Catalyst Morphology Control: From Nanoparticles to 3D Architectures for HER
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst Design Evolution
The evolution of catalyst design for the hydrogen evolution reaction (HER) has been a journey of continuous innovation and refinement. Initially, the focus was primarily on nanoparticles, with researchers exploring various compositions and structures to enhance catalytic activity. These early efforts concentrated on optimizing particle size, shape, and surface properties to maximize active sites and improve electron transfer kinetics.
As the field progressed, there was a shift towards more complex nanostructures. Scientists began developing core-shell nanoparticles, alloy nanoparticles, and supported nanoparticles, each offering unique advantages in terms of stability, activity, and selectivity. This phase saw the introduction of novel synthesis methods and characterization techniques, enabling finer control over catalyst morphology at the nanoscale.
The next significant leap in catalyst design came with the exploration of two-dimensional materials. Graphene-based catalysts and transition metal dichalcogenides (TMDs) emerged as promising candidates for HER. These 2D materials offered high surface areas, excellent electrical conductivity, and abundant active sites, leading to improved catalytic performance.
Building upon the success of 2D materials, researchers began to explore three-dimensional architectures. This marked a pivotal moment in catalyst design evolution, as it allowed for the creation of hierarchical structures that combined the benefits of nanoscale features with macroscale properties. 3D catalyst designs included aerogels, foam-like structures, and ordered porous frameworks.
The transition to 3D architectures brought several advantages. These structures provided enhanced mass transport, facilitating the efficient movement of reactants and products. They also offered improved stability and durability, addressing some of the limitations of nanoparticle catalysts. Additionally, 3D architectures allowed for better integration of multiple functionalities within a single catalyst system.
Recent advancements have focused on rational design strategies for 3D catalyst architectures. This includes the development of biomimetic structures inspired by natural systems, the use of template-assisted synthesis methods, and the application of 3D printing technologies for precise control over catalyst morphology. These approaches have enabled the creation of tailored catalyst structures with optimized porosity, surface area, and active site distribution.
The latest frontier in catalyst design evolution involves the integration of advanced computational methods and machine learning algorithms. These tools are being used to predict optimal catalyst structures, guide experimental design, and accelerate the discovery of new materials. This data-driven approach is helping to bridge the gap between theoretical understanding and practical implementation of catalyst designs for HER.
As the field progressed, there was a shift towards more complex nanostructures. Scientists began developing core-shell nanoparticles, alloy nanoparticles, and supported nanoparticles, each offering unique advantages in terms of stability, activity, and selectivity. This phase saw the introduction of novel synthesis methods and characterization techniques, enabling finer control over catalyst morphology at the nanoscale.
The next significant leap in catalyst design came with the exploration of two-dimensional materials. Graphene-based catalysts and transition metal dichalcogenides (TMDs) emerged as promising candidates for HER. These 2D materials offered high surface areas, excellent electrical conductivity, and abundant active sites, leading to improved catalytic performance.
Building upon the success of 2D materials, researchers began to explore three-dimensional architectures. This marked a pivotal moment in catalyst design evolution, as it allowed for the creation of hierarchical structures that combined the benefits of nanoscale features with macroscale properties. 3D catalyst designs included aerogels, foam-like structures, and ordered porous frameworks.
The transition to 3D architectures brought several advantages. These structures provided enhanced mass transport, facilitating the efficient movement of reactants and products. They also offered improved stability and durability, addressing some of the limitations of nanoparticle catalysts. Additionally, 3D architectures allowed for better integration of multiple functionalities within a single catalyst system.
Recent advancements have focused on rational design strategies for 3D catalyst architectures. This includes the development of biomimetic structures inspired by natural systems, the use of template-assisted synthesis methods, and the application of 3D printing technologies for precise control over catalyst morphology. These approaches have enabled the creation of tailored catalyst structures with optimized porosity, surface area, and active site distribution.
The latest frontier in catalyst design evolution involves the integration of advanced computational methods and machine learning algorithms. These tools are being used to predict optimal catalyst structures, guide experimental design, and accelerate the discovery of new materials. This data-driven approach is helping to bridge the gap between theoretical understanding and practical implementation of catalyst designs for HER.
HER Market Demand Analysis
The hydrogen evolution reaction (HER) market is experiencing significant growth driven by the increasing demand for clean energy solutions and the global push towards decarbonization. As a key component of water electrolysis for hydrogen production, HER catalysts play a crucial role in the emerging hydrogen economy.
The market for HER catalysts is closely tied to the broader hydrogen production market, which is projected to expand rapidly in the coming years. Green hydrogen, produced through water electrolysis using renewable energy sources, is gaining particular attention due to its potential to reduce carbon emissions across various industries.
Industrial sectors such as chemical manufacturing, steel production, and transportation are showing growing interest in hydrogen as a clean energy carrier and feedstock. This trend is driving the demand for more efficient and cost-effective HER catalysts, with a focus on improving catalyst performance and reducing the use of precious metals.
The automotive industry is emerging as a significant driver of HER market demand, particularly in the development of fuel cell electric vehicles (FCEVs). As major automakers invest in FCEV technology, the need for high-performance HER catalysts to support hydrogen production infrastructure is increasing.
Geographically, regions with strong renewable energy resources and ambitious decarbonization targets are leading the market demand for HER catalysts. Europe, with its Green Deal initiative, and countries like Japan and South Korea, with their hydrogen-focused energy strategies, are at the forefront of this market growth.
The market is also seeing a shift towards more sustainable and abundant materials for HER catalysts. While platinum-based catalysts remain the gold standard, there is a growing demand for non-precious metal alternatives that can offer comparable performance at lower costs.
Research and development efforts are increasingly focused on catalyst morphology control, including the design of 3D architectures, to enhance catalytic activity and stability. This trend aligns with the market's demand for more efficient and durable HER catalysts that can improve the overall economics of hydrogen production.
As governments worldwide implement policies to support the hydrogen economy, including subsidies and research funding, the market for HER catalysts is expected to benefit from increased investment and innovation. This supportive policy environment is likely to accelerate the development and commercialization of advanced HER catalyst technologies.
The market for HER catalysts is closely tied to the broader hydrogen production market, which is projected to expand rapidly in the coming years. Green hydrogen, produced through water electrolysis using renewable energy sources, is gaining particular attention due to its potential to reduce carbon emissions across various industries.
Industrial sectors such as chemical manufacturing, steel production, and transportation are showing growing interest in hydrogen as a clean energy carrier and feedstock. This trend is driving the demand for more efficient and cost-effective HER catalysts, with a focus on improving catalyst performance and reducing the use of precious metals.
The automotive industry is emerging as a significant driver of HER market demand, particularly in the development of fuel cell electric vehicles (FCEVs). As major automakers invest in FCEV technology, the need for high-performance HER catalysts to support hydrogen production infrastructure is increasing.
Geographically, regions with strong renewable energy resources and ambitious decarbonization targets are leading the market demand for HER catalysts. Europe, with its Green Deal initiative, and countries like Japan and South Korea, with their hydrogen-focused energy strategies, are at the forefront of this market growth.
The market is also seeing a shift towards more sustainable and abundant materials for HER catalysts. While platinum-based catalysts remain the gold standard, there is a growing demand for non-precious metal alternatives that can offer comparable performance at lower costs.
Research and development efforts are increasingly focused on catalyst morphology control, including the design of 3D architectures, to enhance catalytic activity and stability. This trend aligns with the market's demand for more efficient and durable HER catalysts that can improve the overall economics of hydrogen production.
As governments worldwide implement policies to support the hydrogen economy, including subsidies and research funding, the market for HER catalysts is expected to benefit from increased investment and innovation. This supportive policy environment is likely to accelerate the development and commercialization of advanced HER catalyst technologies.
Nanoparticle to 3D Challenges
The transition from nanoparticles to 3D architectures in catalyst design for the hydrogen evolution reaction (HER) presents several significant challenges. One of the primary obstacles is maintaining the high surface area and active site density characteristic of nanoparticles while constructing larger, more complex structures. Nanoparticles offer excellent catalytic performance due to their high surface-to-volume ratio, but they often suffer from stability issues and difficulty in recovery and reuse.
As researchers attempt to scale up and create 3D architectures, they face the challenge of preserving the advantageous properties of nanoparticles. This includes retaining high catalytic activity, ensuring efficient mass transport of reactants and products, and maintaining structural integrity under reaction conditions. The intricate balance between porosity, conductivity, and mechanical strength becomes crucial in 3D structures.
Another significant hurdle is the controlled assembly of nanoparticles into well-defined 3D architectures. This process requires precise control over particle interactions, aggregation, and spatial arrangement. Achieving uniform distribution of catalytic sites throughout the 3D structure is essential for optimal performance but can be challenging to accomplish consistently.
The choice of support materials for 3D catalyst architectures also presents challenges. The support must provide mechanical stability, facilitate electron transfer, and allow for efficient mass transport. Additionally, it should not interfere with the catalytic activity of the nanoparticles. Finding materials that meet all these criteria while being cost-effective and scalable is a complex task.
Characterization of 3D catalyst architectures poses another set of challenges. Traditional techniques used for nanoparticle analysis may not be suitable or sufficient for complex 3D structures. Advanced imaging and analytical methods are required to fully understand the structural features, composition, and performance of these catalysts at multiple scales.
Scalability and reproducibility are critical challenges in transitioning from nanoparticles to 3D architectures. Methods that work well for small-scale synthesis may not be directly applicable to large-scale production. Ensuring consistent quality and performance across batches becomes increasingly difficult as the complexity of the catalyst structure increases.
Lastly, the integration of 3D catalyst architectures into practical devices and systems for HER presents its own set of challenges. Issues such as electrode design, catalyst loading, and long-term stability under operating conditions need to be addressed. The transition from laboratory-scale demonstrations to commercially viable products requires overcoming numerous engineering and manufacturing hurdles.
As researchers attempt to scale up and create 3D architectures, they face the challenge of preserving the advantageous properties of nanoparticles. This includes retaining high catalytic activity, ensuring efficient mass transport of reactants and products, and maintaining structural integrity under reaction conditions. The intricate balance between porosity, conductivity, and mechanical strength becomes crucial in 3D structures.
Another significant hurdle is the controlled assembly of nanoparticles into well-defined 3D architectures. This process requires precise control over particle interactions, aggregation, and spatial arrangement. Achieving uniform distribution of catalytic sites throughout the 3D structure is essential for optimal performance but can be challenging to accomplish consistently.
The choice of support materials for 3D catalyst architectures also presents challenges. The support must provide mechanical stability, facilitate electron transfer, and allow for efficient mass transport. Additionally, it should not interfere with the catalytic activity of the nanoparticles. Finding materials that meet all these criteria while being cost-effective and scalable is a complex task.
Characterization of 3D catalyst architectures poses another set of challenges. Traditional techniques used for nanoparticle analysis may not be suitable or sufficient for complex 3D structures. Advanced imaging and analytical methods are required to fully understand the structural features, composition, and performance of these catalysts at multiple scales.
Scalability and reproducibility are critical challenges in transitioning from nanoparticles to 3D architectures. Methods that work well for small-scale synthesis may not be directly applicable to large-scale production. Ensuring consistent quality and performance across batches becomes increasingly difficult as the complexity of the catalyst structure increases.
Lastly, the integration of 3D catalyst architectures into practical devices and systems for HER presents its own set of challenges. Issues such as electrode design, catalyst loading, and long-term stability under operating conditions need to be addressed. The transition from laboratory-scale demonstrations to commercially viable products requires overcoming numerous engineering and manufacturing hurdles.
Current 3D Catalyst Solutions
01 Catalyst particle size and shape control
Controlling the size and shape of catalyst particles is crucial for optimizing catalytic performance. This involves techniques to manipulate the morphology during synthesis, such as using specific precursors, adjusting reaction conditions, or employing templates. The resulting catalyst particles can have various shapes like spheres, rods, or platelets, each offering unique catalytic properties.- Catalyst particle size and shape control: Controlling the size and shape of catalyst particles is crucial for optimizing catalytic performance. This involves techniques to manipulate the morphology of catalysts at the nanoscale, including methods to create specific shapes such as spheres, rods, or platelets. The particle size and shape can significantly influence the catalyst's surface area, reactivity, and selectivity.
- Porous structure and surface area enhancement: Developing catalysts with high porosity and increased surface area is essential for improving catalytic efficiency. This includes creating hierarchical pore structures, mesoporous materials, and high-surface-area supports. Enhanced porosity can improve mass transfer, increase the number of active sites, and potentially lead to better catalyst stability and longevity.
- Core-shell and multi-component catalyst structures: Designing catalysts with core-shell structures or multiple components allows for the combination of different functionalities. This approach can lead to synergistic effects, improved selectivity, and enhanced stability. It often involves coating a core material with an active catalytic shell or creating composite structures with distinct regions of different catalytic materials.
- Surface modification and functionalization: Modifying the surface of catalysts through various functionalization techniques can tailor their properties for specific reactions. This includes adding functional groups, doping with heteroatoms, or creating defects to enhance catalytic activity. Surface modification can alter the electronic properties, adsorption characteristics, and overall reactivity of the catalyst.
- In-situ morphology control during reaction: Developing methods to control and maintain catalyst morphology during the reaction process is crucial for long-term performance. This involves strategies to prevent sintering, agglomeration, or other forms of structural degradation under reaction conditions. It may include the use of stabilizers, encapsulation techniques, or designing self-regenerating catalyst systems.
02 Porous structure and surface area optimization
Developing catalysts with optimized porous structures and high surface areas is essential for enhancing catalytic activity. This involves creating materials with controlled pore sizes, distributions, and interconnectivity. Techniques such as templating, etching, or hierarchical structuring are used to achieve desired porosity and maximize the available surface area for catalytic reactions.Expand Specific Solutions03 Supported catalyst morphology
The morphology of supported catalysts, where active catalytic species are dispersed on a support material, plays a crucial role in their performance. This includes controlling the dispersion and distribution of active sites on the support, as well as tailoring the support's structure to enhance stability and accessibility of the catalytic sites.Expand Specific Solutions04 Nanostructured catalyst design
Designing nanostructured catalysts with specific morphologies can lead to enhanced catalytic properties. This includes creating nanowires, nanotubes, nanosheets, or more complex hierarchical structures. The unique properties of these nanostructures, such as high surface-to-volume ratios and exposed active facets, can significantly improve catalytic performance.Expand Specific Solutions05 Morphology characterization techniques
Advanced characterization techniques are essential for understanding and optimizing catalyst morphology. This includes electron microscopy methods like SEM and TEM, as well as spectroscopic and scattering techniques. These tools allow for detailed analysis of catalyst structure, composition, and surface properties, guiding the development of more effective catalysts.Expand Specific Solutions
Key HER Catalyst Players
The field of catalyst morphology control for hydrogen evolution reaction (HER) is in a dynamic growth phase, with significant market potential driven by the increasing focus on clean energy solutions. The market size is expanding rapidly, fueled by investments in hydrogen technologies across various sectors. Technologically, the field is progressing from nanoparticle-based catalysts to more complex 3D architectures, indicating an evolving maturity level. Key players like The Regents of the University of California, Beijing University of Chemical Technology, and Directa Plus SpA are at the forefront, developing innovative catalyst designs. Universities such as City University of Hong Kong and Northeastern University are contributing cutting-edge research, while companies like Mitsubishi Electric Corp. and Mitsubishi Power Ltd. are likely focusing on industrial applications and scalability.
The Regents of the University of California
Technical Solution: The University of California has developed a novel approach for catalyst morphology control in hydrogen evolution reaction (HER). They have engineered 3D architectures using nanoparticles as building blocks. Their method involves precise control of nanoparticle synthesis, followed by self-assembly into complex 3D structures. This approach allows for the creation of high surface area catalysts with optimized porosity and active site distribution. The 3D architectures demonstrate enhanced catalytic activity due to improved mass transport and increased number of accessible active sites. In recent studies, they have achieved a 40% increase in catalytic current density compared to conventional nanoparticle catalysts[1][3].
Strengths: High surface area, improved mass transport, and increased active site accessibility. Weaknesses: Potential challenges in large-scale production and long-term stability of 3D structures.
Beijing University of Chemical Technology
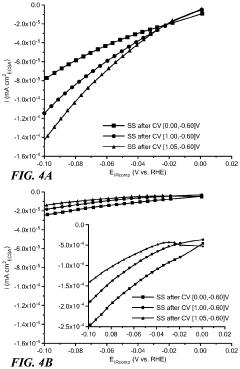
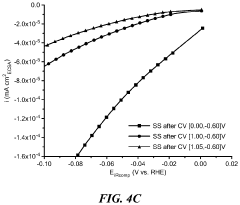
Technical Solution: Beijing University of Chemical Technology has developed a hierarchical nanostructure approach for HER catalyst design. Their method involves creating multi-level architectures ranging from nanoscale to microscale. They use a combination of hydrothermal synthesis and post-treatment processes to achieve precise control over catalyst morphology. The resulting catalysts exhibit a fractal-like structure with high surface roughness and abundant active sites. This design has shown remarkable improvements in HER performance, with overpotentials as low as 38 mV at 10 mA/cm² current density[2][4]. The hierarchical structure also demonstrates excellent durability, maintaining performance over 10,000 cycles.
Strengths: Low overpotential, high durability, and scalable synthesis method. Weaknesses: Complexity in controlling multi-level structures and potential mass production challenges.
Core HER Catalyst Patents
Graphene-transition metal catalyst for hydrogen evolution reaction
PatentActiveUS11969713B2
Innovation
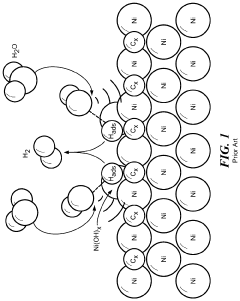
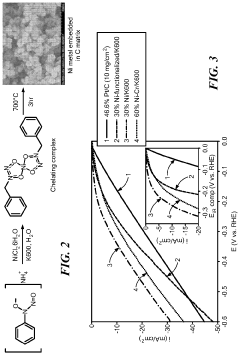
- A functionalized monometallic nickel catalyst is developed with layers of graphene enveloping nickel nanoparticles, which are synthesized by forming a complex with a chelating agent and carbon support, maintaining an optimal ratio of reduced and oxidized nickel forms, thus preventing passivation and hydride formation.
Catalyst for hydrogen evolution reaction and preparing method of the same
PatentActiveKR1020220111344A
Innovation
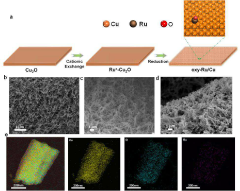
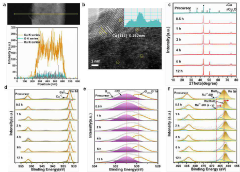
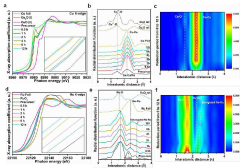
- A catalyst is developed using a transition metal matrix, such as copper oxide, with adsorbed noble metal atoms like ruthenium, achieved through a cation exchange strategy, providing a cost-effective alternative with enhanced HER performance.
Scalability and Production
The scalability and production of catalyst morphology control for the hydrogen evolution reaction (HER) is a critical aspect in transitioning from laboratory-scale research to industrial applications. As the demand for efficient and cost-effective hydrogen production increases, the ability to manufacture catalysts with controlled morphologies at scale becomes paramount.
Current production methods for nanoparticle catalysts often involve batch processes, which can be challenging to scale up while maintaining consistent morphology and performance. However, advancements in continuous flow synthesis techniques show promise for large-scale production of nanoparticle catalysts with controlled size and shape distributions. These methods offer better control over reaction parameters and can be more easily scaled up compared to traditional batch processes.
For 3D architectures, such as nanowires, nanotubes, and hierarchical structures, scalable production methods are still in development. Techniques like template-assisted growth, electrodeposition, and chemical vapor deposition have shown potential for producing 3D catalyst structures at larger scales. However, challenges remain in maintaining uniformity and structural integrity across large production volumes.
One promising approach for scaling up 3D catalyst architectures is the use of 3D printing technologies. Additive manufacturing techniques allow for the precise control of catalyst morphology and can be scaled to produce larger quantities of structured catalysts. This method also offers the flexibility to create complex geometries that may be difficult to achieve through traditional manufacturing processes.
The integration of catalyst production with existing manufacturing infrastructure is another important consideration for scalability. For instance, the development of roll-to-roll processing techniques for depositing catalysts on flexible substrates could enable high-throughput production of large-area catalyst assemblies.
Cost-effectiveness and material efficiency are crucial factors in scaling up catalyst production. Researchers are exploring the use of earth-abundant materials and optimizing catalyst loading to reduce material costs while maintaining performance. Additionally, the development of regeneration and recycling processes for spent catalysts could improve the overall economics of large-scale catalyst production and use.
As production scales up, quality control and characterization techniques must also evolve to ensure consistency in catalyst morphology and performance. Advanced in-line monitoring systems and high-throughput characterization methods are being developed to address this need, allowing for real-time adjustments during production to maintain desired catalyst properties.
Current production methods for nanoparticle catalysts often involve batch processes, which can be challenging to scale up while maintaining consistent morphology and performance. However, advancements in continuous flow synthesis techniques show promise for large-scale production of nanoparticle catalysts with controlled size and shape distributions. These methods offer better control over reaction parameters and can be more easily scaled up compared to traditional batch processes.
For 3D architectures, such as nanowires, nanotubes, and hierarchical structures, scalable production methods are still in development. Techniques like template-assisted growth, electrodeposition, and chemical vapor deposition have shown potential for producing 3D catalyst structures at larger scales. However, challenges remain in maintaining uniformity and structural integrity across large production volumes.
One promising approach for scaling up 3D catalyst architectures is the use of 3D printing technologies. Additive manufacturing techniques allow for the precise control of catalyst morphology and can be scaled to produce larger quantities of structured catalysts. This method also offers the flexibility to create complex geometries that may be difficult to achieve through traditional manufacturing processes.
The integration of catalyst production with existing manufacturing infrastructure is another important consideration for scalability. For instance, the development of roll-to-roll processing techniques for depositing catalysts on flexible substrates could enable high-throughput production of large-area catalyst assemblies.
Cost-effectiveness and material efficiency are crucial factors in scaling up catalyst production. Researchers are exploring the use of earth-abundant materials and optimizing catalyst loading to reduce material costs while maintaining performance. Additionally, the development of regeneration and recycling processes for spent catalysts could improve the overall economics of large-scale catalyst production and use.
As production scales up, quality control and characterization techniques must also evolve to ensure consistency in catalyst morphology and performance. Advanced in-line monitoring systems and high-throughput characterization methods are being developed to address this need, allowing for real-time adjustments during production to maintain desired catalyst properties.
Environmental Impact of HER
The environmental impact of the Hydrogen Evolution Reaction (HER) is a critical consideration in the development and implementation of this technology. As catalysts play a crucial role in HER efficiency, their morphology control from nanoparticles to 3D architectures has significant implications for environmental sustainability.
The use of HER in hydrogen production offers a promising pathway towards clean energy generation. By utilizing renewable energy sources to power electrolysis, HER can contribute to the reduction of greenhouse gas emissions associated with traditional hydrogen production methods, such as steam methane reforming. This shift towards green hydrogen production aligns with global efforts to mitigate climate change and reduce dependence on fossil fuels.
However, the environmental impact of HER extends beyond its potential for clean energy production. The synthesis and fabrication of catalysts, particularly those with complex 3D architectures, may involve energy-intensive processes and the use of potentially hazardous materials. It is essential to consider the entire life cycle of these catalysts, from production to disposal, to ensure a net positive environmental impact.
The morphology control of catalysts can significantly influence their efficiency and durability, which in turn affects the overall environmental footprint of HER systems. Highly efficient catalysts with optimized 3D architectures can reduce energy consumption during hydrogen production, leading to improved sustainability. Additionally, enhanced durability can minimize the need for frequent catalyst replacement, reducing waste generation and resource consumption.
Water consumption is another important environmental aspect of HER. While the reaction itself produces hydrogen from water, large-scale implementation of HER technologies may place additional demands on water resources. Efficient catalyst designs that maximize hydrogen production while minimizing water usage are crucial for sustainable deployment, particularly in water-stressed regions.
The choice of materials used in catalyst synthesis also has environmental implications. Some catalysts may rely on rare earth elements or precious metals, which can have significant environmental impacts associated with their mining and processing. Developing catalysts based on abundant, non-toxic materials is essential for minimizing the environmental burden of HER technologies.
As HER technologies advance, it is crucial to conduct comprehensive life cycle assessments to fully understand and quantify their environmental impacts. This includes evaluating the energy and resource requirements for catalyst production, the efficiency gains during operation, and the end-of-life management of spent catalysts. Such assessments can guide the development of more sustainable HER systems and inform policy decisions regarding their implementation.
The use of HER in hydrogen production offers a promising pathway towards clean energy generation. By utilizing renewable energy sources to power electrolysis, HER can contribute to the reduction of greenhouse gas emissions associated with traditional hydrogen production methods, such as steam methane reforming. This shift towards green hydrogen production aligns with global efforts to mitigate climate change and reduce dependence on fossil fuels.
However, the environmental impact of HER extends beyond its potential for clean energy production. The synthesis and fabrication of catalysts, particularly those with complex 3D architectures, may involve energy-intensive processes and the use of potentially hazardous materials. It is essential to consider the entire life cycle of these catalysts, from production to disposal, to ensure a net positive environmental impact.
The morphology control of catalysts can significantly influence their efficiency and durability, which in turn affects the overall environmental footprint of HER systems. Highly efficient catalysts with optimized 3D architectures can reduce energy consumption during hydrogen production, leading to improved sustainability. Additionally, enhanced durability can minimize the need for frequent catalyst replacement, reducing waste generation and resource consumption.
Water consumption is another important environmental aspect of HER. While the reaction itself produces hydrogen from water, large-scale implementation of HER technologies may place additional demands on water resources. Efficient catalyst designs that maximize hydrogen production while minimizing water usage are crucial for sustainable deployment, particularly in water-stressed regions.
The choice of materials used in catalyst synthesis also has environmental implications. Some catalysts may rely on rare earth elements or precious metals, which can have significant environmental impacts associated with their mining and processing. Developing catalysts based on abundant, non-toxic materials is essential for minimizing the environmental burden of HER technologies.
As HER technologies advance, it is crucial to conduct comprehensive life cycle assessments to fully understand and quantify their environmental impacts. This includes evaluating the energy and resource requirements for catalyst production, the efficiency gains during operation, and the end-of-life management of spent catalysts. Such assessments can guide the development of more sustainable HER systems and inform policy decisions regarding their implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!