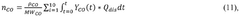How to Compare Catalysts by Cost per kg H2 Produced — Economic Benchmarking
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst Benchmarking Goals
The primary goal of catalyst benchmarking in hydrogen production is to establish a standardized methodology for comparing the economic efficiency of different catalysts. This approach aims to provide a clear and objective basis for evaluating catalysts based on their cost-effectiveness in producing hydrogen, specifically focusing on the cost per kilogram of hydrogen produced.
One of the key objectives is to develop a comprehensive framework that takes into account various factors affecting catalyst performance and cost. This includes considering the initial cost of the catalyst, its longevity, activity, selectivity, and regeneration potential. By incorporating these elements, the benchmarking process can offer a more holistic view of a catalyst's economic value over its entire lifecycle.
Another crucial aim is to create a level playing field for comparison across different types of catalysts and production processes. This involves standardizing the evaluation criteria and testing conditions to ensure that the results are comparable and reproducible. Such standardization is essential for making informed decisions when selecting catalysts for industrial applications.
The benchmarking goals also include identifying the most cost-effective catalysts for specific hydrogen production methods, such as steam methane reforming, water electrolysis, or biomass gasification. This targeted approach allows for more precise comparisons within each production category, leading to optimized catalyst selection for different industrial scenarios.
Furthermore, the benchmarking process seeks to highlight areas for potential improvement in catalyst design and manufacturing. By analyzing the cost structure and performance metrics of various catalysts, researchers and manufacturers can identify key factors that contribute to lower hydrogen production costs. This insight can drive innovation in catalyst development, focusing efforts on enhancing the most critical aspects of catalyst performance.
An additional objective is to provide industry stakeholders with a reliable tool for assessing the economic viability of new catalyst technologies. This is particularly important in the rapidly evolving field of hydrogen production, where novel catalysts are continually being developed. The benchmarking methodology aims to offer a quick and accurate means of evaluating these new catalysts against established alternatives.
Lastly, the benchmarking goals include the development of a dynamic model that can adapt to changing market conditions, such as fluctuations in raw material costs or energy prices. This flexibility ensures that the economic comparisons remain relevant and accurate over time, providing ongoing value to the hydrogen production industry.
One of the key objectives is to develop a comprehensive framework that takes into account various factors affecting catalyst performance and cost. This includes considering the initial cost of the catalyst, its longevity, activity, selectivity, and regeneration potential. By incorporating these elements, the benchmarking process can offer a more holistic view of a catalyst's economic value over its entire lifecycle.
Another crucial aim is to create a level playing field for comparison across different types of catalysts and production processes. This involves standardizing the evaluation criteria and testing conditions to ensure that the results are comparable and reproducible. Such standardization is essential for making informed decisions when selecting catalysts for industrial applications.
The benchmarking goals also include identifying the most cost-effective catalysts for specific hydrogen production methods, such as steam methane reforming, water electrolysis, or biomass gasification. This targeted approach allows for more precise comparisons within each production category, leading to optimized catalyst selection for different industrial scenarios.
Furthermore, the benchmarking process seeks to highlight areas for potential improvement in catalyst design and manufacturing. By analyzing the cost structure and performance metrics of various catalysts, researchers and manufacturers can identify key factors that contribute to lower hydrogen production costs. This insight can drive innovation in catalyst development, focusing efforts on enhancing the most critical aspects of catalyst performance.
An additional objective is to provide industry stakeholders with a reliable tool for assessing the economic viability of new catalyst technologies. This is particularly important in the rapidly evolving field of hydrogen production, where novel catalysts are continually being developed. The benchmarking methodology aims to offer a quick and accurate means of evaluating these new catalysts against established alternatives.
Lastly, the benchmarking goals include the development of a dynamic model that can adapt to changing market conditions, such as fluctuations in raw material costs or energy prices. This flexibility ensures that the economic comparisons remain relevant and accurate over time, providing ongoing value to the hydrogen production industry.
H2 Production Market Analysis
The hydrogen production market has been experiencing significant growth in recent years, driven by the increasing demand for clean energy solutions and the global push towards decarbonization. As of 2021, the global hydrogen production market was valued at approximately $130 billion, with projections indicating a compound annual growth rate (CAGR) of 9.2% through 2030.
The market is segmented based on production methods, with steam methane reforming (SMR) currently dominating the industry, accounting for about 76% of global hydrogen production. However, there is a growing shift towards greener production methods, particularly electrolysis, which is expected to see the fastest growth rate in the coming years.
Geographically, Asia-Pacific leads the market, with China being the largest producer and consumer of hydrogen. Europe and North America follow closely, with both regions investing heavily in hydrogen infrastructure and production capabilities. The European Union's ambitious hydrogen strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, significantly boosting the market potential in the region.
Key industry players include Air Liquide, Linde plc, Air Products and Chemicals, Inc., and Plug Power Inc. These companies are actively investing in research and development to improve production efficiency and reduce costs, particularly in green hydrogen technologies.
The cost of hydrogen production varies significantly depending on the production method and region. Currently, grey hydrogen produced through SMR costs between $1-2 per kg, while green hydrogen from electrolysis ranges from $3-8 per kg. However, the cost of green hydrogen is expected to decrease substantially, potentially reaching parity with grey hydrogen by 2030 in some regions.
Market trends indicate a growing focus on developing more efficient and cost-effective catalysts for hydrogen production. This aligns with the need for economic benchmarking of catalysts based on the cost per kg of H2 produced. As the industry moves towards larger-scale green hydrogen production, the efficiency and cost-effectiveness of catalysts will play a crucial role in determining the overall economic viability of hydrogen as a clean energy carrier.
The market analysis reveals a strong correlation between government policies and market growth. Countries with supportive regulatory frameworks and incentives for clean hydrogen production are seeing faster market expansion. For instance, Japan's Basic Hydrogen Strategy and Germany's National Hydrogen Strategy have significantly boosted their respective hydrogen markets.
The market is segmented based on production methods, with steam methane reforming (SMR) currently dominating the industry, accounting for about 76% of global hydrogen production. However, there is a growing shift towards greener production methods, particularly electrolysis, which is expected to see the fastest growth rate in the coming years.
Geographically, Asia-Pacific leads the market, with China being the largest producer and consumer of hydrogen. Europe and North America follow closely, with both regions investing heavily in hydrogen infrastructure and production capabilities. The European Union's ambitious hydrogen strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, significantly boosting the market potential in the region.
Key industry players include Air Liquide, Linde plc, Air Products and Chemicals, Inc., and Plug Power Inc. These companies are actively investing in research and development to improve production efficiency and reduce costs, particularly in green hydrogen technologies.
The cost of hydrogen production varies significantly depending on the production method and region. Currently, grey hydrogen produced through SMR costs between $1-2 per kg, while green hydrogen from electrolysis ranges from $3-8 per kg. However, the cost of green hydrogen is expected to decrease substantially, potentially reaching parity with grey hydrogen by 2030 in some regions.
Market trends indicate a growing focus on developing more efficient and cost-effective catalysts for hydrogen production. This aligns with the need for economic benchmarking of catalysts based on the cost per kg of H2 produced. As the industry moves towards larger-scale green hydrogen production, the efficiency and cost-effectiveness of catalysts will play a crucial role in determining the overall economic viability of hydrogen as a clean energy carrier.
The market analysis reveals a strong correlation between government policies and market growth. Countries with supportive regulatory frameworks and incentives for clean hydrogen production are seeing faster market expansion. For instance, Japan's Basic Hydrogen Strategy and Germany's National Hydrogen Strategy have significantly boosted their respective hydrogen markets.
Current Catalyst Challenges
The current challenges in catalyst development for hydrogen production are multifaceted and complex. One of the primary issues is the high cost of catalysts, particularly those containing precious metals like platinum and palladium. These materials, while highly effective, significantly increase the overall cost of hydrogen production, making it less economically viable on a large scale.
Catalyst stability and longevity present another significant challenge. Many catalysts degrade over time, losing their effectiveness and requiring frequent replacement. This not only increases operational costs but also impacts the continuity of hydrogen production processes. Researchers are striving to develop catalysts that maintain their activity over extended periods, even under harsh reaction conditions.
Efficiency is a crucial factor that continues to challenge catalyst developers. While current catalysts can facilitate hydrogen production, there is still considerable room for improvement in terms of reaction rates and energy efficiency. Enhancing catalytic activity to increase hydrogen yield per unit of energy input remains a key focus area for researchers and industry professionals.
The scalability of catalyst production is another hurdle that needs to be overcome. Many promising catalysts developed in laboratory settings face difficulties when scaled up for industrial applications. Issues such as uniform catalyst distribution, heat and mass transfer limitations, and maintaining catalyst performance at larger scales need to be addressed to bridge the gap between research and practical implementation.
Environmental concerns also pose challenges in catalyst development. There is a growing need for catalysts that are not only effective and economical but also environmentally friendly. This includes developing catalysts that use abundant, non-toxic materials and those that can operate under milder conditions, reducing overall energy consumption and environmental impact.
Lastly, the lack of standardized methods for comparing catalyst performance across different studies and technologies hinders progress in the field. Developing a unified framework for economic benchmarking of catalysts based on cost per kg of hydrogen produced is crucial. This would enable more accurate comparisons between different catalytic systems and guide research efforts towards the most promising and cost-effective solutions.
Catalyst stability and longevity present another significant challenge. Many catalysts degrade over time, losing their effectiveness and requiring frequent replacement. This not only increases operational costs but also impacts the continuity of hydrogen production processes. Researchers are striving to develop catalysts that maintain their activity over extended periods, even under harsh reaction conditions.
Efficiency is a crucial factor that continues to challenge catalyst developers. While current catalysts can facilitate hydrogen production, there is still considerable room for improvement in terms of reaction rates and energy efficiency. Enhancing catalytic activity to increase hydrogen yield per unit of energy input remains a key focus area for researchers and industry professionals.
The scalability of catalyst production is another hurdle that needs to be overcome. Many promising catalysts developed in laboratory settings face difficulties when scaled up for industrial applications. Issues such as uniform catalyst distribution, heat and mass transfer limitations, and maintaining catalyst performance at larger scales need to be addressed to bridge the gap between research and practical implementation.
Environmental concerns also pose challenges in catalyst development. There is a growing need for catalysts that are not only effective and economical but also environmentally friendly. This includes developing catalysts that use abundant, non-toxic materials and those that can operate under milder conditions, reducing overall energy consumption and environmental impact.
Lastly, the lack of standardized methods for comparing catalyst performance across different studies and technologies hinders progress in the field. Developing a unified framework for economic benchmarking of catalysts based on cost per kg of hydrogen produced is crucial. This would enable more accurate comparisons between different catalytic systems and guide research efforts towards the most promising and cost-effective solutions.
Economic Evaluation Methods
01 Catalyst composition optimization
Optimizing the composition of catalysts can significantly reduce the cost per kg of H2 produced. This involves developing novel catalyst formulations, adjusting the ratios of active components, and incorporating cost-effective materials while maintaining or improving catalytic performance. Such optimizations can lead to more efficient hydrogen production processes and lower overall catalyst costs.- Catalyst composition optimization: Improving catalyst composition can significantly reduce the cost per kg of H2 produced. This involves developing novel catalyst materials or enhancing existing ones to increase catalytic activity, selectivity, and durability. Optimized compositions can lead to higher hydrogen yields and lower catalyst consumption, thereby reducing overall production costs.
- Catalyst support and structure design: The design of catalyst supports and structures plays a crucial role in reducing costs. By optimizing the surface area, porosity, and stability of catalyst supports, the efficiency of hydrogen production can be improved. Advanced structures such as nanoparticles or core-shell configurations can enhance catalyst performance and longevity, leading to lower costs per kg of H2 produced.
- Process optimization and integration: Optimizing the overall hydrogen production process and integrating it with other systems can reduce catalyst-related costs. This includes improving reaction conditions, developing more efficient reactor designs, and implementing heat recovery systems. Such optimizations can lead to higher hydrogen yields and lower energy consumption, thereby reducing the overall cost per kg of H2 produced.
- Catalyst regeneration and recycling: Developing effective methods for catalyst regeneration and recycling can significantly reduce the cost of hydrogen production. By extending catalyst lifespan and reusing spent catalysts, the overall catalyst consumption and replacement costs can be minimized. This approach helps in lowering the long-term operational costs associated with hydrogen production.
- Alternative low-cost catalyst materials: Exploring and developing alternative low-cost catalyst materials can help reduce the overall cost of hydrogen production. This includes investigating non-noble metal catalysts, earth-abundant materials, or bio-inspired catalysts that can offer comparable performance to traditional expensive catalysts. The use of such materials can significantly lower the catalyst cost component in hydrogen production.
02 Catalyst support material selection
The choice of catalyst support material plays a crucial role in determining the cost-effectiveness of hydrogen production. Utilizing inexpensive, abundant, and durable support materials can significantly reduce the overall catalyst cost while maintaining high catalytic activity. This approach focuses on identifying and developing support materials that enhance catalyst stability and performance without substantially increasing production costs.Expand Specific Solutions03 Catalyst recycling and regeneration
Implementing effective catalyst recycling and regeneration techniques can substantially reduce the cost per kg of H2 produced. This involves developing methods to recover and reactivate spent catalysts, extending their useful life, and minimizing the need for frequent catalyst replacement. Such approaches can significantly lower the long-term costs associated with catalyst usage in hydrogen production processes.Expand Specific Solutions04 Process optimization for catalyst efficiency
Optimizing the hydrogen production process to maximize catalyst efficiency can lead to reduced catalyst costs per kg of H2 produced. This includes fine-tuning reaction conditions, improving reactor designs, and implementing advanced control strategies to enhance catalyst performance and longevity. By increasing the overall efficiency of the catalytic process, the amount of catalyst required per unit of hydrogen produced can be minimized.Expand Specific Solutions05 Low-cost catalyst alternatives
Developing and utilizing low-cost catalyst alternatives can significantly reduce the overall cost of hydrogen production. This approach focuses on identifying and synthesizing catalysts using abundant, inexpensive materials that can match or exceed the performance of traditional, more expensive catalysts. By replacing costly noble metals with more economical alternatives, the cost per kg of H2 produced can be substantially lowered.Expand Specific Solutions
Key H2 Catalyst Manufacturers
The economic benchmarking of catalysts for hydrogen production is currently in a growth phase, with increasing market size driven by the global push for clean energy solutions. The technology maturity varies among key players, with established companies like China Petroleum & Chemical Corp., BASF Corp., and BP Plc leading in commercial applications. Emerging players such as Susteon, Inc. and IFP Energies Nouvelles are focusing on innovative catalyst designs to improve efficiency and reduce costs. Research institutions like The University of Liverpool and Northwestern University are contributing to advancing the fundamental understanding of catalyst mechanisms, potentially leading to breakthrough technologies in the near future.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a cost-effective catalyst benchmarking approach for hydrogen production. Their method involves a comprehensive analysis of catalyst performance, including activity, selectivity, and stability, combined with economic factors such as raw material costs and operational expenses. Sinopec's approach utilizes advanced high-throughput experimentation techniques to rapidly screen and evaluate multiple catalyst formulations[1]. They have implemented a standardized testing protocol that simulates industrial conditions, allowing for accurate comparisons of different catalysts' hydrogen production efficiency. The company has also integrated machine learning algorithms to predict catalyst performance and optimize formulations, potentially reducing development time and costs[3].
Strengths: Extensive R&D resources, access to large-scale industrial data, and integrated supply chain. Weaknesses: Potential bias towards petroleum-based feedstocks, slower adoption of cutting-edge technologies compared to smaller, more agile competitors.
BASF Corp.
Technical Solution: BASF Corp. has developed a sophisticated catalyst benchmarking system for hydrogen production, focusing on economic efficiency. Their approach incorporates a holistic view of the entire production process, considering not only the catalyst cost but also its impact on overall plant economics. BASF utilizes advanced process simulation tools to model various scenarios, allowing for accurate predictions of catalyst performance under different operating conditions[2]. They have implemented a life cycle assessment (LCA) methodology to evaluate the environmental impact of catalysts, which is increasingly important for sustainable hydrogen production. BASF's benchmarking system also includes accelerated aging tests to predict long-term catalyst stability and performance, crucial for calculating the true cost per kg of H2 produced over the catalyst's lifetime[4].
Strengths: Comprehensive approach considering both economic and environmental factors, strong expertise in catalyst development and manufacturing. Weaknesses: Potential overemphasis on traditional chemical processes, may be slower to adapt to emerging hydrogen production technologies.
Innovative Catalyst Designs
Semi-continuous process for co-production of co 2-free hydrogen and high value carbon via hydrocarbon pyrolysis
PatentWO2024156001A1
Innovation
- A semi-continuous hydrocarbon pyrolysis process using a fluidized-bed reactor with a pyrolysis catalyst, such as Fe/θ-Al2O3, to convert hydrocarbons into CO2-free hydrogen and high-value carbon materials like carbon nanotubes, where the catalyst is continuously reused and regenerated, allowing for efficient production and collection of both products.
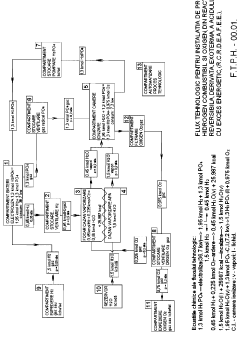
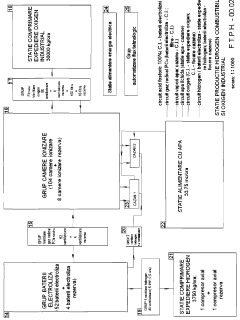
TECHNOLOGY BASED ON EXOTHERMAL, DERIVED REVERSIBLE CHEMICAL REACTION OF PHOSPHORIC ACID WITH ENERGETIC EXCESS (<b>RCR DEAFEE</b>) FOR PRODUCING COMBUSTIBLE HYDROGEN AND INDUSTRIAL OXYGEN
PatentUndeterminedRO126560A2
Innovation
- The RCRDEAFEE method employs a reversible, exothermic chemical reaction of phosphoric acid to produce hydrogen and oxygen, utilizing water as the primary raw material, with a chemical process that includes electrolysis, combustion, and ionization chamber reactions to achieve a theoretical energy surplus and low-cost production.
Sustainability in Catalysis
Sustainability in catalysis has become a crucial focus in the field of hydrogen production, particularly when comparing catalysts based on their cost-effectiveness per kilogram of hydrogen produced. This economic benchmarking approach not only addresses the financial aspects but also aligns with broader environmental and social sustainability goals.
The evaluation of catalysts for hydrogen production must consider multiple factors beyond mere cost. Efficiency, longevity, and environmental impact are key components in determining a catalyst's overall sustainability. Catalysts that demonstrate high activity and selectivity while requiring minimal energy input contribute significantly to reducing the carbon footprint of hydrogen production processes.
Life cycle assessment (LCA) plays a vital role in comprehensively analyzing the sustainability of catalysts. This approach examines the environmental impacts from raw material extraction to catalyst synthesis, use, and eventual disposal or recycling. Catalysts composed of abundant, non-toxic elements that can be easily recycled or regenerated are increasingly favored for their reduced environmental burden and improved long-term economic viability.
The concept of atom economy is particularly relevant in sustainable catalysis for hydrogen production. Catalysts that maximize the utilization of input materials and minimize waste generation contribute to both economic efficiency and environmental stewardship. This principle encourages the development of catalytic systems that operate under mild conditions, reducing energy requirements and associated costs.
Innovations in catalyst design are driving progress towards more sustainable hydrogen production methods. Nanotechnology has enabled the creation of catalysts with increased surface area and enhanced reactivity, potentially lowering the required catalyst loading and improving cost-effectiveness. Additionally, the development of bio-inspired catalysts mimicking natural enzymatic processes offers promising avenues for sustainable hydrogen production with minimal environmental impact.
The integration of renewable energy sources in catalyst production and hydrogen generation processes further enhances sustainability. Solar-powered electrolysis and photocatalytic water splitting represent cutting-edge approaches that align economic benchmarking with environmental considerations. These technologies have the potential to significantly reduce the carbon intensity of hydrogen production, making it a more viable clean energy carrier.
In conclusion, the sustainability aspect of catalysis in hydrogen production extends beyond simple cost comparisons. It encompasses a holistic view of economic, environmental, and social factors, driving innovation towards more efficient, cleaner, and economically viable hydrogen production methods. As research progresses, the synergy between cost-effectiveness and sustainability will likely become an increasingly important criterion in catalyst development and selection for industrial-scale hydrogen production.
The evaluation of catalysts for hydrogen production must consider multiple factors beyond mere cost. Efficiency, longevity, and environmental impact are key components in determining a catalyst's overall sustainability. Catalysts that demonstrate high activity and selectivity while requiring minimal energy input contribute significantly to reducing the carbon footprint of hydrogen production processes.
Life cycle assessment (LCA) plays a vital role in comprehensively analyzing the sustainability of catalysts. This approach examines the environmental impacts from raw material extraction to catalyst synthesis, use, and eventual disposal or recycling. Catalysts composed of abundant, non-toxic elements that can be easily recycled or regenerated are increasingly favored for their reduced environmental burden and improved long-term economic viability.
The concept of atom economy is particularly relevant in sustainable catalysis for hydrogen production. Catalysts that maximize the utilization of input materials and minimize waste generation contribute to both economic efficiency and environmental stewardship. This principle encourages the development of catalytic systems that operate under mild conditions, reducing energy requirements and associated costs.
Innovations in catalyst design are driving progress towards more sustainable hydrogen production methods. Nanotechnology has enabled the creation of catalysts with increased surface area and enhanced reactivity, potentially lowering the required catalyst loading and improving cost-effectiveness. Additionally, the development of bio-inspired catalysts mimicking natural enzymatic processes offers promising avenues for sustainable hydrogen production with minimal environmental impact.
The integration of renewable energy sources in catalyst production and hydrogen generation processes further enhances sustainability. Solar-powered electrolysis and photocatalytic water splitting represent cutting-edge approaches that align economic benchmarking with environmental considerations. These technologies have the potential to significantly reduce the carbon intensity of hydrogen production, making it a more viable clean energy carrier.
In conclusion, the sustainability aspect of catalysis in hydrogen production extends beyond simple cost comparisons. It encompasses a holistic view of economic, environmental, and social factors, driving innovation towards more efficient, cleaner, and economically viable hydrogen production methods. As research progresses, the synergy between cost-effectiveness and sustainability will likely become an increasingly important criterion in catalyst development and selection for industrial-scale hydrogen production.
Catalyst Lifecycle Assessment
Catalyst lifecycle assessment is a crucial aspect of evaluating the economic viability and environmental impact of hydrogen production processes. This assessment involves analyzing the entire lifespan of a catalyst, from its production to its eventual disposal or recycling. The primary goal is to determine the overall cost-effectiveness and sustainability of the catalyst in hydrogen production.
The lifecycle of a catalyst typically begins with raw material extraction and processing. These materials are then synthesized into the catalyst through various chemical processes. The production phase is critical in determining the initial cost of the catalyst, which directly impacts the overall cost per kg of hydrogen produced. Factors such as energy consumption, resource availability, and manufacturing complexity all contribute to this initial cost.
Once the catalyst is in use, its performance characteristics become paramount. The activity, selectivity, and stability of the catalyst directly influence the efficiency of hydrogen production. Higher activity catalysts can produce more hydrogen per unit time, potentially offsetting higher initial costs. Selectivity ensures that the desired hydrogen product is maximized while minimizing unwanted by-products. Stability determines the catalyst's lifespan, with more stable catalysts requiring less frequent replacement and thus reducing long-term costs.
Deactivation mechanisms play a significant role in catalyst lifecycle assessment. Catalysts may lose effectiveness due to poisoning, sintering, or fouling. Understanding these mechanisms is crucial for predicting catalyst lifespan and planning maintenance or replacement schedules. Strategies to mitigate deactivation, such as regeneration processes, can extend catalyst life and improve overall economic performance.
The end-of-life phase of a catalyst is equally important in lifecycle assessment. Options for spent catalysts include disposal, recycling, or regeneration. Recycling and regeneration can recover valuable materials and potentially reduce the overall environmental impact and cost of catalyst use. However, these processes come with their own economic and energy costs, which must be factored into the overall assessment.
Environmental considerations are increasingly significant in catalyst lifecycle assessments. This includes evaluating the carbon footprint of catalyst production, use, and disposal. Catalysts that enable more efficient hydrogen production with lower greenhouse gas emissions may be preferred, even if they have higher upfront costs. Additionally, the use of abundant, non-toxic materials in catalyst design can improve sustainability and reduce long-term environmental risks.
In conclusion, a comprehensive catalyst lifecycle assessment provides valuable insights for comparing catalysts based on their cost per kg of hydrogen produced. By considering all phases of the catalyst's life, from production to disposal, researchers and industry professionals can make informed decisions that balance economic efficiency with environmental sustainability in hydrogen production processes.
The lifecycle of a catalyst typically begins with raw material extraction and processing. These materials are then synthesized into the catalyst through various chemical processes. The production phase is critical in determining the initial cost of the catalyst, which directly impacts the overall cost per kg of hydrogen produced. Factors such as energy consumption, resource availability, and manufacturing complexity all contribute to this initial cost.
Once the catalyst is in use, its performance characteristics become paramount. The activity, selectivity, and stability of the catalyst directly influence the efficiency of hydrogen production. Higher activity catalysts can produce more hydrogen per unit time, potentially offsetting higher initial costs. Selectivity ensures that the desired hydrogen product is maximized while minimizing unwanted by-products. Stability determines the catalyst's lifespan, with more stable catalysts requiring less frequent replacement and thus reducing long-term costs.
Deactivation mechanisms play a significant role in catalyst lifecycle assessment. Catalysts may lose effectiveness due to poisoning, sintering, or fouling. Understanding these mechanisms is crucial for predicting catalyst lifespan and planning maintenance or replacement schedules. Strategies to mitigate deactivation, such as regeneration processes, can extend catalyst life and improve overall economic performance.
The end-of-life phase of a catalyst is equally important in lifecycle assessment. Options for spent catalysts include disposal, recycling, or regeneration. Recycling and regeneration can recover valuable materials and potentially reduce the overall environmental impact and cost of catalyst use. However, these processes come with their own economic and energy costs, which must be factored into the overall assessment.
Environmental considerations are increasingly significant in catalyst lifecycle assessments. This includes evaluating the carbon footprint of catalyst production, use, and disposal. Catalysts that enable more efficient hydrogen production with lower greenhouse gas emissions may be preferred, even if they have higher upfront costs. Additionally, the use of abundant, non-toxic materials in catalyst design can improve sustainability and reduce long-term environmental risks.
In conclusion, a comprehensive catalyst lifecycle assessment provides valuable insights for comparing catalysts based on their cost per kg of hydrogen produced. By considering all phases of the catalyst's life, from production to disposal, researchers and industry professionals can make informed decisions that balance economic efficiency with environmental sustainability in hydrogen production processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!