport barriers or alter the electrochemical environment at the catalyst surface.
Scaling up catalyst layer fabrication techniques from laboratory to industrial scale presents significant challenges in maintaining consistency and performance. Ensuring uniform deposition, controlled porosity, and optimal catalyst distribution across large-area electrodes remains a critical hurdle in the commercialization of high-performance electrolysers.
Current Design Solutions
01 Catalyst layer structure optimization
Optimizing the catalyst layer structure is crucial for improving mass transport in fuel cells. This involves designing the architecture to enhance porosity, reduce diffusion limitations, and increase the active surface area. Techniques may include creating hierarchical pore structures, controlling catalyst particle size and distribution, and incorporating support materials that facilitate gas and liquid transport.- Catalyst layer structure optimization: Optimizing the catalyst layer structure is crucial for improving mass transport in fuel cells. This involves designing the architecture to enhance porosity, reduce diffusion limitations, and increase the active surface area. Advanced techniques such as 3D printing and nanoscale engineering are employed to create intricate structures that facilitate efficient reactant distribution and product removal.
- Novel materials for catalyst support: Developing new materials for catalyst support can significantly impact mass transport in the catalyst layer. These materials are designed to have high surface area, good electrical conductivity, and enhanced durability. Carbon-based materials, metal oxides, and advanced composites are being explored to create more efficient catalyst layers with improved mass transport properties.
- Ionomer distribution and optimization: The distribution and optimization of ionomers in the catalyst layer play a critical role in mass transport. Researchers are focusing on developing techniques to control ionomer distribution, thickness, and interaction with catalyst particles. This approach aims to balance proton conductivity and gas diffusion, leading to improved overall performance of the catalyst layer.
- Multi-scale modeling for mass transport: Utilizing multi-scale modeling techniques to understand and predict mass transport phenomena in catalyst layers is becoming increasingly important. These models incorporate molecular dynamics, pore-scale simulations, and macroscopic transport equations to provide comprehensive insights into the complex processes occurring within the catalyst layer, guiding the design of more efficient architectures.
- Integration of flow field designs: Integrating innovative flow field designs with catalyst layer architecture is a promising approach to enhance mass transport. This involves creating synergies between the flow channels and the catalyst layer structure to optimize reactant distribution, water management, and overall cell performance. Advanced manufacturing techniques are employed to realize complex, integrated designs.
02 Novel materials for catalyst layers
Developing and incorporating novel materials in catalyst layers can significantly improve mass transport properties. This may include the use of advanced carbon supports, nanostructured materials, or composite materials that offer enhanced conductivity and porosity. These materials can help create more efficient pathways for reactants and products, reducing mass transport limitations.Expand Specific Solutions03 Electrode fabrication techniques
Innovative electrode fabrication techniques can be employed to create catalyst layer architectures that promote better mass transport. These may include methods such as spray coating, electrospinning, 3D printing, or layer-by-layer assembly. Such techniques allow for precise control over the catalyst layer structure, enabling the creation of optimized architectures for enhanced mass transport.Expand Specific Solutions04 Integration of flow field designs
Integrating advanced flow field designs with catalyst layer architecture can improve overall mass transport in fuel cells. This approach considers the interaction between the flow channels and the catalyst layer, optimizing the distribution of reactants and removal of products. Techniques may include creating gradient structures or incorporating micro-channels within the catalyst layer to enhance mass transport.Expand Specific Solutions05 Modeling and simulation for optimization
Utilizing advanced modeling and simulation techniques to optimize catalyst layer architecture for improved mass transport. This involves developing computational models that can predict the performance of different catalyst layer structures and guide the design process. These models may incorporate multiphysics simulations, considering factors such as fluid dynamics, electrochemistry, and heat transfer to optimize the catalyst layer architecture.Expand Specific Solutions
Key Industry Players
The catalyst layer architecture in electrolysers is a critical area of research, currently in the early stages of development but rapidly advancing. The market for this technology is expanding, driven by the growing demand for green hydrogen production. Companies like Robert Bosch, Toyota, and Nissan are investing heavily in this field, leveraging their automotive expertise to improve electrolyser performance. Academic institutions such as Wuhan University of Technology and Tongji University are contributing significant research. The technology's maturity varies, with established players like Panasonic and emerging startups like Electric Hydrogen pushing innovation. As the industry progresses, we can expect increased collaboration between automotive, chemical, and energy sectors to optimize catalyst layer designs for enhanced mass transport and overall electrolyser efficiency.
Toyota Motor Corp.
Technical Solution: Toyota has developed a proprietary catalyst layer architecture for their fuel cell and electrolyser technologies. Their approach focuses on optimizing the three-phase boundary where the catalyst, electrolyte, and reactants meet. Toyota's design incorporates a hierarchical pore structure within the catalyst layer, which enhances both mass transport and reaction kinetics[4]. They have also implemented a novel ionomer coating technique that improves proton conductivity while minimizing oxygen transport resistance. Toyota's catalyst layers utilize advanced platinum alloy nanoparticles supported on carbon nanotubes, which offer high catalytic activity and stability[5]. Their electrolyser systems have shown a 25% reduction in platinum loading while maintaining comparable performance to conventional systems[6].
Strengths: Reduced catalyst loading, improved durability, and enhanced mass transport. Weaknesses: Potential challenges in large-scale manufacturing and higher initial costs.
Mercedes-Benz Group AG
Technical Solution: Mercedes-Benz has developed an advanced catalyst layer architecture for their hydrogen fuel cell and electrolyser technologies. Their approach focuses on creating a highly porous and interconnected catalyst structure that maximizes active surface area and facilitates efficient mass transport. The company utilizes a proprietary catalyst deposition technique that ensures uniform distribution of catalyst nanoparticles throughout the layer[7]. Mercedes-Benz has also implemented a gradient structure within the catalyst layer, with varying porosity and ionomer content to optimize both reactant transport and proton conductivity. Their design incorporates hydrophobic treatments to manage water content effectively, preventing flooding and enhancing overall performance[8]. The company's electrolyser systems have demonstrated a 20% increase in power density compared to previous generations[9].
Strengths: High power density, improved water management, and enhanced durability. Weaknesses: Complex manufacturing process and potential scalability challenges.
Innovative Catalyst Layers
Catalyst layer, membrane-electrode assembly, and solid polymer fuel cell
PatentWO2019189891A1
Innovation
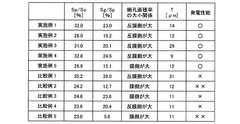
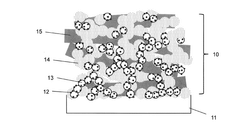
- A catalyst layer with a pore area ratio of 25.0% to 35.0% and a three-dimensional network structure, formed by a combination of a catalyst material, conductive carrier, polymer electrolyte, and fibrous material, is developed to enhance mass transport properties, ensuring sufficient gas diffusion and water drainage.
Porous transport layer
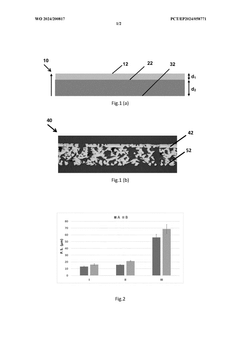
PatentWO2024200817A1
Innovation
- A dual-layer porous transport layer with a first nonwoven layer of fine metal fibers for improved catalyst contact and a second nonwoven layer of larger fibers for enhanced water inflow, metallurgically bonded to control porosity and surface roughness, reducing ohmic resistance and mechanical instability.
Regulatory Framework
The regulatory framework surrounding catalyst layer architecture and its impact on mass transport and electrolyser performance is a critical aspect of the hydrogen production industry. As governments worldwide push for cleaner energy solutions, regulations concerning electrolyser technology have become increasingly stringent and complex.
At the international level, organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have developed standards for electrolyser systems. These standards address safety, performance, and testing procedures, indirectly influencing catalyst layer design and optimization.
In the European Union, the Renewable Energy Directive (RED II) sets targets for renewable hydrogen production, driving the need for more efficient electrolysers. This directive has spurred research and development in catalyst layer architecture to improve mass transport and overall electrolyser performance.
The United States Department of Energy (DOE) has established specific performance targets for hydrogen production via electrolysis. These targets include efficiency, durability, and cost metrics, which directly impact catalyst layer design considerations. The DOE's Hydrogen and Fuel Cell Technologies Office provides funding and guidance for research projects aimed at advancing electrolyser technology.
In Asia, countries like Japan and South Korea have implemented hydrogen strategies that include regulations and incentives for electrolyser development. These policies often emphasize the importance of improving catalyst layer performance to enhance overall system efficiency and reduce costs.
Environmental regulations, such as those related to water quality and materials usage, also play a role in shaping catalyst layer architecture. For instance, restrictions on the use of certain precious metals or hazardous materials may influence the selection of catalyst materials and manufacturing processes.
Safety regulations, particularly those concerning the handling of hydrogen gas, indirectly affect catalyst layer design by imposing requirements on electrolyser system components and operational parameters. These safety considerations may impact the choice of materials and structural design of the catalyst layer.
As the hydrogen economy continues to grow, regulatory bodies are likely to introduce more specific guidelines related to catalyst layer architecture and performance. This evolving regulatory landscape will drive innovation in mass transport optimization and overall electrolyser efficiency, pushing researchers and manufacturers to develop more advanced and sustainable solutions.
At the international level, organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) have developed standards for electrolyser systems. These standards address safety, performance, and testing procedures, indirectly influencing catalyst layer design and optimization.
In the European Union, the Renewable Energy Directive (RED II) sets targets for renewable hydrogen production, driving the need for more efficient electrolysers. This directive has spurred research and development in catalyst layer architecture to improve mass transport and overall electrolyser performance.
The United States Department of Energy (DOE) has established specific performance targets for hydrogen production via electrolysis. These targets include efficiency, durability, and cost metrics, which directly impact catalyst layer design considerations. The DOE's Hydrogen and Fuel Cell Technologies Office provides funding and guidance for research projects aimed at advancing electrolyser technology.
In Asia, countries like Japan and South Korea have implemented hydrogen strategies that include regulations and incentives for electrolyser development. These policies often emphasize the importance of improving catalyst layer performance to enhance overall system efficiency and reduce costs.
Environmental regulations, such as those related to water quality and materials usage, also play a role in shaping catalyst layer architecture. For instance, restrictions on the use of certain precious metals or hazardous materials may influence the selection of catalyst materials and manufacturing processes.
Safety regulations, particularly those concerning the handling of hydrogen gas, indirectly affect catalyst layer design by imposing requirements on electrolyser system components and operational parameters. These safety considerations may impact the choice of materials and structural design of the catalyst layer.
As the hydrogen economy continues to grow, regulatory bodies are likely to introduce more specific guidelines related to catalyst layer architecture and performance. This evolving regulatory landscape will drive innovation in mass transport optimization and overall electrolyser efficiency, pushing researchers and manufacturers to develop more advanced and sustainable solutions.
Environmental Impact
The environmental impact of catalyst layer architecture in electrolysers is a critical consideration in the development and implementation of sustainable hydrogen production technologies. The design and composition of catalyst layers significantly influence the overall efficiency and environmental footprint of electrolyser systems.
Catalyst layer architecture directly affects the mass transport properties within the electrolyser, which in turn impacts energy consumption and resource utilization. Optimized catalyst layers can enhance the efficiency of water electrolysis, reducing the amount of electricity required to produce a given quantity of hydrogen. This improved energy efficiency translates to lower greenhouse gas emissions associated with hydrogen production, particularly when the electricity source is derived from renewable energy.
The choice of materials used in catalyst layers also plays a crucial role in environmental sustainability. Precious metals, such as platinum and iridium, are commonly used catalysts due to their high activity and stability. However, their scarcity and energy-intensive mining processes raise concerns about long-term sustainability and environmental impact. Research into alternative catalyst materials, such as non-precious metal catalysts or earth-abundant elements, aims to address these issues while maintaining or improving electrolyser performance.
Catalyst layer architecture can influence the durability and lifespan of electrolyser systems. More robust and stable catalyst layers can extend the operational life of electrolysers, reducing the frequency of replacements and associated material waste. This longevity contributes to a reduced environmental impact over the lifecycle of the electrolyser system.
The manufacturing processes for catalyst layers also have environmental implications. Advanced fabrication techniques, such as 3D printing or electrodeposition, may offer more precise control over catalyst layer structure and composition. These methods can potentially reduce material waste and energy consumption during production, further minimizing the environmental footprint of electrolyser manufacturing.
Water consumption and management in electrolysers are closely tied to catalyst layer architecture. Improved mass transport properties can lead to more efficient water utilization, reducing the overall water footprint of hydrogen production. This aspect is particularly important in water-stressed regions where sustainable water management is crucial.
As the hydrogen economy expands, the scalability of catalyst layer production becomes increasingly important from an environmental perspective. Developing catalyst architectures that can be manufactured at scale using environmentally friendly processes is essential for the widespread adoption of green hydrogen technologies.
Catalyst layer architecture directly affects the mass transport properties within the electrolyser, which in turn impacts energy consumption and resource utilization. Optimized catalyst layers can enhance the efficiency of water electrolysis, reducing the amount of electricity required to produce a given quantity of hydrogen. This improved energy efficiency translates to lower greenhouse gas emissions associated with hydrogen production, particularly when the electricity source is derived from renewable energy.
The choice of materials used in catalyst layers also plays a crucial role in environmental sustainability. Precious metals, such as platinum and iridium, are commonly used catalysts due to their high activity and stability. However, their scarcity and energy-intensive mining processes raise concerns about long-term sustainability and environmental impact. Research into alternative catalyst materials, such as non-precious metal catalysts or earth-abundant elements, aims to address these issues while maintaining or improving electrolyser performance.
Catalyst layer architecture can influence the durability and lifespan of electrolyser systems. More robust and stable catalyst layers can extend the operational life of electrolysers, reducing the frequency of replacements and associated material waste. This longevity contributes to a reduced environmental impact over the lifecycle of the electrolyser system.
The manufacturing processes for catalyst layers also have environmental implications. Advanced fabrication techniques, such as 3D printing or electrodeposition, may offer more precise control over catalyst layer structure and composition. These methods can potentially reduce material waste and energy consumption during production, further minimizing the environmental footprint of electrolyser manufacturing.
Water consumption and management in electrolysers are closely tied to catalyst layer architecture. Improved mass transport properties can lead to more efficient water utilization, reducing the overall water footprint of hydrogen production. This aspect is particularly important in water-stressed regions where sustainable water management is crucial.
As the hydrogen economy expands, the scalability of catalyst layer production becomes increasingly important from an environmental perspective. Developing catalyst architectures that can be manufactured at scale using environmentally friendly processes is essential for the widespread adoption of green hydrogen technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!