Best Catalysts for Green Hydrogen Electrolysis: Non-Precious Options that Scale
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Green H2 Catalysis Background and Objectives
Green hydrogen production through water electrolysis has emerged as a promising solution for sustainable energy systems. The evolution of this technology can be traced back to the early 19th century when water electrolysis was first discovered. However, it is only in recent decades that significant advancements have been made in improving the efficiency and scalability of this process.
The primary objective of research in green hydrogen catalysis is to develop non-precious catalysts that can efficiently split water molecules into hydrogen and oxygen. These catalysts must be cost-effective, durable, and capable of operating at high current densities to enable large-scale hydrogen production. The focus on non-precious materials is driven by the need to reduce the overall cost of electrolyzers and make green hydrogen economically competitive with fossil fuel-derived hydrogen.
Current technological trends in this field include the exploration of transition metal-based catalysts, such as nickel, iron, and cobalt compounds, as alternatives to precious metal catalysts like platinum and iridium. Researchers are also investigating novel nanostructured materials and composite catalysts to enhance catalytic activity and stability. Additionally, there is growing interest in developing bifunctional catalysts that can perform both hydrogen and oxygen evolution reactions, simplifying electrolyzer design and reducing costs.
The development of scalable non-precious catalysts for green hydrogen production is expected to play a crucial role in the global transition to a hydrogen-based economy. This technology has the potential to revolutionize various sectors, including transportation, industry, and energy storage. As countries worldwide set ambitious targets for carbon neutrality, the demand for efficient and cost-effective green hydrogen production methods is likely to increase significantly in the coming years.
To achieve the goals of this research, scientists and engineers are focusing on several key areas. These include optimizing catalyst composition and structure, improving catalyst synthesis methods, and enhancing the understanding of reaction mechanisms at the molecular level. Advanced characterization techniques and computational modeling are being employed to accelerate the discovery and development of novel catalytic materials.
In conclusion, the research on non-precious catalysts for green hydrogen electrolysis represents a critical step towards sustainable energy production. By addressing the challenges of cost, efficiency, and scalability, this technology has the potential to make a significant impact on global efforts to mitigate climate change and establish a clean energy future.
The primary objective of research in green hydrogen catalysis is to develop non-precious catalysts that can efficiently split water molecules into hydrogen and oxygen. These catalysts must be cost-effective, durable, and capable of operating at high current densities to enable large-scale hydrogen production. The focus on non-precious materials is driven by the need to reduce the overall cost of electrolyzers and make green hydrogen economically competitive with fossil fuel-derived hydrogen.
Current technological trends in this field include the exploration of transition metal-based catalysts, such as nickel, iron, and cobalt compounds, as alternatives to precious metal catalysts like platinum and iridium. Researchers are also investigating novel nanostructured materials and composite catalysts to enhance catalytic activity and stability. Additionally, there is growing interest in developing bifunctional catalysts that can perform both hydrogen and oxygen evolution reactions, simplifying electrolyzer design and reducing costs.
The development of scalable non-precious catalysts for green hydrogen production is expected to play a crucial role in the global transition to a hydrogen-based economy. This technology has the potential to revolutionize various sectors, including transportation, industry, and energy storage. As countries worldwide set ambitious targets for carbon neutrality, the demand for efficient and cost-effective green hydrogen production methods is likely to increase significantly in the coming years.
To achieve the goals of this research, scientists and engineers are focusing on several key areas. These include optimizing catalyst composition and structure, improving catalyst synthesis methods, and enhancing the understanding of reaction mechanisms at the molecular level. Advanced characterization techniques and computational modeling are being employed to accelerate the discovery and development of novel catalytic materials.
In conclusion, the research on non-precious catalysts for green hydrogen electrolysis represents a critical step towards sustainable energy production. By addressing the challenges of cost, efficiency, and scalability, this technology has the potential to make a significant impact on global efforts to mitigate climate change and establish a clean energy future.
Market Analysis for Non-Precious Catalysts
The market for non-precious catalysts in green hydrogen electrolysis is experiencing rapid growth, driven by the increasing demand for sustainable energy solutions and the need to reduce production costs. As the global focus on decarbonization intensifies, the hydrogen economy is gaining momentum, with green hydrogen production through electrolysis at its core.
Currently, the market is dominated by precious metal catalysts, particularly platinum and iridium, due to their high efficiency and stability. However, the scarcity and high cost of these materials present significant barriers to large-scale adoption of hydrogen electrolysis technologies. This has created a substantial opportunity for non-precious catalysts to enter and disrupt the market.
The potential market size for non-precious catalysts in green hydrogen electrolysis is substantial. The global green hydrogen market is projected to grow significantly in the coming years, with electrolysis playing a crucial role in this expansion. As the demand for electrolyzers increases, the market for catalysts, including non-precious alternatives, is expected to grow proportionally.
Key market drivers for non-precious catalysts include cost reduction, scalability, and sustainability. Industries such as transportation, power generation, and industrial processes are increasingly looking to integrate green hydrogen into their operations, creating a diverse and expanding customer base for non-precious catalyst technologies.
Geographically, regions with strong renewable energy infrastructure and ambitious decarbonization targets, such as Europe, North America, and parts of Asia, are expected to be primary markets for non-precious catalysts. These areas are investing heavily in hydrogen technologies and are likely to be early adopters of cost-effective, scalable solutions.
The competitive landscape is evolving rapidly, with both established chemical companies and innovative startups entering the non-precious catalyst market. Research institutions and universities are also playing a significant role in developing and commercializing new catalyst technologies.
Market challenges include performance parity with precious metal catalysts, long-term stability, and integration with existing electrolysis systems. Overcoming these hurdles will be crucial for widespread market adoption. Additionally, regulatory frameworks and government incentives will play a significant role in shaping market dynamics and accelerating the transition to non-precious catalysts.
In conclusion, the market for non-precious catalysts in green hydrogen electrolysis presents a significant opportunity for innovation and growth. As technology advances and production scales up, non-precious catalysts have the potential to revolutionize the economics of green hydrogen production, making it more accessible and economically viable across various industries and applications.
Currently, the market is dominated by precious metal catalysts, particularly platinum and iridium, due to their high efficiency and stability. However, the scarcity and high cost of these materials present significant barriers to large-scale adoption of hydrogen electrolysis technologies. This has created a substantial opportunity for non-precious catalysts to enter and disrupt the market.
The potential market size for non-precious catalysts in green hydrogen electrolysis is substantial. The global green hydrogen market is projected to grow significantly in the coming years, with electrolysis playing a crucial role in this expansion. As the demand for electrolyzers increases, the market for catalysts, including non-precious alternatives, is expected to grow proportionally.
Key market drivers for non-precious catalysts include cost reduction, scalability, and sustainability. Industries such as transportation, power generation, and industrial processes are increasingly looking to integrate green hydrogen into their operations, creating a diverse and expanding customer base for non-precious catalyst technologies.
Geographically, regions with strong renewable energy infrastructure and ambitious decarbonization targets, such as Europe, North America, and parts of Asia, are expected to be primary markets for non-precious catalysts. These areas are investing heavily in hydrogen technologies and are likely to be early adopters of cost-effective, scalable solutions.
The competitive landscape is evolving rapidly, with both established chemical companies and innovative startups entering the non-precious catalyst market. Research institutions and universities are also playing a significant role in developing and commercializing new catalyst technologies.
Market challenges include performance parity with precious metal catalysts, long-term stability, and integration with existing electrolysis systems. Overcoming these hurdles will be crucial for widespread market adoption. Additionally, regulatory frameworks and government incentives will play a significant role in shaping market dynamics and accelerating the transition to non-precious catalysts.
In conclusion, the market for non-precious catalysts in green hydrogen electrolysis presents a significant opportunity for innovation and growth. As technology advances and production scales up, non-precious catalysts have the potential to revolutionize the economics of green hydrogen production, making it more accessible and economically viable across various industries and applications.
Current Challenges in Scalable Green H2 Production
The production of green hydrogen through electrolysis faces several significant challenges that hinder its scalability and widespread adoption. One of the primary obstacles is the high cost associated with the electrolysis process, particularly due to the use of precious metal catalysts such as platinum and iridium. These materials, while highly effective, are scarce and expensive, making large-scale implementation economically unfeasible.
The durability and stability of catalysts present another major hurdle. Non-precious catalysts often suffer from rapid degradation under the harsh conditions of electrolysis, leading to decreased efficiency and frequent replacement needs. This not only increases operational costs but also impacts the long-term viability of green hydrogen production systems.
Efficiency remains a critical challenge in scaling up green hydrogen production. Current non-precious catalysts generally exhibit lower catalytic activity compared to their precious metal counterparts, resulting in higher energy requirements and reduced hydrogen yield. This efficiency gap needs to be bridged to make large-scale green hydrogen production economically competitive with traditional fossil fuel-based methods.
The complexity of integrating electrolysis systems with renewable energy sources poses additional challenges. Intermittent nature of renewable energy supplies can lead to fluctuations in power input, affecting the stability and performance of electrolysis systems. Developing robust catalysts that can maintain high efficiency under variable operating conditions is crucial for seamless integration with renewable energy sources.
Scalability itself presents unique challenges in terms of manufacturing and material availability. As production scales up, ensuring a steady supply of catalyst materials becomes increasingly important. Some promising non-precious catalysts may face limitations in large-scale synthesis or may rely on elements that could become scarce with increased demand.
Environmental and safety concerns also play a role in scaling green hydrogen production. While the process is considered clean, the long-term environmental impacts of large-scale catalyst production and disposal need to be carefully assessed. Additionally, ensuring the safety of large-scale hydrogen production and storage facilities remains a critical consideration.
Addressing these challenges requires a multifaceted approach, combining advances in materials science, process engineering, and system integration. Developing novel, high-performance non-precious catalysts that can match or exceed the efficiency of precious metal catalysts while maintaining durability and scalability is key to overcoming these obstacles and realizing the potential of green hydrogen as a sustainable energy carrier.
The durability and stability of catalysts present another major hurdle. Non-precious catalysts often suffer from rapid degradation under the harsh conditions of electrolysis, leading to decreased efficiency and frequent replacement needs. This not only increases operational costs but also impacts the long-term viability of green hydrogen production systems.
Efficiency remains a critical challenge in scaling up green hydrogen production. Current non-precious catalysts generally exhibit lower catalytic activity compared to their precious metal counterparts, resulting in higher energy requirements and reduced hydrogen yield. This efficiency gap needs to be bridged to make large-scale green hydrogen production economically competitive with traditional fossil fuel-based methods.
The complexity of integrating electrolysis systems with renewable energy sources poses additional challenges. Intermittent nature of renewable energy supplies can lead to fluctuations in power input, affecting the stability and performance of electrolysis systems. Developing robust catalysts that can maintain high efficiency under variable operating conditions is crucial for seamless integration with renewable energy sources.
Scalability itself presents unique challenges in terms of manufacturing and material availability. As production scales up, ensuring a steady supply of catalyst materials becomes increasingly important. Some promising non-precious catalysts may face limitations in large-scale synthesis or may rely on elements that could become scarce with increased demand.
Environmental and safety concerns also play a role in scaling green hydrogen production. While the process is considered clean, the long-term environmental impacts of large-scale catalyst production and disposal need to be carefully assessed. Additionally, ensuring the safety of large-scale hydrogen production and storage facilities remains a critical consideration.
Addressing these challenges requires a multifaceted approach, combining advances in materials science, process engineering, and system integration. Developing novel, high-performance non-precious catalysts that can match or exceed the efficiency of precious metal catalysts while maintaining durability and scalability is key to overcoming these obstacles and realizing the potential of green hydrogen as a sustainable energy carrier.
Existing Non-Precious Catalyst Solutions
01 Novel catalyst materials for efficient hydrogen production
Development of advanced catalyst materials to enhance the efficiency of hydrogen production through electrolysis. These materials aim to improve the catalytic activity, stability, and selectivity of the electrodes, leading to increased hydrogen yield and reduced energy consumption in the electrolysis process.- Novel catalyst materials for efficient hydrogen production: Development of new catalyst materials to enhance the efficiency of hydrogen production through electrolysis. These materials aim to improve the catalytic activity, stability, and selectivity of the electrodes, leading to increased hydrogen yield and reduced energy consumption in the electrolysis process.
- Scalable electrode designs for large-scale electrolysis: Innovative electrode designs that can be easily scaled up for industrial-scale hydrogen production. These designs focus on increasing the active surface area, improving mass transfer, and enhancing the overall performance of electrolyzers while maintaining cost-effectiveness for large-scale applications.
- Advanced membrane technologies for electrolysis efficiency: Development of high-performance membranes that improve the separation of hydrogen and oxygen during electrolysis. These membranes aim to reduce gas crossover, enhance ion conductivity, and increase the overall efficiency of the electrolysis process, particularly in large-scale operations.
- System integration and process optimization for scalability: Innovative approaches to integrate electrolysis systems with renewable energy sources and optimize the overall process for large-scale hydrogen production. This includes advanced control systems, energy management strategies, and modular designs that facilitate easy scaling and maintenance of electrolysis plants.
- Cost-effective manufacturing techniques for electrolysis components: Development of novel manufacturing methods and materials to reduce the production costs of key electrolysis components. These techniques aim to improve the scalability of green hydrogen production by making the manufacturing process more efficient and economical, particularly for large-scale applications.
02 Scalable electrode designs for large-scale electrolysis
Innovative electrode designs that can be easily scaled up for industrial-scale hydrogen production. These designs focus on increasing the active surface area, improving mass transfer, and enhancing the overall performance of electrolyzers while maintaining cost-effectiveness for large-scale applications.Expand Specific Solutions03 Integration of renewable energy sources with electrolysis systems
Development of integrated systems that combine renewable energy sources, such as solar or wind power, with electrolysis units. These systems aim to optimize the use of intermittent renewable energy for green hydrogen production, addressing challenges related to energy storage and grid stability.Expand Specific Solutions04 Advanced membrane technologies for improved electrolyte separation
Development of high-performance membrane materials and designs to enhance the separation of hydrogen and oxygen during electrolysis. These advanced membranes aim to increase the purity of produced hydrogen, improve system efficiency, and extend the lifespan of electrolysis units.Expand Specific Solutions05 Process optimization and control systems for scalable electrolysis
Implementation of advanced process control and optimization techniques to enhance the scalability and efficiency of electrolysis systems. These innovations focus on real-time monitoring, adaptive control strategies, and predictive maintenance to maximize hydrogen production while minimizing operational costs and downtime.Expand Specific Solutions
Key Players in Green Hydrogen Catalyst Industry
The research on non-precious catalysts for green hydrogen electrolysis is in a rapidly evolving phase, with significant market potential due to the growing focus on sustainable energy solutions. The global market for green hydrogen is projected to expand substantially, driven by decarbonization efforts. Technologically, the field is advancing, with various institutions and companies making progress. Key players like King Fahd University of Petroleum & Minerals, Industrial Technology Research Institute, and Nanyang Technological University are contributing to catalyst development. Companies such as AbbVie and Industrie De Nora are also involved, indicating a mix of academic and industrial efforts. While promising advancements have been made, the technology is still maturing, with ongoing research aimed at improving efficiency and scalability.
The Regents of the University of California
Technical Solution: The University of California has developed a novel non-precious metal catalyst for green hydrogen electrolysis based on nickel and iron. Their approach involves creating a nanostructured nickel-iron hydroxide material with high surface area and abundant active sites. This catalyst demonstrates excellent activity and stability for the oxygen evolution reaction (OER) in alkaline electrolyzers[1]. The team has optimized the Ni:Fe ratio and synthesis conditions to achieve performance comparable to precious metal catalysts. Additionally, they have incorporated the catalyst into a membrane electrode assembly (MEA) design that enhances mass transport and reduces overpotential[2]. Scalability has been addressed through the development of a continuous flow synthesis method that can produce large quantities of the catalyst material[3].
Strengths: Low-cost materials, high catalytic activity, good stability, and scalable synthesis. Weaknesses: Performance in acidic conditions may be limited, and long-term durability in industrial settings needs further validation.
Industrial Technology Research Institute
Technical Solution: The Industrial Technology Research Institute (ITRI) has made significant progress in developing non-precious catalysts for green hydrogen electrolysis. Their approach focuses on transition metal-based materials, particularly cobalt and manganese oxides. ITRI has engineered these materials at the nanoscale to create high-surface-area structures with optimized electronic properties[4]. The institute has also developed a novel doping strategy to enhance the catalytic activity and stability of these materials. Their catalysts have shown promising performance in both alkaline and polymer electrolyte membrane (PEM) electrolyzers[5]. To address scalability, ITRI has implemented a spray pyrolysis technique that allows for continuous production of catalyst particles with controlled composition and morphology[6].
Strengths: Cost-effective materials, versatility in different electrolyte environments, and scalable production method. Weaknesses: Activity still lower than some precious metal catalysts, and long-term stability in highly corrosive conditions needs improvement.
Innovative Catalysts for Efficient Electrolysis
An electrolyzer system with nonprecious electrocatalysts for green h 2 production by electrolysis of water
PatentWO2024003930A1
Innovation
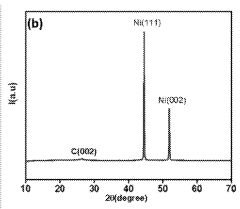
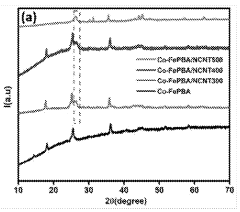
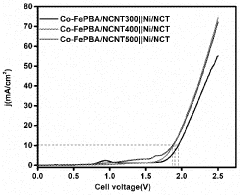
- An electrolyzer system using Prussian blue analogues (PBA) and nitrogen-doped carbon nanotubes (NCNT) as the anode and metallic nickel particles encapsulated in nitrogen-doped carbon tubules as the cathode, supported by carbon materials, which reduces the cost and enhances stability and selectivity towards oxygen evolution over chlorine evolution.
High-efficiency electrolysis system for green hydrogen production using sustainable catalysts
PatentPendingIN202341048312A
Innovation
- A high-efficiency electrolysis system utilizing sustainable catalysts, such as transition metal oxides, metal phosphides, and carbon-based materials, which are abundant and environmentally friendly, reducing reliance on precious metals and enhancing system stability and durability.
Environmental Impact of Catalyst Materials
The environmental impact of catalyst materials for green hydrogen electrolysis is a critical consideration in the pursuit of sustainable energy solutions. Non-precious catalysts, while offering cost advantages, may present unique environmental challenges throughout their lifecycle. The extraction and processing of raw materials for these catalysts can have significant ecological implications, including habitat disruption, water pollution, and energy-intensive mining operations.
During the manufacturing phase, the production of non-precious catalysts often involves complex chemical processes that may generate hazardous waste and emissions. These byproducts require careful management to minimize environmental harm. Additionally, the energy consumption associated with catalyst synthesis contributes to the overall carbon footprint of the electrolysis process.
In operational use, non-precious catalysts generally demonstrate lower environmental impact compared to their precious metal counterparts. They typically require less frequent replacement, reducing the need for continuous resource extraction and manufacturing. However, the potential leaching of catalyst materials into water systems during electrolysis must be carefully monitored and mitigated to prevent aquatic ecosystem contamination.
The end-of-life phase for non-precious catalysts presents both challenges and opportunities. Recycling and recovery processes for these materials are often less developed compared to precious metals, potentially leading to increased waste. However, advancements in recycling technologies are gradually improving the recoverability of non-precious catalyst components, promoting a more circular economy approach.
When scaling up green hydrogen production, the environmental impact of catalyst materials becomes even more pronounced. Large-scale deployment necessitates careful consideration of land use for catalyst production facilities and the associated infrastructure. The increased demand for raw materials may also lead to more extensive mining operations, potentially exacerbating environmental degradation in resource-rich regions.
To address these concerns, researchers are exploring bio-inspired catalysts and earth-abundant materials that offer reduced environmental impact. These innovative approaches aim to minimize the use of harmful chemicals in catalyst synthesis and improve the overall sustainability of the electrolysis process. Furthermore, the development of catalysts with enhanced durability and efficiency can significantly reduce the environmental footprint of green hydrogen production over time.
In conclusion, while non-precious catalysts for green hydrogen electrolysis offer promising environmental benefits compared to traditional precious metal alternatives, their impact across the entire lifecycle must be carefully assessed and managed. Continued research and development efforts are essential to optimize these materials for both performance and environmental sustainability, ensuring that the transition to green hydrogen aligns with broader ecological preservation goals.
During the manufacturing phase, the production of non-precious catalysts often involves complex chemical processes that may generate hazardous waste and emissions. These byproducts require careful management to minimize environmental harm. Additionally, the energy consumption associated with catalyst synthesis contributes to the overall carbon footprint of the electrolysis process.
In operational use, non-precious catalysts generally demonstrate lower environmental impact compared to their precious metal counterparts. They typically require less frequent replacement, reducing the need for continuous resource extraction and manufacturing. However, the potential leaching of catalyst materials into water systems during electrolysis must be carefully monitored and mitigated to prevent aquatic ecosystem contamination.
The end-of-life phase for non-precious catalysts presents both challenges and opportunities. Recycling and recovery processes for these materials are often less developed compared to precious metals, potentially leading to increased waste. However, advancements in recycling technologies are gradually improving the recoverability of non-precious catalyst components, promoting a more circular economy approach.
When scaling up green hydrogen production, the environmental impact of catalyst materials becomes even more pronounced. Large-scale deployment necessitates careful consideration of land use for catalyst production facilities and the associated infrastructure. The increased demand for raw materials may also lead to more extensive mining operations, potentially exacerbating environmental degradation in resource-rich regions.
To address these concerns, researchers are exploring bio-inspired catalysts and earth-abundant materials that offer reduced environmental impact. These innovative approaches aim to minimize the use of harmful chemicals in catalyst synthesis and improve the overall sustainability of the electrolysis process. Furthermore, the development of catalysts with enhanced durability and efficiency can significantly reduce the environmental footprint of green hydrogen production over time.
In conclusion, while non-precious catalysts for green hydrogen electrolysis offer promising environmental benefits compared to traditional precious metal alternatives, their impact across the entire lifecycle must be carefully assessed and managed. Continued research and development efforts are essential to optimize these materials for both performance and environmental sustainability, ensuring that the transition to green hydrogen aligns with broader ecological preservation goals.
Economic Feasibility of Non-Precious Catalysts
The economic feasibility of non-precious catalysts for green hydrogen electrolysis is a critical factor in the widespread adoption of this technology. Traditional precious metal catalysts, such as platinum and iridium, have long been the standard for electrolysis due to their high efficiency and stability. However, their scarcity and high cost have hindered the large-scale implementation of green hydrogen production.
Non-precious catalysts, primarily based on abundant transition metals like nickel, iron, and cobalt, offer a promising alternative. These materials are significantly less expensive and more readily available, potentially reducing the overall cost of electrolysis systems. For instance, nickel-based catalysts have shown comparable activity to platinum in alkaline electrolyzers, while being up to 1000 times less expensive per unit mass.
The economic advantages of non-precious catalysts extend beyond material costs. Their abundance allows for easier scaling of production, reducing supply chain risks and price volatility associated with precious metals. This stability in material costs can provide greater predictability for long-term investments in hydrogen infrastructure.
However, the economic feasibility of non-precious catalysts must be evaluated holistically. While material costs are lower, these catalysts often require higher loadings to achieve comparable performance to precious metal counterparts. This can partially offset the cost savings in some cases. Additionally, the durability and longevity of non-precious catalysts under industrial conditions are still being optimized, which could impact long-term operational costs.
Research and development efforts are focused on enhancing the activity and stability of non-precious catalysts to further improve their economic viability. Strategies such as nanostructuring, doping, and creating multi-metallic compounds are showing promising results in bridging the performance gap with precious metals while maintaining cost advantages.
The economic feasibility of non-precious catalysts is also influenced by the broader context of renewable energy integration and carbon pricing mechanisms. As the cost of renewable electricity decreases and carbon taxes increase, the overall economics of green hydrogen production improve, making the use of non-precious catalysts even more attractive.
In conclusion, while challenges remain, the economic potential of non-precious catalysts for green hydrogen electrolysis is significant. Their successful development and implementation could be a key enabler in making green hydrogen economically competitive with fossil fuel-based alternatives, driving the transition to a sustainable hydrogen economy.
Non-precious catalysts, primarily based on abundant transition metals like nickel, iron, and cobalt, offer a promising alternative. These materials are significantly less expensive and more readily available, potentially reducing the overall cost of electrolysis systems. For instance, nickel-based catalysts have shown comparable activity to platinum in alkaline electrolyzers, while being up to 1000 times less expensive per unit mass.
The economic advantages of non-precious catalysts extend beyond material costs. Their abundance allows for easier scaling of production, reducing supply chain risks and price volatility associated with precious metals. This stability in material costs can provide greater predictability for long-term investments in hydrogen infrastructure.
However, the economic feasibility of non-precious catalysts must be evaluated holistically. While material costs are lower, these catalysts often require higher loadings to achieve comparable performance to precious metal counterparts. This can partially offset the cost savings in some cases. Additionally, the durability and longevity of non-precious catalysts under industrial conditions are still being optimized, which could impact long-term operational costs.
Research and development efforts are focused on enhancing the activity and stability of non-precious catalysts to further improve their economic viability. Strategies such as nanostructuring, doping, and creating multi-metallic compounds are showing promising results in bridging the performance gap with precious metals while maintaining cost advantages.
The economic feasibility of non-precious catalysts is also influenced by the broader context of renewable energy integration and carbon pricing mechanisms. As the cost of renewable electricity decreases and carbon taxes increase, the overall economics of green hydrogen production improve, making the use of non-precious catalysts even more attractive.
In conclusion, while challenges remain, the economic potential of non-precious catalysts for green hydrogen electrolysis is significant. Their successful development and implementation could be a key enabler in making green hydrogen economically competitive with fossil fuel-based alternatives, driving the transition to a sustainable hydrogen economy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!