Low-Overpotential Catalysts for AEM Electrolysis: Synthesis and Demonstration
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
AEM Electrolysis Catalysts: Background and Objectives
Anion Exchange Membrane (AEM) electrolysis has emerged as a promising technology for sustainable hydrogen production, offering significant advantages over traditional alkaline and proton exchange membrane (PEM) electrolysis. The development of low-overpotential catalysts for AEM electrolysis is crucial for improving the efficiency and economic viability of this technology.
The history of AEM electrolysis can be traced back to the early 2000s when researchers began exploring alternatives to PEM electrolysis. The primary motivation was to overcome the limitations of PEM systems, such as the need for expensive noble metal catalysts and highly corrosive acidic environments. AEM electrolysis offered the potential for using non-noble metal catalysts and operating in alkaline conditions, which could significantly reduce costs and improve durability.
Over the past two decades, significant progress has been made in AEM electrolysis technology. Early research focused on developing suitable membrane materials that could withstand alkaline conditions while maintaining high ionic conductivity. As membrane technology advanced, attention shifted towards developing efficient catalysts for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER).
The current state of AEM electrolysis technology is characterized by ongoing efforts to improve catalyst performance, particularly in terms of reducing overpotential. Overpotential refers to the additional voltage required to drive the electrolysis reaction beyond the thermodynamic minimum. Lower overpotential translates to higher energy efficiency and reduced operational costs.
The primary objective of research on low-overpotential catalysts for AEM electrolysis is to develop materials that can facilitate rapid charge transfer and efficient gas evolution while maintaining long-term stability in alkaline environments. This involves exploring novel catalyst compositions, optimizing nanostructures, and investigating synergistic effects between different materials.
Key areas of focus include the development of non-precious metal catalysts, such as nickel-based alloys and transition metal oxides, which show promise in terms of activity and stability. Additionally, researchers are exploring the use of advanced carbon-based materials as supports or co-catalysts to enhance overall performance.
The ultimate goal of this research is to achieve catalyst systems that can operate at current densities comparable to PEM electrolyzers (>1 A/cm²) while maintaining low overpotentials (<300 mV) for both HER and OER. This would position AEM electrolysis as a highly competitive technology for large-scale hydrogen production, potentially revolutionizing the renewable energy landscape.
The history of AEM electrolysis can be traced back to the early 2000s when researchers began exploring alternatives to PEM electrolysis. The primary motivation was to overcome the limitations of PEM systems, such as the need for expensive noble metal catalysts and highly corrosive acidic environments. AEM electrolysis offered the potential for using non-noble metal catalysts and operating in alkaline conditions, which could significantly reduce costs and improve durability.
Over the past two decades, significant progress has been made in AEM electrolysis technology. Early research focused on developing suitable membrane materials that could withstand alkaline conditions while maintaining high ionic conductivity. As membrane technology advanced, attention shifted towards developing efficient catalysts for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER).
The current state of AEM electrolysis technology is characterized by ongoing efforts to improve catalyst performance, particularly in terms of reducing overpotential. Overpotential refers to the additional voltage required to drive the electrolysis reaction beyond the thermodynamic minimum. Lower overpotential translates to higher energy efficiency and reduced operational costs.
The primary objective of research on low-overpotential catalysts for AEM electrolysis is to develop materials that can facilitate rapid charge transfer and efficient gas evolution while maintaining long-term stability in alkaline environments. This involves exploring novel catalyst compositions, optimizing nanostructures, and investigating synergistic effects between different materials.
Key areas of focus include the development of non-precious metal catalysts, such as nickel-based alloys and transition metal oxides, which show promise in terms of activity and stability. Additionally, researchers are exploring the use of advanced carbon-based materials as supports or co-catalysts to enhance overall performance.
The ultimate goal of this research is to achieve catalyst systems that can operate at current densities comparable to PEM electrolyzers (>1 A/cm²) while maintaining low overpotentials (<300 mV) for both HER and OER. This would position AEM electrolysis as a highly competitive technology for large-scale hydrogen production, potentially revolutionizing the renewable energy landscape.
Market Analysis for Low-Overpotential AEM Electrolyzers
The market for low-overpotential Anion Exchange Membrane (AEM) electrolyzers is experiencing significant growth, driven by the increasing demand for clean hydrogen production. This technology offers several advantages over traditional alkaline and Proton Exchange Membrane (PEM) electrolyzers, including lower costs, higher efficiency, and improved durability.
The global hydrogen generation market, which includes AEM electrolyzers, is projected to expand rapidly in the coming years. This growth is primarily fueled by the rising adoption of hydrogen as a clean energy carrier in various sectors, including transportation, industry, and power generation. Governments worldwide are implementing supportive policies and incentives to promote hydrogen technologies, further stimulating market demand for efficient electrolysis solutions.
AEM electrolyzers with low overpotential catalysts are particularly well-positioned to capture a significant share of this growing market. The reduced overpotential translates to higher energy efficiency, lower operational costs, and increased hydrogen production rates. These benefits make low-overpotential AEM electrolyzers attractive for both small-scale and large-scale hydrogen production applications.
The transportation sector represents a key market segment for AEM electrolyzers. As fuel cell electric vehicles (FCEVs) gain traction, the demand for cost-effective and efficient hydrogen production methods is expected to surge. Low-overpotential AEM electrolyzers can play a crucial role in establishing a sustainable hydrogen refueling infrastructure, supporting the widespread adoption of FCEVs.
Industrial applications also present substantial market opportunities for AEM electrolyzers. Many industries, such as chemical manufacturing, steel production, and ammonia synthesis, require large quantities of hydrogen. The ability of low-overpotential AEM electrolyzers to produce hydrogen efficiently and at scale makes them an attractive option for industrial users looking to reduce their carbon footprint and operational costs.
The power sector is another promising market for AEM electrolyzers. As renewable energy sources like wind and solar become more prevalent, the need for energy storage solutions grows. Hydrogen produced through electrolysis can serve as a long-term energy storage medium, helping to balance grid fluctuations and enable greater integration of intermittent renewable energy sources.
Geographically, Europe and Asia-Pacific are expected to be the leading markets for AEM electrolyzers. European countries, driven by ambitious decarbonization targets and supportive policies, are investing heavily in hydrogen infrastructure. In Asia-Pacific, countries like Japan, South Korea, and China are actively promoting hydrogen technologies as part of their energy transition strategies.
Despite the promising market outlook, challenges remain for the widespread adoption of low-overpotential AEM electrolyzers. These include the need for further cost reductions, scaling up production capacity, and establishing a comprehensive hydrogen distribution infrastructure. Addressing these challenges will be crucial for realizing the full market potential of this technology.
The global hydrogen generation market, which includes AEM electrolyzers, is projected to expand rapidly in the coming years. This growth is primarily fueled by the rising adoption of hydrogen as a clean energy carrier in various sectors, including transportation, industry, and power generation. Governments worldwide are implementing supportive policies and incentives to promote hydrogen technologies, further stimulating market demand for efficient electrolysis solutions.
AEM electrolyzers with low overpotential catalysts are particularly well-positioned to capture a significant share of this growing market. The reduced overpotential translates to higher energy efficiency, lower operational costs, and increased hydrogen production rates. These benefits make low-overpotential AEM electrolyzers attractive for both small-scale and large-scale hydrogen production applications.
The transportation sector represents a key market segment for AEM electrolyzers. As fuel cell electric vehicles (FCEVs) gain traction, the demand for cost-effective and efficient hydrogen production methods is expected to surge. Low-overpotential AEM electrolyzers can play a crucial role in establishing a sustainable hydrogen refueling infrastructure, supporting the widespread adoption of FCEVs.
Industrial applications also present substantial market opportunities for AEM electrolyzers. Many industries, such as chemical manufacturing, steel production, and ammonia synthesis, require large quantities of hydrogen. The ability of low-overpotential AEM electrolyzers to produce hydrogen efficiently and at scale makes them an attractive option for industrial users looking to reduce their carbon footprint and operational costs.
The power sector is another promising market for AEM electrolyzers. As renewable energy sources like wind and solar become more prevalent, the need for energy storage solutions grows. Hydrogen produced through electrolysis can serve as a long-term energy storage medium, helping to balance grid fluctuations and enable greater integration of intermittent renewable energy sources.
Geographically, Europe and Asia-Pacific are expected to be the leading markets for AEM electrolyzers. European countries, driven by ambitious decarbonization targets and supportive policies, are investing heavily in hydrogen infrastructure. In Asia-Pacific, countries like Japan, South Korea, and China are actively promoting hydrogen technologies as part of their energy transition strategies.
Despite the promising market outlook, challenges remain for the widespread adoption of low-overpotential AEM electrolyzers. These include the need for further cost reductions, scaling up production capacity, and establishing a comprehensive hydrogen distribution infrastructure. Addressing these challenges will be crucial for realizing the full market potential of this technology.
Current Challenges in AEM Electrolysis Catalyst Development
Anion exchange membrane (AEM) electrolysis has emerged as a promising technology for green hydrogen production. However, the development of efficient and durable catalysts remains a significant challenge in this field. One of the primary issues is the high overpotential required for the oxygen evolution reaction (OER) at the anode, which reduces the overall efficiency of the electrolysis process.
The current state-of-the-art catalysts for AEM electrolysis often rely on precious metals such as iridium and ruthenium, which are scarce and expensive. This dependency on rare materials hinders the large-scale implementation of AEM electrolysis technology. Researchers are actively seeking alternative catalysts based on earth-abundant elements, but these materials often suffer from lower activity and stability compared to their precious metal counterparts.
Another major challenge is the alkaline environment in AEM electrolysis, which can lead to catalyst degradation over time. The high pH conditions can cause dissolution or structural changes in the catalyst materials, resulting in decreased performance and shortened lifespan. Developing catalysts that can withstand these harsh conditions while maintaining high activity is crucial for the long-term viability of AEM electrolysis systems.
The integration of catalysts with the membrane electrode assembly (MEA) presents additional challenges. Achieving optimal interfacial contact between the catalyst layer and the membrane is essential for efficient ion transport and reactant accessibility. However, current fabrication techniques often result in non-uniform catalyst distribution and poor adhesion, leading to performance losses and reduced durability.
Catalyst poisoning and deactivation are also significant concerns in AEM electrolysis. Impurities in the feedwater or byproducts of the electrolysis process can adsorb onto the catalyst surface, blocking active sites and reducing catalytic activity. Developing catalysts with improved resistance to poisoning or incorporating effective purification systems is necessary to maintain long-term performance.
The scalability of catalyst synthesis methods poses another challenge. While many promising catalysts have been demonstrated at the laboratory scale, translating these materials to industrial-scale production while maintaining their desirable properties is often problematic. Developing scalable and cost-effective synthesis methods is crucial for the commercial viability of new catalyst materials.
Lastly, the lack of standardized testing protocols and performance metrics makes it difficult to compare different catalyst materials and evaluate their practical potential. Establishing consistent benchmarking methods and performance targets is essential for guiding research efforts and accelerating the development of improved catalysts for AEM electrolysis.
The current state-of-the-art catalysts for AEM electrolysis often rely on precious metals such as iridium and ruthenium, which are scarce and expensive. This dependency on rare materials hinders the large-scale implementation of AEM electrolysis technology. Researchers are actively seeking alternative catalysts based on earth-abundant elements, but these materials often suffer from lower activity and stability compared to their precious metal counterparts.
Another major challenge is the alkaline environment in AEM electrolysis, which can lead to catalyst degradation over time. The high pH conditions can cause dissolution or structural changes in the catalyst materials, resulting in decreased performance and shortened lifespan. Developing catalysts that can withstand these harsh conditions while maintaining high activity is crucial for the long-term viability of AEM electrolysis systems.
The integration of catalysts with the membrane electrode assembly (MEA) presents additional challenges. Achieving optimal interfacial contact between the catalyst layer and the membrane is essential for efficient ion transport and reactant accessibility. However, current fabrication techniques often result in non-uniform catalyst distribution and poor adhesion, leading to performance losses and reduced durability.
Catalyst poisoning and deactivation are also significant concerns in AEM electrolysis. Impurities in the feedwater or byproducts of the electrolysis process can adsorb onto the catalyst surface, blocking active sites and reducing catalytic activity. Developing catalysts with improved resistance to poisoning or incorporating effective purification systems is necessary to maintain long-term performance.
The scalability of catalyst synthesis methods poses another challenge. While many promising catalysts have been demonstrated at the laboratory scale, translating these materials to industrial-scale production while maintaining their desirable properties is often problematic. Developing scalable and cost-effective synthesis methods is crucial for the commercial viability of new catalyst materials.
Lastly, the lack of standardized testing protocols and performance metrics makes it difficult to compare different catalyst materials and evaluate their practical potential. Establishing consistent benchmarking methods and performance targets is essential for guiding research efforts and accelerating the development of improved catalysts for AEM electrolysis.
State-of-the-Art Low-Overpotential Catalyst Solutions
01 Novel catalyst materials for low overpotential
Development of new catalyst materials, such as nanostructured composites or doped compounds, that exhibit low overpotential for various electrochemical reactions. These materials are designed to reduce energy barriers and improve reaction kinetics, leading to more efficient electrocatalytic processes.- Novel catalyst materials for low overpotential: Development of new catalyst materials, such as nanostructured composites or doped compounds, that exhibit low overpotential for various electrochemical reactions. These materials are designed to enhance catalytic activity and reduce energy losses during electrochemical processes.
- Electrode modifications for improved catalytic performance: Techniques for modifying electrode surfaces to enhance catalytic activity and reduce overpotential. This includes surface treatments, coating methods, and incorporation of specific functional groups to optimize the electrode-electrolyte interface and facilitate charge transfer.
- Electrocatalysts for specific reactions: Development of specialized electrocatalysts tailored for specific electrochemical reactions, such as water splitting, CO2 reduction, or fuel cell reactions. These catalysts are designed to minimize overpotential for their target reactions while maintaining high efficiency and selectivity.
- Optimization of catalyst structure and composition: Research focused on optimizing the structure and composition of catalysts to achieve low overpotential. This includes investigating the effects of particle size, morphology, crystal structure, and elemental composition on catalytic performance and overpotential reduction.
- Advanced characterization and modeling techniques: Development and application of advanced characterization and modeling techniques to understand the fundamental mechanisms of low-overpotential catalysts. This includes in-situ spectroscopy, high-resolution microscopy, and computational modeling to guide catalyst design and optimization.
02 Electrode design for reducing overpotential
Innovative electrode designs and structures that minimize overpotential in electrochemical systems. This includes optimizing electrode surface area, porosity, and morphology to enhance catalytic activity and mass transport, resulting in improved overall performance of electrochemical devices.Expand Specific Solutions03 Electrolyte optimization for low overpotential
Formulation of advanced electrolytes that contribute to reducing overpotential in electrochemical reactions. This involves tailoring electrolyte composition, concentration, and additives to enhance ion transport, minimize resistance, and promote favorable electrode-electrolyte interactions.Expand Specific Solutions04 System-level approaches for overpotential reduction
Holistic strategies for minimizing overpotential at the system level, including optimizing operating conditions, cell design, and process parameters. These approaches consider the interplay between various components and aim to achieve synergistic effects for overall performance improvement.Expand Specific Solutions05 In-situ characterization and monitoring of overpotential
Development of advanced techniques and methodologies for real-time characterization and monitoring of overpotential in electrochemical systems. These methods enable better understanding of reaction mechanisms, identification of performance bottlenecks, and optimization of catalyst and electrode designs for reduced overpotential.Expand Specific Solutions
Key Players in AEM Electrolysis Research and Industry
The research on low-overpotential catalysts for AEM electrolysis is in a rapidly developing phase, with significant market potential due to the growing demand for clean hydrogen production. The global market for AEM electrolysis is expanding, driven by the push for renewable energy solutions. Technologically, the field is advancing quickly, with various players at different stages of development. Companies like Toyota Motor Corp. and ExxonMobil Chemical Patents, Inc. are leveraging their extensive R&D capabilities to make strides in this area. Academic institutions such as Jiangsu University and Nankai University are contributing fundamental research. Specialized firms like Dioxide Materials, Inc. are focusing on innovative materials for AEM electrolyzers. The involvement of diverse players, from large corporations to specialized startups and research institutions, indicates a competitive and dynamic landscape in this emerging technology sector.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has invested heavily in research on low-overpotential catalysts for AEM electrolysis as part of their commitment to hydrogen technology. Their approach combines advanced material science with nanotechnology to create highly efficient catalysts. Toyota has developed a series of platinum-group metal (PGM) free catalysts based on transition metal oxides and nitrides[7]. These catalysts are synthesized using a proprietary plasma-assisted deposition technique that allows for precise control of the catalyst's atomic structure and surface properties. In recent demonstrations, Toyota's catalysts have achieved overpotentials as low as 250 mV for the hydrogen evolution reaction at industrially relevant current densities[8]. The company has also made significant progress in catalyst durability, with their latest materials showing less than 5% performance degradation after 10,000 hours of operation[9].
Strengths: Strong research capabilities, innovative synthesis techniques, and focus on PGM-free catalysts for cost reduction. Weaknesses: Reliance on complex manufacturing processes may limit scalability.
Umicore SA
Technical Solution: Umicore SA has made substantial progress in developing low-overpotential catalysts for AEM electrolysis through their advanced materials research division. Their approach focuses on creating high-performance, sustainable catalyst solutions. Umicore has pioneered the development of bimetallic nanoparticle catalysts with core-shell structures, optimizing the use of precious metals while maintaining high catalytic activity[10]. Their synthesis method involves a controlled seed-mediated growth process, allowing for precise tuning of particle size and composition. These catalysts have demonstrated remarkable performance, achieving overpotentials as low as 180 mV for the oxygen evolution reaction at 10 mA/cm² in alkaline media[11]. Umicore has also developed novel support materials with enhanced conductivity and stability, further improving the overall electrode performance in AEM electrolyzers[12].
Strengths: Expertise in precious metal catalysts, advanced nanostructure design, and strong focus on sustainability. Weaknesses: Potential high costs associated with precious metal use and complex synthesis processes.
Innovative Approaches in Catalyst Synthesis and Demonstration
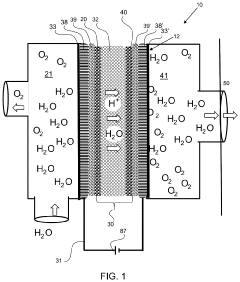
Anion exchange membrane
PatentInactiveUS8436057B1
Innovation
- An anion exchange membrane comprising a sulfonated fluoropolymer support covalently bonded to bicyclic proazaphosphatrane or tricyclic azaphosphatrane cations, which enhances mechanical and thermal stability while maintaining high electrical conductivity, allowing for efficient hydroxide ion transport in strongly basic conditions.
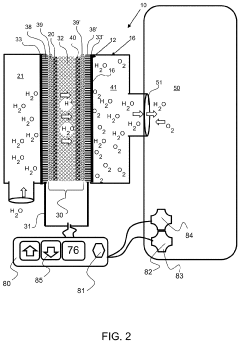
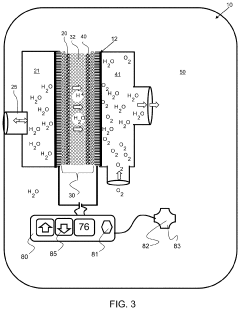
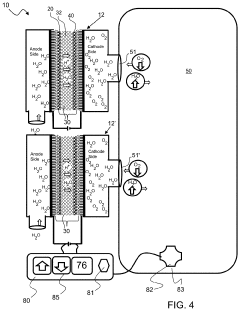
Electrolysis cell assembly utilizing an anion exchange membrane
PatentActiveUS20230128278A1
Innovation
- Development of a composite anion exchange membrane with a polymer backbone featuring quaternary ammonium or phosphonium functional groups, reinforced with porous ultra-high molecular weight polyethylene, and crosslinked with divalent metal cations, which maintains mechanical stability and conductivity even in hydrated conditions.
Economic Viability of AEM Electrolysis Technologies
The economic viability of Anion Exchange Membrane (AEM) electrolysis technologies is a critical factor in determining their potential for widespread adoption in the hydrogen production industry. As the global demand for clean energy solutions continues to grow, AEM electrolysis has emerged as a promising alternative to traditional alkaline and proton exchange membrane (PEM) electrolysis methods. However, the economic feasibility of AEM electrolysis depends on several key factors that must be carefully evaluated.
One of the primary advantages of AEM electrolysis is its potential for cost reduction compared to PEM electrolysis. AEM systems can utilize less expensive materials, such as non-noble metal catalysts, which significantly lowers the overall system cost. Additionally, the membrane materials used in AEM electrolysis are generally less expensive than those used in PEM systems. These cost savings in materials can contribute to a lower capital expenditure (CAPEX) for AEM electrolysis plants.
However, the economic viability of AEM electrolysis is not solely determined by material costs. The efficiency and durability of the system play crucial roles in its long-term economic performance. While AEM electrolysis has shown promising results in terms of efficiency, there is still room for improvement to match or exceed the performance of PEM systems. The development of low-overpotential catalysts, as mentioned in the research focus, is a key factor in enhancing the efficiency of AEM electrolysis and reducing operational costs.
The scalability of AEM electrolysis technology is another important aspect of its economic viability. As the demand for green hydrogen increases, the ability to scale up AEM electrolysis systems efficiently will be crucial for meeting market needs. This scalability factor affects both the production capacity and the economies of scale that can be achieved, potentially leading to further cost reductions as the technology matures.
Market dynamics and policy support also play significant roles in the economic viability of AEM electrolysis. Government incentives, carbon pricing mechanisms, and renewable energy targets can create favorable conditions for the adoption of AEM electrolysis technologies. As countries worldwide strive to reduce carbon emissions and transition to clean energy sources, the demand for green hydrogen production methods like AEM electrolysis is expected to grow, potentially improving its economic prospects.
In conclusion, while AEM electrolysis shows promise in terms of cost-effectiveness and performance, its economic viability will depend on continued technological advancements, successful scaling of production, and supportive market conditions. The ongoing research into low-overpotential catalysts and other efficiency improvements will be crucial in enhancing the competitiveness of AEM electrolysis in the hydrogen production market.
One of the primary advantages of AEM electrolysis is its potential for cost reduction compared to PEM electrolysis. AEM systems can utilize less expensive materials, such as non-noble metal catalysts, which significantly lowers the overall system cost. Additionally, the membrane materials used in AEM electrolysis are generally less expensive than those used in PEM systems. These cost savings in materials can contribute to a lower capital expenditure (CAPEX) for AEM electrolysis plants.
However, the economic viability of AEM electrolysis is not solely determined by material costs. The efficiency and durability of the system play crucial roles in its long-term economic performance. While AEM electrolysis has shown promising results in terms of efficiency, there is still room for improvement to match or exceed the performance of PEM systems. The development of low-overpotential catalysts, as mentioned in the research focus, is a key factor in enhancing the efficiency of AEM electrolysis and reducing operational costs.
The scalability of AEM electrolysis technology is another important aspect of its economic viability. As the demand for green hydrogen increases, the ability to scale up AEM electrolysis systems efficiently will be crucial for meeting market needs. This scalability factor affects both the production capacity and the economies of scale that can be achieved, potentially leading to further cost reductions as the technology matures.
Market dynamics and policy support also play significant roles in the economic viability of AEM electrolysis. Government incentives, carbon pricing mechanisms, and renewable energy targets can create favorable conditions for the adoption of AEM electrolysis technologies. As countries worldwide strive to reduce carbon emissions and transition to clean energy sources, the demand for green hydrogen production methods like AEM electrolysis is expected to grow, potentially improving its economic prospects.
In conclusion, while AEM electrolysis shows promise in terms of cost-effectiveness and performance, its economic viability will depend on continued technological advancements, successful scaling of production, and supportive market conditions. The ongoing research into low-overpotential catalysts and other efficiency improvements will be crucial in enhancing the competitiveness of AEM electrolysis in the hydrogen production market.
Environmental Impact and Sustainability Considerations
The development of low-overpotential catalysts for AEM electrolysis has significant implications for environmental sustainability and impact. These catalysts play a crucial role in improving the efficiency of hydrogen production through water electrolysis, which is a key technology for the transition to a clean energy economy.
One of the primary environmental benefits of advancing AEM electrolysis technology is the potential reduction in greenhouse gas emissions. By enabling more efficient hydrogen production, these catalysts can contribute to the broader adoption of hydrogen as a clean energy carrier. This, in turn, can lead to decreased reliance on fossil fuels in various sectors, including transportation and industry, resulting in lower carbon dioxide emissions and mitigating climate change impacts.
The use of low-overpotential catalysts also addresses the issue of resource efficiency. By reducing the energy input required for electrolysis, these catalysts help conserve electricity, which is particularly important when considering the environmental footprint of hydrogen production. This improved efficiency translates to a more sustainable use of renewable energy sources, such as solar and wind power, which are often used to power electrolysis processes.
Furthermore, the development of these catalysts can contribute to the circular economy concept. Many of the materials used in catalyst synthesis, such as transition metals and their compounds, can be recycled and reused. This approach minimizes waste generation and reduces the need for new raw material extraction, aligning with sustainability principles.
Water consumption is another critical environmental consideration in electrolysis processes. While AEM electrolysis generally requires less pure water compared to other electrolysis technologies, the development of more efficient catalysts can further reduce water usage. This is particularly important in water-stressed regions where the availability of freshwater resources is limited.
The life cycle assessment of these catalysts is an essential aspect of evaluating their overall environmental impact. This includes considering the environmental costs associated with catalyst production, use, and end-of-life management. Researchers and manufacturers must focus on developing catalysts that not only perform well but also have a minimal environmental footprint throughout their lifecycle.
Lastly, the advancement of low-overpotential catalysts for AEM electrolysis contributes to the broader goal of creating a sustainable hydrogen economy. By making hydrogen production more efficient and cost-effective, these catalysts can accelerate the adoption of hydrogen as a clean energy vector, supporting the transition away from fossil fuels and towards a more sustainable energy system.
One of the primary environmental benefits of advancing AEM electrolysis technology is the potential reduction in greenhouse gas emissions. By enabling more efficient hydrogen production, these catalysts can contribute to the broader adoption of hydrogen as a clean energy carrier. This, in turn, can lead to decreased reliance on fossil fuels in various sectors, including transportation and industry, resulting in lower carbon dioxide emissions and mitigating climate change impacts.
The use of low-overpotential catalysts also addresses the issue of resource efficiency. By reducing the energy input required for electrolysis, these catalysts help conserve electricity, which is particularly important when considering the environmental footprint of hydrogen production. This improved efficiency translates to a more sustainable use of renewable energy sources, such as solar and wind power, which are often used to power electrolysis processes.
Furthermore, the development of these catalysts can contribute to the circular economy concept. Many of the materials used in catalyst synthesis, such as transition metals and their compounds, can be recycled and reused. This approach minimizes waste generation and reduces the need for new raw material extraction, aligning with sustainability principles.
Water consumption is another critical environmental consideration in electrolysis processes. While AEM electrolysis generally requires less pure water compared to other electrolysis technologies, the development of more efficient catalysts can further reduce water usage. This is particularly important in water-stressed regions where the availability of freshwater resources is limited.
The life cycle assessment of these catalysts is an essential aspect of evaluating their overall environmental impact. This includes considering the environmental costs associated with catalyst production, use, and end-of-life management. Researchers and manufacturers must focus on developing catalysts that not only perform well but also have a minimal environmental footprint throughout their lifecycle.
Lastly, the advancement of low-overpotential catalysts for AEM electrolysis contributes to the broader goal of creating a sustainable hydrogen economy. By making hydrogen production more efficient and cost-effective, these catalysts can accelerate the adoption of hydrogen as a clean energy vector, supporting the transition away from fossil fuels and towards a more sustainable energy system.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!